N-Doped TiO2-Coated Ceramic Membrane for Carbamazepine Degradation in Different Water Qualities
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Water pH
2.2. Water Hardness
- Addition of 120 mg/L Ca2+ to DI water spiked by 1 mg/L CBZ and pH = 7 adjusted by 1 mM PBS.
- Addition of 120 mg/L Ca2+ to DI water spiked by 1 mg/L CBZ and pH = 8.5 adjusted by 2 mM BBS. Phosphate buffer could not be used in this case due to the precipitation of CaPO4.
- Addition of 120 mg/L Mg2+ to DI water spiked by 1 mg/L CBZ and pH = 8.5 adjusted by 2 mM BBS. Borate buffer was used to create identical conditions to those in the experiment with calcium.
2.3. Effect of Dissolved Organic Matter (DOM)
2.4. Effect of Chloride
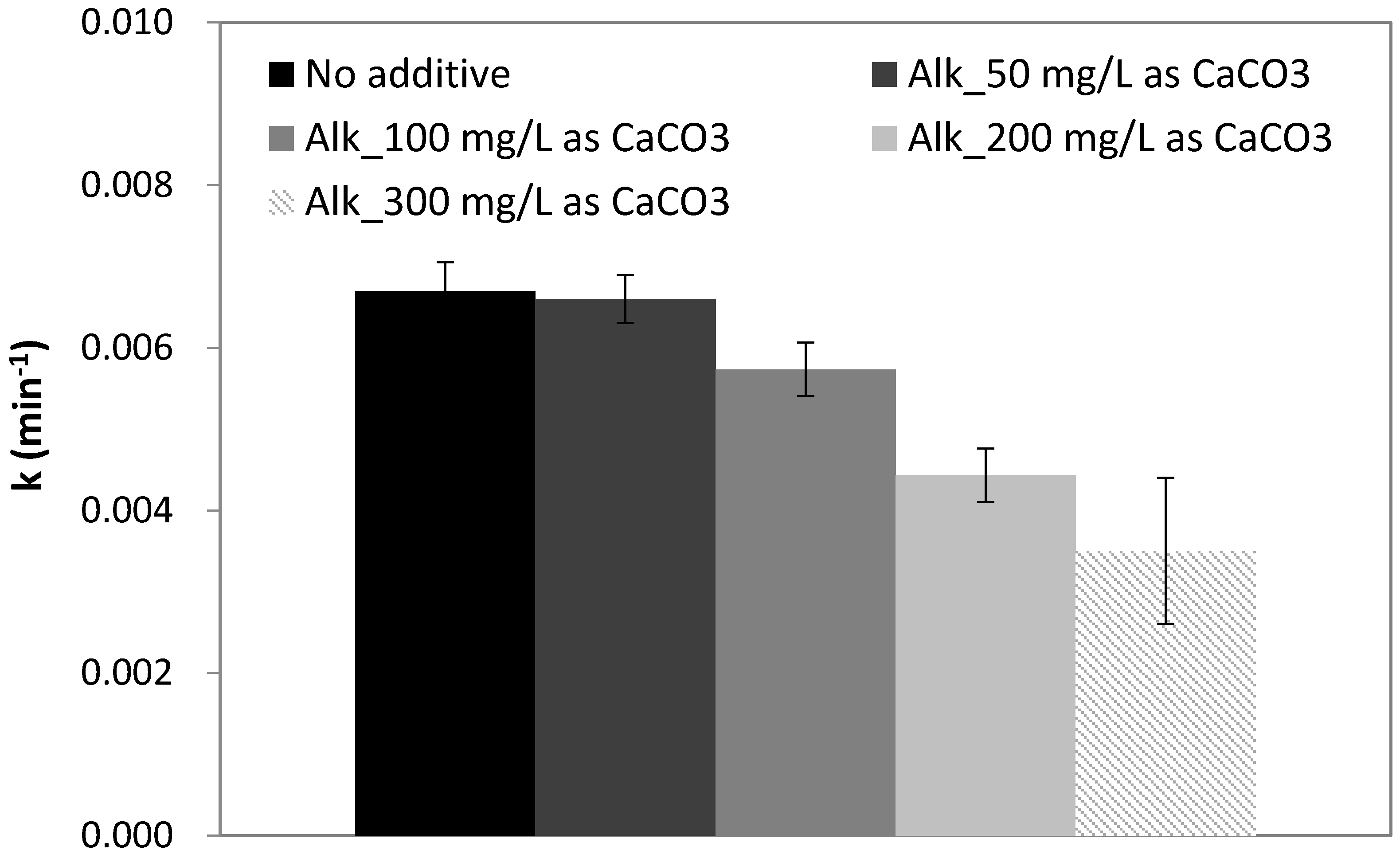
2.5. Effect of Alkalinity
2.6. Summary of Water Quality Results
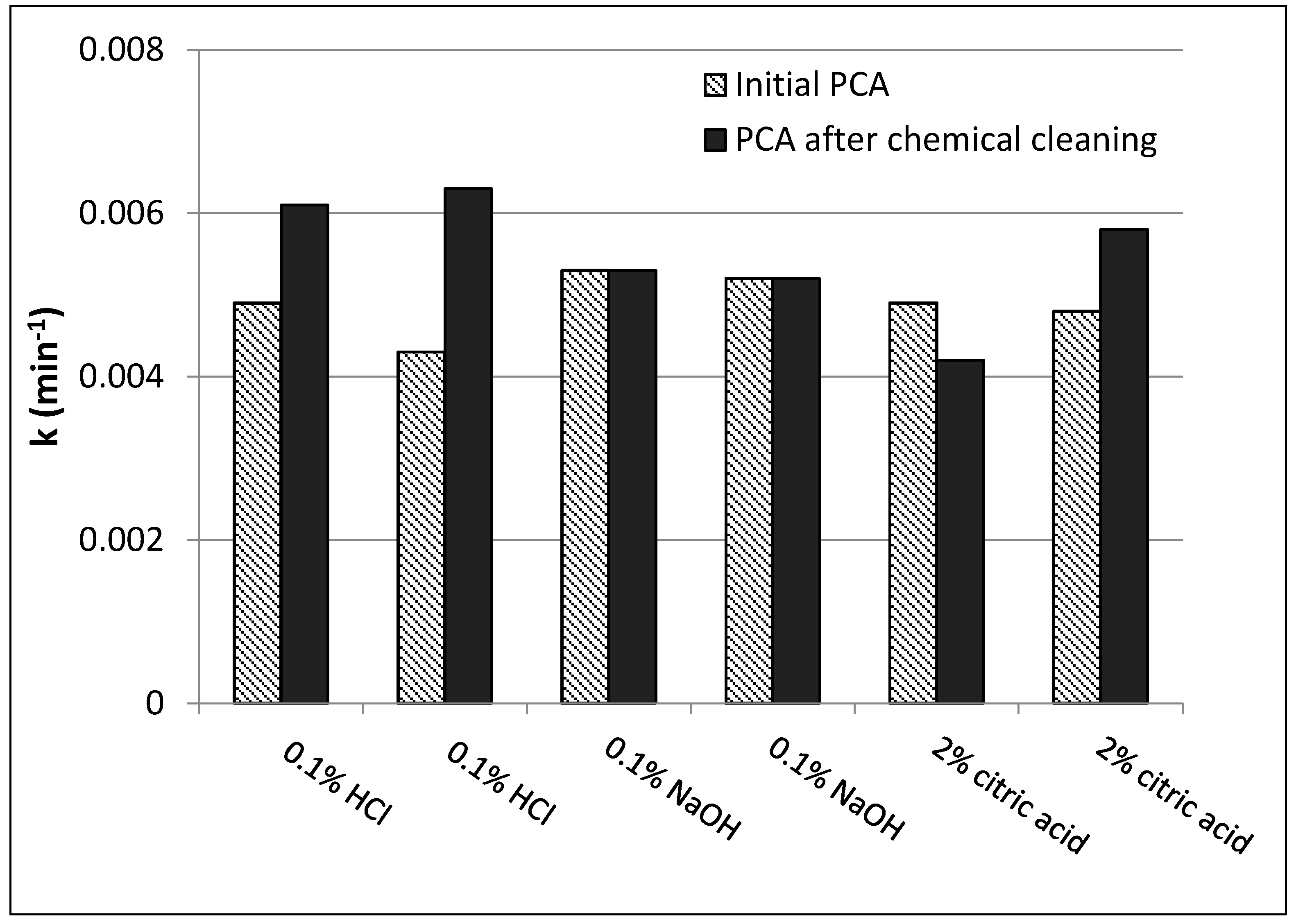
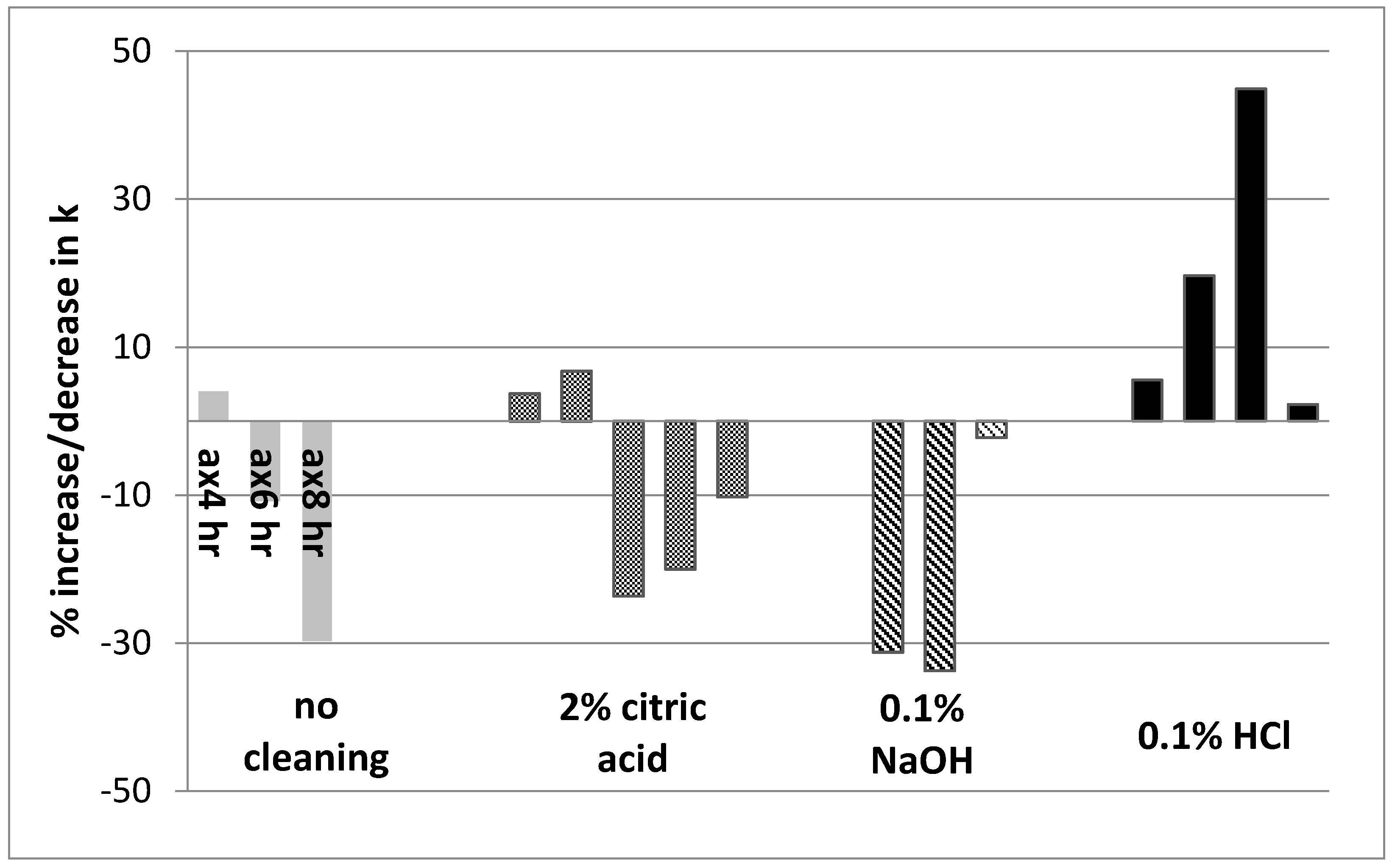
2.7. Membrane Regeneration by Chemical Cleaning
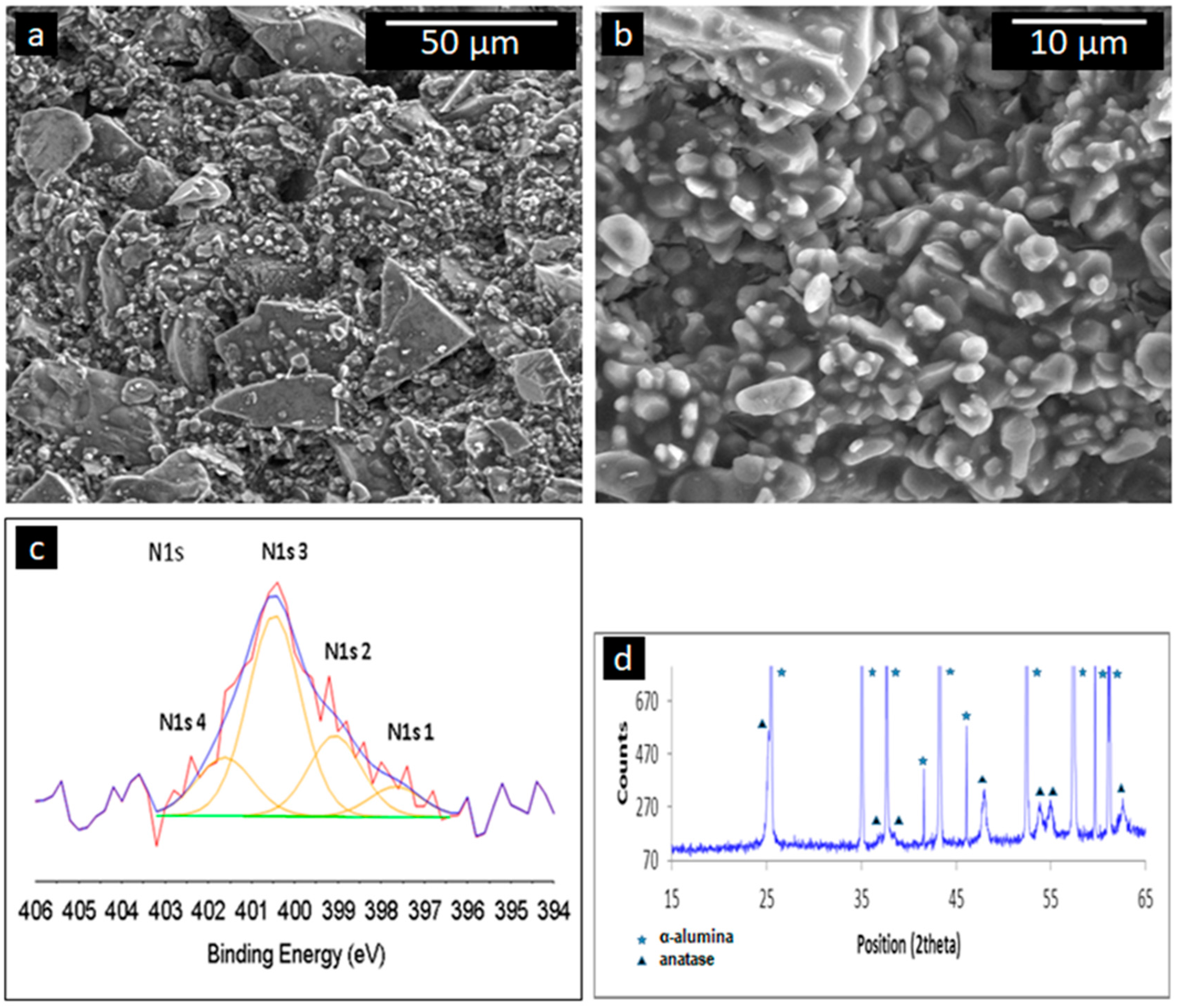
2.8. Surface Analysis
3. Materials and Methods
3.1. Carbamazepine as a Model Pollutant
3.2. Chemicals and Reagents
3.3. Deposition Technique
3.4. Photocatalytic Activity of N-Doped TiO2-Coated Al2O3 Membrane
3.5. Water Analysis
3.6. Surface Characterization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, X.; Li, C.; Grätzel, M.; Kostecki, R.; Mao, S.S. Nanomaterials for renewable energy production and storage. Chem. Soc. Rev. 2012, 41, 7909–7937. [Google Scholar] [CrossRef] [PubMed]
