A Review of the Structure, Preparation, and Application of NLCs, PNPs, and PLNs
Abstract
:1. Introduction
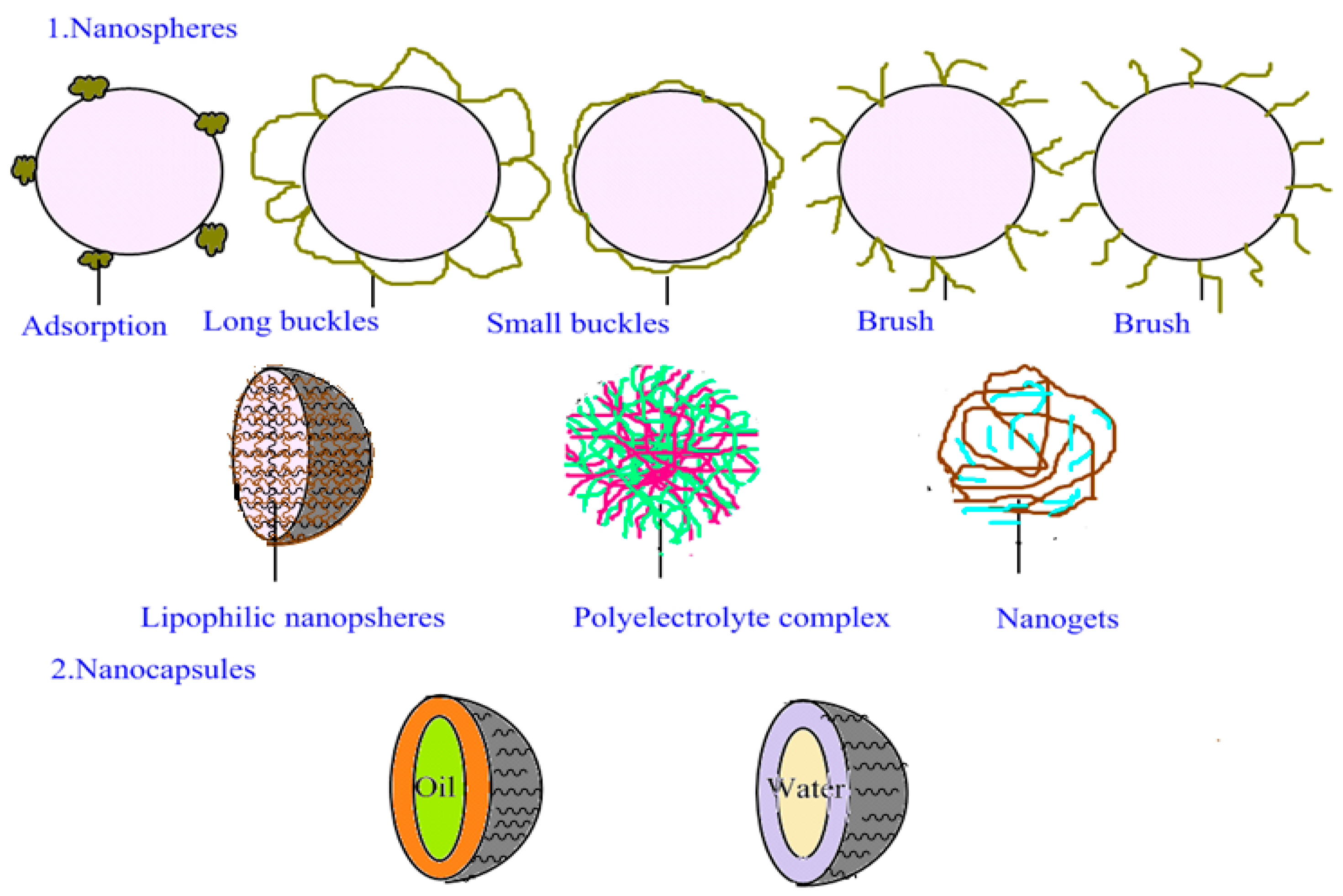
2. Structural Models of the Drug Carriers
2.1. Structural Model of NLCs
2.2. Structural Model of PNPs
2.3. Structural Model of PLNs
3. Methods for Preparing Drug Carriers
3.1. Methods for Preparing NLCs
3.1.1. High-Pressure Homogenization Method
3.1.2. Ultrasonic Emulsion Evaporation Method
3.1.3. Solvent Dispersion
3.1.4. Film-Ultrasonic Method
3.1.5. High-Temperature Emulsion Evaporation—Low-Temperature Curing
3.1.6. Microemulsion Method
3.1.7. Melt Emulsification Method
3.2. Methods for Preparing PNPs
3.2.1. Emulsion Evaporation Method
3.2.2. Double-Emulsion Evaporation Method
3.2.3. Dialysis Method
3.2.4. Improved Thin-Film Dispersion Method
3.2.5. Nanoprecipitation Method
3.2.6. Supercritical Fluid Technology
3.3. Methods for Preparing PLNs
3.3.1. Two-Step Method
3.3.2. Double-Emulsion Solvent Evaporation Method
3.3.3. One-Step Methods
3.3.4. Emulsion Evaporation Method
3.3.5. Modified Solvent Extraction/Evaporation Method
3.3.6. Ultrasonic Method
3.3.7. High-Pressure Homogenization Method
3.3.8. Thin-Film Hydration and Ultrasonic Dispersion
3.3.9. Nanoprecipitation Method
4. Applications of Nanostructured Lipid Carriers
4.1. Applications of NLCs
4.2. Applications of PNPs
4.2.1. Carriers for Antitumor Drugs
4.2.2. Carriers for Antibiotic Drugs
4.2.3. Carriers for Skin Protein Drugs
4.2.4. Carriers for Transdermal Drug Delivery
4.2.5. Applications in Diagnostic Reagents
4.3. Applications of PLNs
4.3.1. Drug Delivery
4.3.2. Gene Delivery
4.3.3. Delivery of Diagnostic Imaging Agents
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| GTCC | GREOIL GTCC |
| APG | Alkyl Polyglycoside |
| ACETEM | Acetylated Mono-and Diglycerides |
| Lipocire DM | Hydrogenated palm kernel glycerides |
| Compritol | Compritol 888ATO |
| mPEG-LPEI-PCL | Methoxy polyethylene-linear polyethyleneimine-poly ε-Caprolactone |
| DESE | A double emulsion solvent evaporation |
References
- Shidhaye, S.S.; Vaidya, R.; Sutar, S.; Patwardhan, A.; Kadam, V.J. Solid lipid nanoparticles and nanostructured lipid carriers—Innovative generations of solid lipid carriers. Curr. Drug Deliv. 2008, 5, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Lancelot, A.; Sierra, T.; Serrano, J.L. Nanostructured liquid–crystalline particles for drug delivery. Expert Opin. Drug Deliv. 2014, 11, 547–564. [Google Scholar] [CrossRef] [PubMed]
- Pardeike, J.; Hommoss, A.; Muller, R.H. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int. J. Pharm. 2009, 366, 170–184. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.H.; Wu, C.T. Optimization of nanostructured lipid carriers for lutein delivery. Colloid Surf. A 2010, 353, 149–156. [Google Scholar] [CrossRef]
- Zhang, W.L.; Gu, X.; Bai, H.; Yang, R.H.; Dong, C.D.; Liu, J.P. Nanostructured lipid carriers constituted from high-density lipoprotein components for delivery of a lipophilic cardiovascular drug. Int. J. Pharm. 2010, 391, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Kretlow, J.D.; Klouda, L.; Mikos, A.G. Injectable matrices and scaffolds for drug delivery in tissue engineering. Adv. Drug Deliv. Rev. 2007, 59, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Z.; Dou, S.; Wang, Y.C.; Long, H.Y.; Xiong, M.H.; Mao, C.Q.; Yao, Y.D.; Wang, J. Single-step assembly of cationic lipid–polymer hybrid nanoparticles for systemic delivery of siRNA. ACS Nano 2012, 6, 4955–4965. [Google Scholar] [CrossRef] [PubMed]
- Hao, T.N.; Qiao, M.X.; Li, Z.; Chen, D.W. Progress in the study of pH and temperature sensitive biodegradable block copolymers. Acta Pharm. Sin. 2008, 43, 123–127. [Google Scholar]
- Beija, M.; Salvayre, R.; Lauth-de Viguerie, N.; Marty, J.D. Colloidal systems for drug delivery: From design to therapy. Trends Biotechnol. 2012, 30, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Peetla, C.; Stine, A.; Labhasetwar, V. Biophysical interactions with model lipid membranes: Applications in drug discovery and drug delivery. Mol. Pharm. 2009, 6, 1264–1276. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.B.; Muller, R.H. Cosmetic features and applications of lipid nanoparticles (SLN®, NLC®). Int. J. Cosmet. Sci. 2008, 30, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Pokharkar, V.B.; Jolly, M.R.; Kumbhar, D.D. Engineering of a hybrid polymer–lipid nanocarrier for the nasal delivery of tenofovir disoproxil fumarate: Physicochemical, molecular, microstructural, and stability evaluation. Eur. J. Pharm. Sci. 2015, 71, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.J.; Kaul, A.; Trivedi, P. l-Cysteine conjugated poly l-lactide nanoparticles containing 5-fluorouracil: Formulation, characterization, release and uptake by tissues in vivo. Drug Deliv. 2015, 22, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Van Kuilenburg, A.B.; Maring, J.G. Evaluation of 5-fluorouracil pharmacokinetic models and therapeutic drug monitoring in cancer patients. Pharmacogenomics 2013, 14, 799–811. [Google Scholar] [CrossRef] [PubMed]
