Fast Preparation of Porous MnO/C Microspheres as Anode Materials for Lithium-Ion Batteries
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of Materials
2.2. Characterization of Materials
2.3. Electrochemical Measurements
3. Results and Discussion
3.1. Characterization of Samples
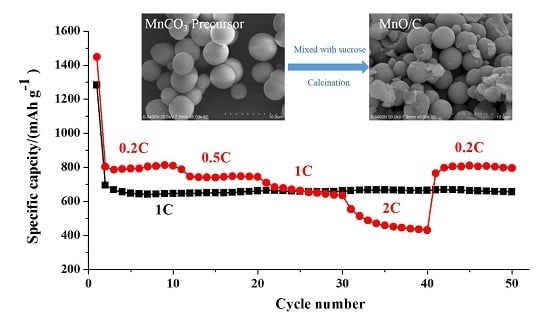
3.2. Electrochemical Performance of the MnO/C Electrode
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Etacheri, V.; Marom, R.; Elazari, R.; Salitra, G.; Aurbach, D. Challenges in the development of advanced Li-ion batteries: A review. Energy Environ. Sci. 2011, 4, 3243–3262. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Park, K. The Li-Ion Rechargeable Battery: A Perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.S.; Hong, Y.J.; Kang, Y.C. Design and Synthesis of Bubble-Nanorod-Structured Fe2O3-Carbon Nanofibers as Advanced Anode Material for Li-Ion Batteries. ACS Nano 2015, 9, 4026–4035. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, C.; Kang, F. Nitrogen-Enriched Porous Carbon Coating for Manganese Oxide Nanostructures toward High-Performance Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2015, 7, 9185–9194. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, Y.; Huang, Z.; Kang, F. MnO-carbon hybrid nanofiber composites as superior anode materials for lithium-ion batteries. Electrochim. Acta 2015, 170, 164–170. [Google Scholar] [CrossRef]
- Lee, R.; Lin, Y.; Weng, Y.; Pan, H.; Lee, J.; Wu, N. Synthesis of high-performance MnOx/carbon composite as lithium-ion battery anode by a facile co-precipitation method: Effects of oxygen stoichiometry and carbon morphology. J. Power Sources 2014, 253, 373–380. [Google Scholar] [CrossRef]
- Li, X.; Zhu, Y.; Zhang, X.; Liang, J.; Qian, Y. MnO@1-D carbon composites from the precursor C4H4MnO6 and their high-performance in lithium batteries. RSC Adv. 2013, 3, 10001–10006. [Google Scholar] [CrossRef]
- Guo, J.; Liu, Q.; Wang, C.; Zachariah, M.R. Interdispersed Amorphous MnOx-Carbon Nanocomposites with Superior Electrochemical Performance as Lithium-Storage Material. Adv. Funct. Mater. 2012, 22, 803–811. [Google Scholar] [CrossRef]
- Wang, S.; Xing, Y.; Xu, H.; Zhang, S. MnO Nanoparticles Interdispersed in 3D Porous Carbon Framework for High Performance Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2014, 6, 12713–12718. [Google Scholar] [CrossRef] [PubMed]
- Qiao, H.; Yao, D.; Cai, Y.; Huang, F.; Wei, Q. One-pot synthesis and electrochemical property of MnO/C hybrid microspheres. Ionics 2013, 19, 595–600. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, X.; Luo, W.; Xia, F.; Huang, Y. Reconstruction of Conformal Nanoscale MnO on Graphene as a High-Capacity and Long-Life Anode Material for Lithium Ion Batteries. Adv. Funct. Mater. 2013, 23, 2436–2444. [Google Scholar] [CrossRef]
- Zhang, S.; He, W.; Zhang, X.; Yang, X. Rational design of carbon-coated hollow MnO nanotubes for Li-ion batteries. J. Mater. Sci. Mater. Electron. 2015, 26, 2189–2197. [Google Scholar] [CrossRef]
- Yang, C.; Gao, Q.; Tian, W.; Tan, Y.; Zhang, T.; Yang, K.; Zhu, L. Superlow load of nanosized MnO on a porous carbon matrix from wood fibre with superior lithium ion storage performance. J. Mater. Chem. A 2014, 2, 19975–19982. [Google Scholar] [CrossRef]