- Avisar, D.; Horovitz, I.; Lozzi, L.; Ruggieri, F.; Baker, M.; Abel, M.-L.; Mamane, H. Impact of water quality on removal of carbamazepine in natural waters by N-doped TiO2 photo-catalytic thin film surfaces. J. Hazard. Mater. 2013, 244, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Malato, S.; Fernández-Ibáñez, P.; Maldonado, M.; Blanco, J.; Gernjak, W. Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catal. Today 2009, 147, 1–59. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Zhou, B.; Chen, H.; Wang, Z.; Cai, W. A TiO2-nanotube-array-based photocatalytic fuel cell using refractory organic compounds as substrates for electricity generation. Chem. Commun. 2011, 47, 10314–10316. [Google Scholar] [CrossRef] [PubMed]
- Mozia, S. Photocatalytic membrane reactors (PMRs) in water and wastewater treatment. A review. Sep. Purif. Technol. 2010, 73, 71–91. [Google Scholar] [CrossRef]
- Ahmed, S.; Rasul, M.; Martens, W.N.; Brown, R.; Hashib, M. Advances in heterogeneous photocatalytic degradation of phenols and dyes in wastewater: A review. Water Air Soil Pollut. 2011, 215, 3–29. [Google Scholar] [CrossRef] [Green Version]
- Bhatkhande, D.S.; Pangarkar, V.G.; Beenackers, A.A. Photocatalytic degradation for environmental applications–A review. J. Chem. Technol. Biotechnol. 2002, 77, 102–116. [Google Scholar] [CrossRef]
- Hu, C.; Guo, J.; Qu, J.; Hu, X. Photocatalytic degradation of pathogenic bacteria with AgI/TiO2 under visible light irradiation. Langmuir 2007, 23, 4982–4987. [Google Scholar] [CrossRef] [PubMed]
- Welker, M.; Steinberg, C. Rates of humic substance photosensitized degradation of microcystin-LR in natural waters. Environ. Sci. Technol. 2000, 34, 3415–3419. [Google Scholar] [CrossRef]