- Kohandel, M.; Haselwandter, C.A.; Kardar, M.; Sengupta, S.; Sivaloganathan, S. Quantitative model for efficient temporal targeting of tumor cells and neovasculature. Comput. Math. Methods Med. 2011, 2011, 790721. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.Z.; Yuet, K.; Zhang, L.; Gu, F.X.; Huynh-Le, M.; Radovic-Moreno, A.F.; Kantoff, P.W.; Bander, N.H.; Langer, R.; Farokhzad, O.C. ChemoRad nanoparticles: A novel multifunctional nanoparticle platform for targeted delivery of concurrent chemoradiation. Nanomedicine 2010, 5, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yu, B.; Weecharangsan, W.; Piao, L.; Darby, M.; Mao, Y.; Koynova, R.; Yang, X.; Li, H.; Xu, S.; et al. Transferrin-conjugated lipid-coated PLGA nanoparticles for targeted delivery of aromatase inhibitor 7α-APTADD to breast cancer cells. Int. J. Pharm. 2010, 390, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, J.; Couvreur, P. Synthesis of poly(alkyl cyanoacrylate)-based colloidal nanomedicines. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009, 1, 111–127. [Google Scholar] [CrossRef] [PubMed]
- Vrignaud, S.; Benoit, J.P.; Saulnier, P. Strategies for the nanoencapsulation of hydrophilic molecules in polymer-based nanoparticles. Biomaterials 2011, 32, 8593–8604. [Google Scholar] [CrossRef] [PubMed]
- Rong, X.; Xie, Y.; Hao, X.; Chen, T.; Wang, Y.; Liu, Y. Applications of polymeric nanocapsules in field of drug delivery systems. Curr. Drug Discov. Technol. 2011, 8, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Wohlgemuth, M.; Machtle, W.; Mayer, C. Improved preparation and physical studies of polybutylcyanoacrylate nanocapsules. J. Microencapsul. 2000, 17, 437–448. [Google Scholar] [PubMed]
- Wibowo, D.; Hui, Y.; Middelberg, A.P.; Zhao, C.X. Interfacial engineering for silica nanocapsules. Adv. Colloid Interface Sci. 2016, 236, 83–100. [Google Scholar] [CrossRef] [PubMed]
- Hillaireau, H.; Le Doan, T.; Appel, M.; Couvreur, P. Hybrid polymer nanocapsules enhance in vitro delivery of azidothymidine-triphosphate to macrophages. J. Control. Release 2006, 116, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Graf, A.; McDowell, A.; Rades, T. Poly(alkylcyanoacrylate) nanoparticles for enhanced delivery of therapeutics—Is there real potential? Expert Opin. Drug Deliv. 2009, 6, 371–387. [Google Scholar] [CrossRef] [PubMed]
- Bouchemal, K.; Briançon, S.; Perrier, E.; Fessi, H.; Bonnet, I.; Zydowicz, N. Synthesis and characterization of polyurethane and poly(ether urethane) nanocapsules using a new technique of interfacial polycondensation combined to spontaneous emulsification. Int. J. Pharm. 2004, 269, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Bouchemal, K.; Briançon, S.; Fessi, H.; Chevalier, Y.; Bonnet, I. Simultaneous emulsification and interfacial polycondensation for the preparation of colloidal suspensions of nanocapsules. Mater. Sci. Eng. C 2006, 26, 472–480. [Google Scholar] [CrossRef]
- Pohlmann, A.R.; Fonseca, F.N.; Paese, K.; Detoni, C.B.; Coradini, K.; Beck, R.C.; Guterres, S.S. Poly(ε-caprolactone) microcapsules and nanocapsules in drug delivery. Expert Opin. Drug Deliv. 2013, 10, 623–638. [Google Scholar] [CrossRef] [PubMed]
- Mora-Huertas, C.E.; Garrigues, O.; Fessi, H.; Elaissari, A. Nanocapsules prepared via nanoprecipitation and emulsification-diffusion methods: Comparative study. Eur. J. Pharm. Biopharm. 2012, 80, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, K.; Caban, S.; Kozlu, S.; Kadayifci, E.; Yerlikaya, F.; Capan, Y. The influence of technological parameters on the physicochemical properties of blank PLGA nanoparticles. Die Pharm. 2010, 65, 665–669. [Google Scholar]