- Liu, B.; Hu, X.; Xu, H.; Luo, W.; Sun, Y.; Huang, Y. Encapsulation of MnO Nanocrystals in Electrospun Carbon Nanofibers as High-Performance Anode Materials for Lithium-Ion Batteries. Sci. Rep. 2014, 4, 4229. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Cheng, H.; Liu, Z.; Yu, Y. Facile Synthesis of Carbon Spheres with Uniformly Dispersed MnO Nanoparticles for Lithium Ion Battery Anode. Electrochim. Acta 2015, 152, 44–52. [Google Scholar] [CrossRef]
- Wang, S.; Ren, Y.; Liu, G.; Xing, Y.; Zhang, S. Peanut-like MnO@C core-shell composites as anode electrodes for high-performance lithium ion batteries. Nanoscale 2014, 6, 3508–3512. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xing, Z.; Wang, L.; Zhu, Y.; Li, Q.; Liang, J.; Yu, Y.; Huang, T.; Tang, K.; Qian, Y.; et al. Synthesis of MnO@C core-shell nanoplates with controllable shell thickness and their electrochemical performance for lithium-ion batteries. J. Mater. Chem. 2012, 22, 17864–17869. [Google Scholar] [CrossRef]
- Chen, W.; Qie, L.; Shen, Y.; Sun, Y.; Yuan, L.; Hu, X.; Zhang, W.; Huang, Y. Superior lithium storage performance in nanoscaled MnO promoted by N-doped carbon webs. Nano Energy 2013, 2, 412–418. [Google Scholar] [CrossRef]
- Gu, X.; Yue, J.; Chen, L.; Liu, S.; Xu, H.; Yang, J.; Qian, Y.; Zhao, X. Coaxial MnO/N-doped carbon nanorods for advanced lithium-ion battery anodes. J. Mater. Chem. A 2015, 3, 1037–1041. [Google Scholar] [CrossRef]
- Masai, H.; Hino, Y.; Yanagida, T.; Fujimoto, Y. Photoluminescence and radioluminescence properties of MnO-doped SnO-ZnO-P2O5 glasses. Opt. Mater. 2015, 42, 381–384. [Google Scholar] [CrossRef]
- Chen, R.; Yan, J.; Liu, Y.; Li, J. Three-Dimensional Nitrogen-Doped Graphene/MnO Nanoparticle Hybrids as a High-Performance Catalyst for Oxygen Reduction Reaction. J. Phys. Chem. C 2015, 119, 8032–8037. [Google Scholar] [CrossRef]
- Sun, X.; Xu, Y.; Ding, P.; Chen, G.; Zheng, X.; Zhang, R.; Li, L. The composite sphere of manganese oxide and carbon nanotubes as a prospective anode material for lithium-ion batteries. J. Power Sources 2014, 255, 163–169. [Google Scholar] [CrossRef]
- Mai, Y.J.; Zhang, D.; Qiao, Y.Q.; Gu, C.D.; Wang, X.L.; Tu, J.P. MnO/reduced graphene oxide sheet hybrid as an anode for Li-ion batteries with enhanced lithium storage performance. J. Power Sources 2012, 216, 201–207. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, X.; Li, F.; Xia, D. Facile synthesis of MnO/C anode materials for lithium-ion batteries. Electrochim. Acta 2011, 56, 6448–6452. [Google Scholar] [CrossRef]
- Su, L.; Zhong, Y.; Wei, J.; Zhou, Z. Preparation and electrochemical Li storage performance of MnO@C nanorods consisting of ultra small MnO nanocrystals. RSC Adv. 2013, 3, 9035–9041. [Google Scholar] [CrossRef]
- Zhu, C.; Sheng, N.; Akiyama, T. MnO nanoparticles embedded in a carbon matrix for a high performance Li ion battery anode. RSC Adv. 2015, 5, 21066–21073. [Google Scholar] [CrossRef]
- Jiang, H.; Hu, Y.; Guo, S.; Yan, C.; Lee, P.S.; Li, C. Rational Design of MnO/Carbon Nanopeapods with Internal Void Space for High-Rate and Long-Life Li-Ion Batteries. ACS Nano 2014, 8, 6038–6046. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, D.; Qiao, L.; Wang, X.; Sun, X.; Wang, P.; He, D. Interconnected porous MnO nanoflakes for high-performance lithium ion battery anodes. J. Mater. Chem. 2012, 22, 9189–9194. [Google Scholar] [CrossRef]