- Doll, T.E.; Frimmel, F.H. Removal of selected persistent organic pollutants by heterogeneous photocatalysis in water. Catal. Today 2005, 101, 195–202. [Google Scholar] [CrossRef]
- Horovitz, I.; Avisar, D.; Baker, M.A.; Grilli, R.; Lozzi, L.; Di Camillo, D.; Mamane, H. Carbamazepine degradation using a N-doped TiO2 coated photocatalytic membrane reactor: Influence of physical parameters. J. Hazard. Mater. 2016, 310, 98–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doll, T.E.; Frimmel, F.H. Photocatalytic degradation of carbamazepine, clofibric acid and iomeprol with P25 and Hombikat UV100 in the presence of natural organic matter (NOM) and other organic water constituents. Water Res. 2005, 39, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Reitberger, T. Generation of free OHaq radicals by black light illumination of Degussa (evonik) P25 TiO2 aqueous suspensions. Catalysts 2013, 3, 418–443. [Google Scholar] [CrossRef]
- Mullet, M.; Fievet, P.; Reggiani, J.; Pagetti, J. Surface electrochemical properties of mixed oxide ceramic membranes: Zeta-potential and surface charge density. J. Membr. Sci. 1997, 123, 255–265. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, X.; Wang, Y.; Hu, X.; Larbot, A.; Persin, M. Electrokinetic characterization of the Al2O3 ceramic MF membrane by streaming potential measurements. Desalination 2009, 235, 102–109. [Google Scholar] [CrossRef]
- Zhang, W.; An, T.; Cui, M.; Sheng, G.; Fu, J. Effects of anions on the photocatalytic and photoelectrocatalytic degradation of reactive dye in a packed-bed reactor. J. Chem. Technol. Biotechnol. 2005, 80, 223–229. [Google Scholar] [CrossRef]