- Moinard-Checot, D.; Chevalier, Y.; Briancon, S.; Beney, L.; Fessi, H. Mechanism of nanocapsules formation by the emulsion-diffusion process. J. Colloid Interface Sci. 2008, 317, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Godinho, B.M.; McCarthy, D.J.; Torres-Fuentes, C.; Beltrán, C.J.; McCarthy, J.; Quinlan, A.; Ogier, J.R.; Darcy, R.; O’Driscoll, C.M.; Cryan, J.F. Differential nanotoxicological and neuroinflammatory liabilities of non-viral vectors for RNA interference in the central nervous system. Biomaterials 2014, 35, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Lin, S.; Zhang, Q.; Chen, H.; Lan, W.; Li, H.; He, J.; Qin, W. Effect of extraction methods on the properties and antioxidant activities of Chuanminshen violaceum polysaccharides. Int. J. Biol. Macromol. 2016, 93, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, G.Q.; Leite, P.C.; Castro, F.; Ferreira, J.R.; Gomez-Lazaro, M.; Aguiar, P.; Barbosa, M.A.; Neidlinger-Wilke, C.; Goncalves, R.M. Anti-inflammatory Chitosan/Poly-γ-glutamic acid nanoparticles control inflammation while remodeling extracellular matrix in degenerated intervertebral disc. Acta Biomater. 2016, 42, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Cheow, W.S.; Hadinoto, K. Factors affecting drug encapsulation and stability of lipid–polymer hybrid nanoparticles. Colloids Surf. B Biointerfaces 2011, 85, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.Y. Strategies for optimizing polymer–lipid hybrid nanoparticle-mediated drug delivery. Expert Opin. Drug Deliv. 2016, 5, 609–612. [Google Scholar] [CrossRef] [PubMed]
- Al-Kattan, A.; Dufour, P.; Dexpert-Ghys, J.; Drouet, C. Preparation and Physicochemical Characteristics of Luminescent Apatite-Based Colloids. J. Phys. Chem. C 2010, 114, 2918–2924. [Google Scholar] [CrossRef]
- Piao, H.; Ouyang, M.; Xia, D.; Quan, P.; Xiao, W.; Song, Y.; Cui, F. In vitro-in vivo study of CoQ10-loaded lipid nanoparticles in comparison with nanocrystals. Int. J. Pharm. 2011, 419, 255–259. [Google Scholar] [CrossRef] [PubMed]
- El-Salamouni, N.S.; Farid, R.M.; El-Kamel, A.H.; El-Gamal, S.S. Effect of sterilization on the physical stability of brimonidine-loaded solid lipid nanoparticles and nanostructured lipid carriers. Int. J. Pharm. 2015, 496, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Gonullu, U.; Uner, M.; Yener, G.; Karaman, E.F.; Aydogmus, Z. Formulation and characterization of solid lipid nanoparticles, nanostructured lipid carriers and nanoemulsion of lornoxicam for transdermal delivery. Acta Pharm. 2015, 65, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Beloqui, A.; Solinis, M.A.; des Rieux, A.; Preat, V.; Rodriguez-Gascon, A. Dextran-protamine coated nanostructured lipid carriers as mucus-penetrating nanoparticles for lipophilic drugs. Int. J. Pharm. 2014, 468, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Muchow, M.; Maincent, P.; Muller, R.H. Lipid nanoparticles with a solid matrix (SLN®, NLC®, LDC®) for oral drug delivery. Drug Dev. Ind. Pharm. 2008, 34, 1394–1405. [Google Scholar] [CrossRef] [PubMed]
- Tolbert, S.H.; McFadden, P.D.; Loy, D.A. New Hybrid Organic/Inorganic Polysilsesquioxane-Silica Particles as Sunscreens. ACS Appl. Mater. Interfaces 2016, 8, 3160–3174. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahab, S.I.; Sheikh, B.Y.; Taha, M.M.; How, C.W.; Abdullah, R.; Yagoub, U.; El-Sunousi, R.; Eid, E.E. Thymoquinone-loaded nanostructured lipid carriers: Preparation, gastroprotection, in vitro toxicity, and pharmacokinetic properties after extravascular administration. Int. J. Nanomed. 2013, 8, 2163–2172. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.O.; Choe, J.; Suh, S.; Ko, S. Positively Charged Nanostructured Lipid Carriers and Their Effect on the Dissolution of Poorly Soluble Drugs. Molecules 2016, 21, 672. [Google Scholar] [CrossRef] [PubMed]
- Hung, L.C.; Basri, M.; Tejo, B.A.; Ismail, R.; Nang, H.L.; Abu, H.H.; May, C.Y. An improved method for the preparations of nanostructured lipid carriers containing heat-sensitive bioactives. Colloids Surf. B Biointerfaces 2011, 87, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Lacerda, S.P.; Cerize, N.N.; Re, M.I. Preparation and characterization of carnauba wax nanostructured lipid carriers containing benzophenone-3. Int. J. Cosmet. Sci. 2011, 33, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Yang, G.; Ren, J.; Guo, T.; Du, Y.; Feng, N. Formulation design, preparation, and in vitro and in vivo characterizations of β-Elemene-loaded nanostructured lipid carriers. Int. J. Nanomed. 2013, 8, 2533–2541. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.H.; Ramasamy, T.; Truong, D.H.; Choi, H.G.; Yong, C.S.; Kim, J.O. Preparation and characterization of fenofibrate-loaded nanostructured lipid carriers for oral bioavailability enhancement. AAPS PharmSciTech 2014, 15, 1509–1515. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhao, L.; Almasy, L.; Garamus, V.M.; Zou, A.; Willumeit, R.; Fan, S. Preparation and characterization of 4-dedimethylamino sancycline (CMT-3) loaded nanostructured lipid carrier (CMT-3/NLC) formulations. Int. J. Pharm. 