- Xu, G.; Xu, Y.; Sun, H.; Fu, F.; Zheng, X.; Huang, L.; Li, J.; Yang, S.; Sun, S. Facile synthesis of porous MnO/C nanotubes as a high capacity anode material for lithium ion batteries. Chem. Commun. 2012, 48, 8502–8504. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qiu, S.; Lu, G.; He, C.; Liu, J.; Luan, L.; Liu, W. Fabrication of porous MnO microspheres with carbon coating for lithium ion battery application. CrystEngComm 2014, 16, 1802–1809. [Google Scholar] [CrossRef]
- Li, X.; Shang, X.; Li, D.; Yue, H.; Wang, S.; Qiao, L.; He, D. Facile Synthesis of Porous MnO Microspheres for High-Performance Lithium-Ion Batteries. Part. Part. Syst. Charact. 2014, 31, 1001–1007. [Google Scholar] [CrossRef]
- Yue, J.; Gu, X.; Chen, L.; Wang, N.; Jiang, X.; Xu, H.; Yang, J.; Qian, Y. General synthesis of hollow MnO2, Mn3O4 and MnO nanospheres as superior anode materials for lithium ion batteries. J. Mater. Chem. A 2014, 2, 17421–17426. [Google Scholar] [CrossRef]
- Xia, Y.; Xiao, Z.; Dou, X.; Huang, H.; Lu, X.; Yan, R.; Gan, Y.; Zhu, W.; Tu, J.; Zhang, W.; et al. Green and Facile Fabrication of Hollow Porous MnO/C Microspheres from Microalgaes for Lithium-Ion Batteries. ACS Nano 2013, 7, 7083–7092. [Google Scholar] [CrossRef] [PubMed]
- Anh, V.; Qian, Y.; Stein, A. Porous Electrode Materials for Lithium-Ion Batteries—How to Prepare Them and What Makes Them Special. Adv. Energy Mater. 2012, 2, 1056–1085. [Google Scholar]
- Zhong, K.; Zhang, B.; Luo, S.; Wen, W.; Li, H.; Huang, X.; Chen, L. Investigation on porous MnO microsphere anode for lithium ion batteries. J. Power Sources 2011, 196, 6802–6808. [Google Scholar] [CrossRef]
- Guo, S.; Lu, G.; Qiu, S.; Liu, J.; Wang, X.; He, C.; Wei, H.; Yan, X.; Guo, Z. Carbon-coated MnO microparticulate porous nanocomposites serving as anode materials with enhanced electrochemical performances. Nano Energy 2014, 9, 41–49. [Google Scholar] [CrossRef]
- Ma, X.; Wan, Q.; Huang, X.; Ding, C.; Jin, Y.; Guan, Y.; Chen, C. Synthesis of three-dimensionally porous MnO thin films for lithium-ion batteries by improved Electrostatic Spray Deposition technique. Electrochim. Acta 2014, 121, 15–20. [Google Scholar] [CrossRef]
- Liang, Y.; Chu, G.; Wang, J.; Huang, Y.; Chen, J.; Sun, B.; Shao, L. Controllable preparation of nano-CaCO3 in a microporous tube-in-tube microchannel reactor. Chem. Eng. Process. 2014, 79, 34–39. [Google Scholar] [CrossRef]
- Li, S.; Xu, H.; Wang, Y.; Luo, G. Controllable preparation of nanoparticles by drops and plugs flow in a microchannel device. Langmuir 2008, 24, 4194–4199. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Le, D.; Ferguson, P.P.; Dahn, J.R. Lithium polyacrylate as a binder for tin-cobalt-carbon negative electrodes in lithium-ion batteries. Electrochim. Acta 2010, 55, 2991–2995. [Google Scholar] [CrossRef]
- Sun, Y.; Hu, X.; Luo, W.; Huang, Y. Porous carbon-modified MnO disks prepared by a microwave-polyol process and their superior lithium-ion storage properties. J. Mater. Chem. 2012, 22, 19190–19195. [Google Scholar] [CrossRef]
- Sun, X.; Xu, Y.; Ding, P.; Chen, G.; Zheng, X. Superior lithium storage of the carbon modified hybrid of manganese monoxide and carbon nanotubes. Mater. Lett. 2013, 113, 186–189. [Google Scholar] [CrossRef]
- Sun, X.; Xu, Y.; Ding, P.; Jia, M.; Ceder, G. The composite rods of MnO and multi-walled carbon nanotubes as anode materials for lithium ion batteries. J. Power Sources 2013, 244, 690–694. [Google Scholar] [CrossRef]
- Zhao, C.; He, L.; Qiao, S.Z.; Middelberg, A.P.J. Nanoparticle synthesis in microreactors. Chem. Eng. Sci. 2011, 66, 1463–1479. [Google Scholar] [CrossRef]