- Kormann, C.; Bahnemann, D.; Hoffmann, M.R. Photolysis of chloroform and other organic molecules in aqueous titanium dioxide suspensions. Environ. Sci. Technol. 1991, 25, 494–500. [Google Scholar] [CrossRef]
- Ku, Y.; Leu, R.-M.; Lee, K.-C. Decomposition of 2-chlorophenol in aqueous solution by UV irradiation with the presence of titanium dioxide. Water Res. 1996, 30, 2569–2578. [Google Scholar] [CrossRef]
- Mills, A.; Morris, S.; Davies, R. Photomineralisation of 4-chlorophenol sensitised by titanium dioxide: A study of the intermediates. J. Photochem. Photobiol. A 1993, 70, 183–191. [Google Scholar] [CrossRef]
- Schmelling, D.C.; Gray, K.A.; Kamat, P.V. The influence of solution matrix on the photocatalytic degradation of TNT in TiO2 slurries. Water Res. 1997, 31, 1439–1447. [Google Scholar] [CrossRef]
- Matthews, R.W. Photo-oxidation of organic material in aqueous suspensions of titanium dioxide. Water Res. 1986, 20, 569–578. [Google Scholar] [CrossRef]
- Tang, W.; Zhang, Z.; An, H.; Quintana, M.; Torres, D. TiO2/UV photodegradation of azo dyes in aqueous solutions. Environ. Technol. 1997, 18, 1–12. [Google Scholar] [CrossRef]
- Chen, S.; Cao, G. Photocatalytic degradation of organophosphorus pesticides using floating photocatalyst TiO2·SiO2/beads by sunlight. Solar Energy 2005, 79, 1–9. [Google Scholar]
- Achilleos, A.; Hapeshi, E.; Xekoukoulotakis, N.; Mantzavinos, D.; Fatta-Kassinos, D. UV-A and solar photodegradation of ibuprofen and carbamazepine catalyzed by TiO2. Sep. Sci. Technol. 2010, 45, 1564–1570. [Google Scholar] [CrossRef]