2013, 450, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Ranpise, N.S.; Korabu, S.S.; Ghodake, V.N. Second generation lipid nanoparticles (NLC) as an oral drug carrier for delivery of lercanidipine hydrochloride. Colloids Surf. B Biointerfaces 2014, 116, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Uprit, S.; Kumar Sahu, R.; Roy, A.; Pare, A. Preparation and characterization of minoxidil loaded nanostructured lipid carrier gel for effective treatment of alopecia. Saudi Pharm. J. SPJ 2013, 21, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Fan, T.; Yang, Y.; Wu, M.; Li, L.; Zhou, Z.; Jian, Y.; Zhang, Q.; Huang, Y. Preparation, macrophages targeting delivery and anti-inflammatory study of pentapeptide grafted nanostructured lipid carriers. Int. J. Pharm. 2013, 450, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Qiao, H.; Ni, J.M.; Shi, Y.B.; Qiang, Y. Preparation of isoliquiritigenin-loaded nanostructured lipid carrier and the in vivo evaluation in tumor-bearing mice. Eur. J. Pharm. Sci. 2013, 49, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Zhang, W.; Liu, J.; Shaw, J.P.; Shen, Y.; Xu, Y.; Lu, H.; Wu, Z. Preparation and characterization of a lovastatin-loaded protein-free nanostructured lipid carrier resembling high-density lipoprotein and evaluation of its targeting to foam cells. AAPS PharmSciTech 2011, 12, 1200–1208. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Chen, Y.; Zhang, Z.; He, J.; Du, M.; Wu, Q. Preparation of tripterine nanostructured lipid carriers and their absorption in rat intestine. Die Pharm. 2012, 67, 304–310. [Google Scholar]
- Zhang, K.; Lv, S.; Li, X.; Feng, Y.; Li, X.; Liu, L.; Li, S.; Li, Y. Preparation, characterization, and in vivo pharmacokinetics of nanostructured lipid carriers loaded with oleanolic acid and gentiopicrin. Int. J. Nanomed. 2013, 8, 3227–3239. [Google Scholar] [CrossRef] [PubMed]
- Emami, J.; Rezazadeh, M.; Varshosaz, J.; Tabbakhian, M.; Aslani, A. Formulation of LDL Targeted Nanostructured Lipid Carriers Loaded with Paclitaxel: A Detailed Study of Preparation, Freeze Drying Condition, and In Vitro Cytotoxicity. J. Nanomater. 2012, 2012, 3. [Google Scholar] [CrossRef]
- Hong, W.; Chen, D.W.; Zhao, X.L.; Qiao, M.X.; Hu, H.Y. Preparation and study in vitro of long-circulating nanoliposomes of curcumin. China J. Chin. Mater. Med. 2008, 33, 889–892. [Google Scholar]
- Patlolla, R.R.; Chougule, M.; Patel, A.R.; Jackson, T.; Tata, P.N.; Singh, M. Formulation, characterization and pulmonary deposition of nebulized celecoxib encapsulated nanostructured lipid carriers. J. Control. Release 2010, 144, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Luan, J.; Zhang, D.; Hao, L.; Qi, L.; Liu, X.; Guo, H.; Li, C.; Guo, Y.; Li, T.; Zhang, Q.; et al. Preparation, characterization and pharmacokinetics of Amoitone B-loaded long circulating nanostructured lipid carriers. Colloids Surf. B Biointerfaces 2014, 114, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Shao, J.; Tan, B.; Guan, S.; Liu, Z.; Zhao, Z.; He, F.; Zhao, J. Targeted lung cancer therapy: Preparation and optimization of transferrin-decorated nanostructured lipid carriers as novel nanomedicine for co-delivery of anticancer drugs and DNA. Int. J. Nanomed. 2015, 10, 1223–1233. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Yi, Y.; Yuan, H.; Han, J.; Zhang, X. Solidification of nanostructured lipid carriers (NLCs) onto pellets by fluid-bed coating: Preparation, in vitro characterization and bioavailability in dogs. Powder Technol. 2013, 247, 120–127. [Google Scholar] [CrossRef]
- Hori, M.; Onishi, H.; Machida, Y. Evaluation of Eudragit-coated chitosan microparticles as an oral immune delivery system. Int. J. Pharm. 2005, 297, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Muller, R.H.; Petersen, R.D.; Hommoss, A.; Pardeike, J. Nanostructured lipid carriers (NLC) in cosmetic dermal products. Adv. Drug Deliv. Rev. 2007, 59, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Pelegri-O’Day, E.M.; Maynard, H.D. Controlled Radical Polymerization as an Enabling Approach for the Next Generation of Protein–Polymer Conjugates. Acc. Chem. Res. 2016, 49, 1777–1785. [Google Scholar] [CrossRef] [PubMed]
- Hauser, M.; Langer, K.; Schonhoff, M. pH-Triggered release from surface-modified poly(lactic-co-glycolic acid) nanoparticles. Beilstein J. Nanotechnol. 2015, 6, 2504–2512. [Google Scholar] [CrossRef] [PubMed]