- Ying, Y.; Chen, G.; Zhao, Y.; Li, S.; Yuan, Q. A high throughput methodology for continuous preparation of monodispersed nanocrystals in microfluidic reactors. Chem. Eng. J. 2008, 135, 209–215. [Google Scholar] [CrossRef]
- Luo, W.; Hu, X.; Sun, Y.; Huang, Y. Controlled Synthesis of Mesoporous MnO/C Networks by Microwave Irradiation and Their Enhanced Lithium-Storage Properties. ACS Appl. Mater. Interfaces 2013, 5, 1997–2003. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Peng, Z.; Wang, Y.; Tang, J.; Zheng, G. MnO Nanoparticle@Mesoporous Carbon Composites Grown on Conducting Substrates Featuring High-performance Lithium-ion Battery, Supercapacitor and Sensor. Sci. Rep. 2013, 3, 2693. [Google Scholar] [CrossRef] [PubMed]
- Su, K.; Wang, C.; Nie, H.; Guan, Y.; Liu, F.; Chen, J. Facile template-free synthesis of 3D porous MnO/C microspheres with controllable pore size for high-performance lithium-ion battery anodes. J. Mater. Chem. A 2014, 2, 10000–10006. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, X.; Wang, R.; Dong, W.; Lu, W.; Wu, X.; Wang, X.; Li, H.; Chen, L. Influences of Additives on the Formation of a Solid Electrolyte Interphase on MnO Electrode Studied by Atomic Force Microscopy and Force Spectroscopy. J. Phys. Chem. C 2014, 118, 20756–20762. [Google Scholar] [CrossRef]
- Tao, X.; Chai, W.; Xu, F.; Luo, J.; Xiao, H.; Liang, C.; Gan, Y.; Huang, H.; Xia, Y.; Zhang, W. Bio-templated Fabrication of Highly Defective Carbon Anchored MnO Anode Materials with High Reversible Capacity. Electrochim. Acta 2015, 169, 159–167. [Google Scholar] [CrossRef]
- Wang, S.; Xiao, C.; Xing, Y.; Xu, H.; Zhang, S. Formation of a stable carbon framework in a MnO yolk-shell sphere to achieve exceptional performance for a Li-ion battery anode. J. Mater. Chem. A 2015, 3, 15591–15597. [Google Scholar] [CrossRef]
- Sun, B.; Chen, Z.; Kim, H.; Ahn, H.; Wang, G. MnO/C core-shell nanorods as high capacity anode materials for lithium-ion batteries. J. Power Sources 2011, 196, 3346–3349. [Google Scholar] [CrossRef]
- Xu, S.; Zhu, Y.; Zhuang, Q.; Wu, C. Hydrothermal synthesis of manganese oxides/carbon nanotubes composites as anode materials for lithium ion batteries. Mater. Res. Bull. 2013, 48, 3479–3484. [Google Scholar] [CrossRef]









| Rs/Ω | Csei/F·cm−2 | Rsei/Ω | CPE Yo/S·sn·cm−2 | Rct/Ω | Zw/S·s0.5·cm−2 |
|---|---|---|---|---|---|
| 3.88 | 6.45 × 10−5 | 23.62 | 3.36 × 10−4 | 16.32 | 0.00889 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, J.; Liang, H.; Gong, X.-N.; Lv, X.-Y.; Long, Y.-F.; Wen, Y.-X. Fast Preparation of Porous MnO/C Microspheres as Anode Materials for Lithium-Ion Batteries. Nanomaterials 2017, 7, 121. https://doi.org/10.3390/nano7060121
Su J, Liang H, Gong X-N, Lv X-Y, Long Y-F, Wen Y-X. Fast Preparation of Porous MnO/C Microspheres as Anode Materials for Lithium-Ion Batteries. Nanomaterials. 2017; 7(6):121. https://doi.org/10.3390/nano7060121
Chicago/Turabian StyleSu, Jing, Hao Liang, Xian-Nian Gong, Xiao-Yan Lv, Yun-Fei Long, and Yan-Xuan Wen. 2017. "Fast Preparation of Porous MnO/C Microspheres as Anode Materials for Lithium-Ion Batteries" Nanomaterials 7, no. 6: 121. https://doi.org/10.3390/nano7060121
APA StyleSu, J., Liang, H., Gong, X.-N., Lv, X.-Y., Long, Y.-F., & Wen, Y.-X. (2017). Fast Preparation of Porous MnO/C Microspheres as Anode Materials for Lithium-Ion Batteries. Nanomaterials, 7(6), 121. https://doi.org/10.3390/nano7060121