- Vogna, D.; Marotta, R.; Andreozzi, R.; Napolitano, A.; d’Ischia, M. Kinetic and chemical assessment of the UV/H2O2 treatment of antiepileptic drug carbamazepine. Chemosphere 2004, 54, 497–505. [Google Scholar] [CrossRef]
- Damen, J.; Ten Cate, J.; Ellingsen, J. Induction of calcium phosphate precipitation by titanium dioxide. J. Dent. Res. 1991, 70, 1346–1349. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.A.; Li, W.; Lee, H.E.; Phillips, B.L.; Lee, Y.J. Phosphate uptake by TiO2: Batch studies and NMR spectroscopic evidence for multisite adsorption. J. Colloid Interface Sci. 2011, 364, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Chusuei, C.C.; Goodman, D.; Van Stipdonk, M.; Justes, D.; Loh, K.; Schweikert, E. Solid–Liquid Adsorption of Calcium Phosphate on TiO2. Langmuir 1999, 15, 7355–7360. [Google Scholar] [CrossRef]
- Tsuru, T.; Hironaka, D.; Yoshioka, T.; Asaeda, M. Effect of divalent cations on permeate volume flux through porous titania membranes. Desalination 2002, 147, 213–216. [Google Scholar] [CrossRef]
- Abrahamse, A.; Lipreau, C.; Li, S.; Heijman, S. Removal of divalent cations reduces fouling of ultrafiltration membranes. J. Membr. Sci. 2008, 323, 153–158. [Google Scholar] [CrossRef]
- Gupta, A.; Pal, A.; Sahoo, C. Photocatalytic degradation of a mixture of Crystal Violet (Basic Violet 3) and Methyl Red dye in aqueous suspensions using Ag+ doped TiO2. Dyes Pigments 2006, 69, 224–232. [Google Scholar] [CrossRef]