- Bernabeu, E.; Gonzalez, L.; Legaspi, M.J.; Moretton, M.A.; Chiappetta, D.A. Paclitaxel-Loaded TPGS-b-PCL Nanoparticles: In Vitro Cytotoxicity and Cellular Uptake in MCF-7 and MDA-MB-231 Cells versus mPEG-b-PCL Nanoparticles and Abraxane®. J. Nanosci. Nanotechnol. 2016, 16, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Gillich, T.; Acikgöz, C.; Isa, L.; Schlüter, A.D.; Spencer, N.D.; Textor, M. PEG-stabilized core–shell nanoparticles: Impact of linear versus dendritic polymer shell architecture on colloidal properties and the reversibility of temperature-induced aggregation. ACS Nano 2013, 7, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Dörlich, R.M.; Brandholt, S.; Schneider, R.; Trouillet, V.; Bruns, M.; Gerthsen, D.; Nienhaus, G.U. Facile preparation of water-soluble fluorescent gold nanoclusters for cellular imaging applications. Nanoscale 2011, 3, 2009–2014. [Google Scholar] [CrossRef] [PubMed]
- Li, J.L.; Zheng, C.L.; Liu, J.P.; Zhu, J.B. Formulation and process optimization of doxorubicin-loaded PLGA nanoparticles and its in vitro release. Acta Pharm. Sin. 2013, 48, 759–766. [Google Scholar]
- Xing, L.; Shi, Q.; Zheng, K.; Shen, M.; Ma, J.; Li, F.; Liu, Y.; Lin, L.; Tu, W.; Duan, Y.; et al. Ultrasound-Mediated Microbubble Destruction (UMMD) Facilitates the Delivery of CA19–9 Targeted and Paclitaxel Loaded mPEG-PLGA-PLL Nanoparticles in Pancreatic Cancer. Theranostics 2016, 6, 1573–1587. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Liu, R.; Liu, Z.; Shen, X.; Wang, Q.; Tan, X. Cationic Poly-l-Lysine-Fe2O3/SiO2 nanoparticles loaded with small interference RNA: Application to silencing gene expression in primary rat neurons. J. Nanosci. Nanotechnol. 2014, 14, 2810–2815. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.J.; Ahn, K.O.; Kim, E.K.; Kim, Y.H. Formation of Cu or Cu2O nanoparticles embedded in a polyimide film for nanofloating gate memory. J. Nanosci. Nanotechnol. 2011, 11, 11100–11103. [Google Scholar] [CrossRef] [PubMed]
- Muller, R.H.; Radtke, M.; Wissing, S.A. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv. Drug Deliv. Rev. 2002, 54 (Suppl. 1), S131–S155. [Google Scholar] [CrossRef]
- Nordlund, G.; Lonneborg, R.; Brzezinski, P. Formation of supported lipid bilayers on silica particles studied using flow cytometry. Langmuir 2009, 25, 4601–4606. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Pan, J.; Feng, S.S. Nanoparticles of lipid monolayer shell and biodegradable polymer core for controlled release of paclitaxel: Effects of surfactants on particles size, characteristics and in vitro performance. Int. J. Pharm. 2010, 395, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Lin, D.; Wu, F.; Guo, L.; He, G.; Ouyang, L.; Song, X.; Huang, W.; Li, X. Discovery and in vivo evaluation of novel RGD-modified lipid–polymer hybrid nanoparticles for targeted drug delivery. Int. J. Mol. Sci. 2014, 15, 17565–17576. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, F.; Li, Y.; Wang, H.; Ren, H.; Chen, J.; Nie, G.; Hao, J. Co-delivery of HIF1alpha siRNA and gemcitabine via biocompatible lipid–polymer hybrid nanoparticles for effective treatment of pancreatic cancer. Biomaterials 2015, 46, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhao, X.; Wang, H.; Zhao, R.; Ji, T. Multiple layer-by-layer lipid–polymer hybrid nanoparticles for improved FOLFIRINOX chemotherapy in pancreatic tumor models. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 527. [Google Scholar] [CrossRef]
- Cai, J.; Huang, H.; Song, W.; Hu, H.; Chen, J.; Zhang, L.; Li, P.; Wu, R.; Wu, C. Preparation and evaluation of lipid polymer nanoparticles for eradicating H. pylori biofilm and impairing antibacterial resistance in vitro. Int. J. Pharm. 2015, 495, 728–737. [Google Scholar] [CrossRef] [PubMed]
- Palange, A.L.; Di Mascolo, D.; Carallo, C.; Gnasso, A.; Decuzzi, P. Lipid–polymer nanoparticles encapsulating curcumin for modulating the vascular deposition of breast cancer cells. Nanomedicine 2014, 10, 991–1002. [Google Scholar] [CrossRef] [PubMed]
- Colombo, S.; Cun, D.; Remaut, K.; Bunker, M.; Zhang, J.; Martin-Bertelsen, B.; Yaghmur, A.; Braeckmans, K.; Nielsen, H.M.; Foged, C. Mechanistic profiling of the siRNA delivery dynamics of lipid–polymer hybrid nanoparticles. J. Control. Release 2015, 201, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Mandal, B.; Mittal, N.K.; Balabathula, P.; Thoma, L.A.; Wood, G.C. Development and in vitro evaluation of core-shell type lipid–polymer hybrid nanoparticles for the delivery of erlotinib in non-small cell lung cancer. Eur. J. Pharm. Sci. 2016, 81, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, T.; Tran, T.H.; Choi, J.Y.; Cho, H.J.; Kim, J.H.; Yong, C.S.; Choi, H.G.; Kim, J.O. Layer-by-layer coated lipid–polymer hybrid nanoparticles designed for use in anticancer drug delivery. Carbohydr. Polym. 2014, 102, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Seedat, N.; Kalhapure, R.S.; Mocktar, C.; Vepuri, S.; Jadhav, M.; Soliman, M.; Govender, T. Co-encapsulation of multi-lipids and polymers enhances the performance of vancomycin in lipid–polymer hybrid nanoparticles: In vitro and in silico studies. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 61, 616–630. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhu, D.; Dong, X.; Sun, H.; Song, C.; Wang, C.; Kong, D. Folate-modified lipid–polymer hybrid nanoparticles for targeted paclitaxel delivery. Int. J. Nanomed. 2015, 10, 2101–2114. [Google Scholar]
- Zhang, L.; Zhu, D.; Dong, X.; Sun, H.; Song, C.; Wang, C.; Kong, D. Development and characterization of single step self-assembled lipid polymer hybrid nanoparticles for effective delivery of methotrexate. RSC Adv. 2015, 5, 62989–62999. [Google Scholar]
- Zhao, P.; Zheng, M.; Yue, C.; Luo, Z.; Gong, P.; Gao, G.; Sheng, Z.; Zheng, C.; Cai, L. Improving drug accumulation and photothermal efficacy in tumor depending on size of ICG loaded lipid–polymer nanoparticles. Biomaterials 2014, 35, 6037–6046. [Google Scholar] [CrossRef] [PubMed]
- De Geest, B.G.; De Koker, S.; Demeester, J.; De Smedt, S.C.; Hennink, W.E. Pulsed in vitro release and in vivo behavior of exploding microcapsules. J. Control. Release 2009, 135, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, K.; Pan, J.; Liu, B.; Feng, S.S. Folic acid conjugated nanoparticles of mixed lipid monolayer shell and biodegradable polymer core for targeted delivery of Docetaxel. Biomaterials 2010, 31, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Hadinoto, K.; Sundaresan, A.; Cheow, W.S. Lipid–polymer hybrid nanoparticles as a new generation therapeutic delivery platform: A review. Eur. J. Pharm. Biopharm. 2013, 85, 427–443. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Tsai, T.H.; Huang, Z.R.; Fang, J.Y. Effects of lipophilic emulsifiers on the oral administration of lovastatin from nanostructured lipid carriers: Physicochemical characterization and pharmacokinetics. Eur. J. Pharm. Biopharm. 2012, 74, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Puglia, C.; Blasi, P.; Rizza, L.; Schoubben, A.; Bonina, F.; Rossi, C.; Ricci, M. Lipid nanoparticles for prolonged topical delivery: An in vitro and in vivo investigation. Int. J. Pharm. 2008, 357, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Junyaprasert, V.B.; Teeranachaideekul, V.; Souto, E.B.; Boonme, P.; Müller, R.H. Q10-loaded NLC versus nanoemulsions: Stability, rheology and in vitro skin permeation. Int. J. Pharm. 2009, 377, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.; Pathak, S.; Sharma, S.; Patravale, V. Design and in vivo pharmacodynamic evaluation of nanostructured lipidcarriers for parenteral delivery of nrtemether: Nanoject. Int. J. Pharm. 2008, 364, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Sha, X.; Shen, A.; Wang, Y.; Sun, Z.; Gu, Z.; Fang, X. Polycation nanostructured lipid carrier, a novel nonviral vector constructed with triolein for efficientgene delivery. Biochem. Biophys. Res. Commun. 2008, 370, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Zhou, H.; Liu, G.; Li, Y.; Yan, Z.; Duan, M. The advantages of a novel CoQ10 delivery system in skin photo-protection. Int. J. Pharm. 2010, 392, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Abraham, G.; McCarroll, J.; Byrne, F.; Saricilar, S.; Kavallaris, M.; Bulmus, V. Block co-polymer nanoparticles with degradable cross-linked core and low-molecular-weight PEG corona for anti-tumour drug delivery. J. Biomater. Sci. Polym. Ed. 2011, 22, 1001–1022. [Google Scholar] [CrossRef] [PubMed]
- Lovejoy, D.B.; Kalinowski, D.S.; Richardson, D.R.; Nie, G.; Zhao, Y. Amphiphilic hyper-branched co-polymer nanoparticles for the controlled delivery of anti-tumor agents. Biomaterials 2010, 31, 7364–7375. [Google Scholar]