- Shirazi, E.; Torabian, A.; Nabi-Bidhendi, G. Carbamazepine Removal from Groundwater: Effectiveness of the TiO2/UV, Nanoparticulate Zero-Valent Iron, and Fenton (NZVI/H2O2) Processes. Clean-Soil Air Water 2013, 41, 1062–1072. [Google Scholar] [CrossRef]
- Kashif, N.; Ouyang, F. Parameters effect on heterogeneous photocatalysed degradation of phenol in aqueous dispersion of TiO2. J. Environ. Sci. 2009, 21, 527–533. [Google Scholar] [CrossRef]
- Li, X.; Fan, C.; Sun, Y. Enhancement of photocatalytic oxidation of humic acid in TiO2 suspensions by increasing cation strength. Chemosphere 2002, 48, 453–460. [Google Scholar] [CrossRef]
- Selvam, K.; Muruganandham, M.; Muthuvel, I.; Swaminathan, M. The influence of inorganic oxidants and metal ions on semiconductor sensitized photodegradation of 4-fluorophenol. Chem. Eng. J. 2007, 128, 51–57. [Google Scholar] [CrossRef]
- Minero, C.; Pramauro, E.; Pelizzetti, E.; Dolci, M.; Marchesini, A. Photosensitized transformations of atrazine under simulated sunlight in aqueous humic acid solution. Chemosphere 1992, 24, 1597–1606. [Google Scholar] [CrossRef]
- Okamura, H.; Sugiyama, Y. Photosensitized degradation of Irgarol 1051 in water. Chemosphere 2004, 57, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Lucia, d.C.C.; Maria, d.C.; Grande, M.A.; Acosta, E.O.; Ginzberg, B. Removal of Cr (VI) and humic acid by heterogeneous photocatalysis in a laboratory reactor and a pilot reactor. Ind. Eng. Chem. Res. 2012, 51, 9468–9474. [Google Scholar]