- Ungaro, F.; d’Angelo, I.; Coletta, C.; d’Emmanuele, V.B.R.; Sorrentino, R.; Perfetto, B.; Tufano, M.A.; Miro, A.; La-Rotonda, M.I.; Quaglia, F. Dry powders based on PLGA nanoparticles for pulmonary delivery of antibiotics: Modulation of encapsulation efficiency, release rate and lung deposition pattern by hydrophilic polymers. J. Control. Release 2012, 157, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Teekamp, N.; Duque, L.F.; Frijlink, H.W.; Hinrichs, W.L.; Olinga, P. Production methods and stabilization strategies for polymer-based nanoparticles and microparticles for parenteral delivery of peptides and proteins. Expert Opin. Drug Deliv. 2015, 12, 1311–1331. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Price, G.J.; Guy, R.H. Disposition of nanoparticles and an associated lipophilic permeant following topical application to the skin. Mol. Pharm. 2009, 6, 1441–1448. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Gao, J.; Zhu, Q.; Zhang, M.; Ding, X.; Wang, X.; Hou, X.; Fan, W.; Ding, B.; Wu, X.; et al. Penetration and distribution of PLGA nanoparticles in the human skin treated with microneedles. Int. J. Pharm. 2010, 402, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Samad, A.; Singh, S.K.; Ahsan, M.N.; Faruk, A.; Ahmed, F.J. Enhanced stability and permeation potential of nanoemulsion containing sefsol-218 oil for topical delivery of amphotericin B. Drug Dev. Ind. Pharm. 2015, 41, 780–790. [Google Scholar] [CrossRef] [PubMed]
- Jeon, W.; Lee, S.; Manjunatha, D.H.; Ban, C. A colorimetric aptasensor for the diagnosis of malaria based on cationic polymers and gold nanoparticles. Anal. Biochem. 2013, 439, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Nezhad, Z.K.; Nagai, N.; Yamamoto, K.; Kaji, H.; Nishizawa, M.; Saya, H.; Nakazawa, T.; Abe, T. Application of clotrimazole via a novel controlled release device provides potent retinal protection. J. Mater. Sci. Mater. Med. 2015, 26, 230. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Eavarone, D.; Capila, I.; Zhao, G.; Watson, N.; Kiziltepe, T.; Sasisekharan, R. Temporal targeting of tumour cells and neovasculature with a nanoscale delivery system. Nature 2005, 436, 568–572. [Google Scholar] [CrossRef] [PubMed]
- Ebos, J.M.; Lee, C.R.; Kerbel, R.S. Tumor and host-mediated pathways of resistance and disease progression in response to antiangiogenic therapy. Clin. Cancer Res. 2009, 15, 5020–5025. [Google Scholar] [CrossRef] [PubMed]
- Al-Dosari, M.S.; Gao, X. Nonviral gene delivery: Principle, limitations, and recent progress. AAPS J. 2009, 11, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; He, Y.Z.; Li, W.; Shen, Y.Z.; Li, Y.R.; Wang, Y.F. A novel polymer–lipid hybrid nanoparticle for efficient nonviral gene delivery. Acta Pharmacol. Sin. 2010, 31, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Mieszawska, A.J.; Gianella, A.; Cormode, D.P.; Zhao, Y.; Meijerink, A.; Langer, R.; Farokhzad, O.C.; Fayad, Z.A.; Mulder, W.J. Engineering of lipid-coated PLGA nanoparticles with a tunable payload of diagnostically active nanocrystals for medical imaging. Chem. Commun. 2012, 48, 5835–5837. [Google Scholar] [CrossRef] [PubMed]


| Drug | Solid Lipid | Liquid Lipid | Surfactant | Method | Entrapment Efficiency/Particle Size | Ref. |
|---|---|---|---|---|---|---|
| CoenzymeQ10 | Hard Stearin | GTCC | Alkyl polyglycoside | HPH | 99.58 ± 0.0061% | [37] |
| Brimonidine base | GMS | Castor oil | Poloxamer188 | HPH | 10.51% | [38] |
| Lornoxicame | Compritol888ATO, Lanette O | OA | Pluronic F68 | HPH | 97.89 ± 0.25% | [49] |
| Quercetin | Imwitor 900 K | MCT | Tween80, Span20, Soybean lecithin | HPH | 91% | [40] |
| Saquinavir mesylate | Precirol ATO5 | Miglyol812 | Tween80, Poloxamer188 | HPH | -- | [41] |
| UvinulT 150 | ACETEM | Odograph, Hydrogenated palm | OlivemR800 OlivemR1000 | HPH | 314 ± 4 nm | [42] |
| thymoquinone | Lipoid S100 | oil | Sorbitol, Thimerosal, Polysorbate80 | HPH | 75 ± 2.4 nm | [43] |
| Docetaxel | Stearic acid, Glycerin monostearate | Olive oil, MCT, OA | Pluronic F68, Cremophor EL | HPH | 60.5 ± 5.0% | [44] |
| β-carotene | Hydrogenated palm kernel | Isopropyl palmitate | Sorbitan monopalmitate, Polysorbate80 | HPH | 259 ± 4.24 nm | [45] |
| Tocolsenzophenone-3 | Glycerides, Carnauba wax | Isodecyloleate | Poloxamer188, Polysorbate80 | HPH | 91% | [46] |
| β-Elemene | GMS | Maisine35-1, Labrafil | Polysorbate80, soybean lecithin | HPH | 138.9 nm | [47] |
| 82.11% | ||||||
| Fenofibrate | Compritol888, ATO | M1944CS, Labrafil | Soya lecithin, Polysorbate80 | HPH | 99% | [48] |
| 84.9 ± 4.9 nm | ||||||
| 4-dedimethylamino sancycline | Stearic acid, Glycerin monostearate | OA. MCT | LutrolF68 | HPH | 90–96% | [49] |
| <200 nm | ||||||
| Lercanidipine HCl | GMS | Linseed oil, Labrafil | Polysorbate80 | Ultrasonication and emulsion evaporation | -- | [50] |
| Minoxidil | Soya lecithin | OA | Polysorbate80 | Ultrasonication and emulsion evaporation | 86.09% | [51] |
| 280 nm | ||||||
| Dexamethasone | glycerol trilaurate | Tristearin, Chain Triglycerides Miglyol812 | Phospholipids | Solvent diffusion | 86.7 ± 3.9% | [52] |
| Isoliquiritigenin | Soya lecithin, Cholesterol | Glycerol | Polysorbate80, Poloxamer188 | Solvent diffusion | 96.74 ± 1.81% | [53] |
| 160.73 ± 6.08 nm | ||||||
| Lovastatin | Cholesteryl oleate, cholesterol | Trioleate | Soybean lecithin | Solvent diffusion | 96.2 ± 1.3% | [54] |
| 13.8 ± 2.2 nm | ||||||
| Celastrol | Precirol ATO-5 | Labrasol | Lecithin, TPGS, Poloxamer188 | Solvent diffusion | 88.6 ± 0.37% | [55] |
| 132.3 ± 25 nm | ||||||
| Gentiopicroside | Glycerin monostearate | OA | Polysorbate80, Poloxamer188 | Solvent diffusion | 38.19 ± 1.61% | [56] |
| 129.9 ± 3.07 nm | ||||||
| Paclitaxel | Cholesterol | OA | Poloxamer188, Polysorbate80 | Solvent diffusion | 72 ± 11.6% | [57] |
| Curcumin | CP | Miglyol812 | Solutol HS15, Soya lecithin | Film-ultrasonic emulsion evaporation | 96.7 ± 0.146% | [58] |
| 135.3 ± 2.52 nm | ||||||
| Celecoxib | Kollicream, CP | Miglyol812ic | Solutol HS15, Soya lecithin | low temperature solidification | 103.5 ± 32.6 nm | [59] |
| Amoitone B | Polyethylene glycol stearate GMS | Caprylic/capric triglyceride | Pluronic F68, Soya lecithin | Emulsion evaporation, low temperature solidification | 68.17 ± 0.94% | [60] |
| 225.7 ± 1.36 nm | ||||||
| Paclitaxel DNA | GMS, Soya lecithin | OA | Polysorbate80 | Microemulsion | 87.1 ± 2.1% | [61] |
| 79 nm | ||||||
| Fenofibrat | Precirol ATO 5 | Captex100 | Polysorbate80 | Melting-emulsification | 8.5% | [62] |
| 227.5 nm |
| Drugs | Polymer | Surfactant | Surface Modification | Method | Entrapment Efficiency/Particle Size | Ref. |
|---|---|---|---|---|---|---|
| Ketoprofen | EudragitE100 Eudragit RS | --- | ---- | emulsion solvent evaporation | 50–150 nm | [64] |
| TanshinoneIIA | PLGA | Span-80 LABRAFILM 1944 CS, PVA | --- | double emulsion evaporation | 98.10% | [65] |
| 188 nm | ||||||
| Bovine albumin | PLA | PVA | Water soluble chitosan polyethylene | Double emulsion evaporation | 100~200 nm | [66] |
| Paclitaxel | LHRy K | PVA | c(RGDyK) | Dialysis | 84.84 ± 2.6% | [67] |
| 131.7 ± 2.3 nm | ||||||
| FA | DMAEMA, HEA | --- | ---- | Dialysis | 275 nm | [68] |
| 4-Bromo-1,8-naphthalic anhydride | PEI | --- | --- | Dialysis | 5~10 nm | [69] |
| HCPT | mPEG-LPEI-PCL | PVA | F-CS | Improved thin film dispersion | 92.6 ± 1.1% | [70] |
| 155 ± 9.6 nm | ||||||
| Paclitaxel | PLGA | Cetyltrimethylammonium bromide | --- | Nanoprecipitation | 321 ± 0.76 nm | [71] |
| DNase I | MePEG-PLGA, PEG | --- | --- | Nanoprecipitation | 89.7 nm | [72] |
| Bovine insulin | PLA | --- | --- | Supercritical fluid technology | >90% | [73] |
| 400~600 nm |
| Drugs | Lipid | Polymer | Method | Entrapment Efficiency/Particle Size | Ref. |
|---|---|---|---|---|---|
| Curcumin | lecithin/cholesterol | PLGA-mPEG, Chol-PEG-RGD | double emulsification | 96.0 ± 0.6% | [77] |
| 216.6 ± 4.7 nm | |||||
| HIF1a siRNA | Lecithin, DSPE-PEG-2000, Cholesterol | mPEG-PLGA | double emulsion | 141.8 nm | [78] |
| 5-fluorouracil | Phospholipids | mPEG-PLA | improved double emulsion | 22.60% | [79] |
| Oxaplatin | Cholesterol | 26.30% | |||
| Camptosar | DSPE-PEG-2000, DSPE-PEG-3400-Mal | 96% | |||
| Amoxicillin | Rhamnolipid | PECS | emulsification, solvent evaporation | 200 nm | [80] |
| Human Fibronectin siRNA | DPPC, DSPE-PEG, Phospholipids, DOTAP | curcumin- PLGA | single emulsion, solvent evaporation | ~150 nm | [81] |
| Erlotinib | HSPC, DSPE-PEG-2000 | PLGA | DESE | 63.3 ± 5.0% | [82] |
| 213 ± 286 nm | |||||
| Doxorubicn | Compritol888, ATO | PCL | single-step sonication | 66% | [83] |
| 170 nm | |||||
| Dextran | Soybean lecithin | CT | HPH | ~265 nm | [84] |
| GTP | HA | 27.80% | |||
| OA | 41.50% | ||||
| Vancomycin | CHT, ALG | EUD | HPH | 54.30% | [85] |
| Paclitaxel | DSPE-PEG2000 | PCL-PEG-PCL | thin-film hydration and ultrasonic dispersion | 69.30% | [86] |
| 279.9 ± 8.7 nm | |||||
| Mitomycin C | Phospholipid, Soybean lecithin | PCL | nanoprecipitation | 80–90% | [87] |
| 150–300 nm | |||||
| Indocyanine green | DSPE-PEG | PLGA | nanoprecipitation | 39 nm, 68 nm | [88] |
| 116 nm |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Cai, T.; Huang, Y.; Xia, X.; Cole, S.P.C.; Cai, Y. A Review of the Structure, Preparation, and Application of NLCs, PNPs, and PLNs. Nanomaterials 2017, 7, 122. https://doi.org/10.3390/nano7060122
Li Q, Cai T, Huang Y, Xia X, Cole SPC, Cai Y. A Review of the Structure, Preparation, and Application of NLCs, PNPs, and PLNs. Nanomaterials. 2017; 7(6):122. https://doi.org/10.3390/nano7060122
Chicago/Turabian StyleLi, Qianwen, Tiange Cai, Yinghong Huang, Xi Xia, Susan P. C. Cole, and Yu Cai. 2017. "A Review of the Structure, Preparation, and Application of NLCs, PNPs, and PLNs" Nanomaterials 7, no. 6: 122. https://doi.org/10.3390/nano7060122