- Selli, E.; Baglio, D.; Montanarella, L.; Bidoglio, G. Role of humic acids in the TiO2-photocatalyzed degradation of tetrachloroethene in water. Water Res. 1999, 33, 1827–1836. [Google Scholar] [CrossRef]
- Vinodgopal, K.; Kamat, P.V. Environmental photochemistry on surfaces. Charge injection from excited fulvic acid into semiconductor colloids. Environ. Sci. Technol. 1992, 26, 1963–1966. [Google Scholar] [CrossRef]
- Nienow, A.M.; Bezares-Cruz, J.C.; Poyer, I.C.; Hua, I.; Jafvert, C.T. Hydrogen peroxide-assisted UV photodegradation of Lindane. Chemosphere 2008, 72, 1700–1705. [Google Scholar] [CrossRef] [PubMed]
- Autin, O.; Hart, J.; Jarvis, P.; MacAdam, J.; Parsons, S.A.; Jefferson, B. The impact of background organic matter and alkalinity on the degradation of the pesticide metaldehyde by two advanced oxidation processes: UV/H2O2 and UV/TiO2. Water Res. 2013, 47, 2041–2049. [Google Scholar] [CrossRef] [PubMed]
- Makita, M.; Harata, A. Photocatalytic decolorization of rhodamine B dye as a model of dissolved organic compounds: Influence of dissolved inorganic chloride salts in seawater of the Sea of Japan. Chem. Eng. Process. 2008, 47, 859–863. [Google Scholar] [CrossRef]
- Yuan, R.; Ramjaun, S.N.; Wang, Z.; Liu, J. Photocatalytic degradation and chlorination of azo dye in saline wastewater: Kinetics and AOX formation. Chem. Eng. J. 2012, 192, 171–178. [Google Scholar] [CrossRef]
- Mopper, K.; Zhou, X. Hydroxyl radical photoproduction in the sea and its potential impact on marine processes. Science 1990, 250, 661–664. [Google Scholar] [CrossRef] [PubMed]
- Vione, D.; Falletti, G.; Maurino, V.; Minero, C.; Pelizzetti, E.; Malandrino, M.; Ajassa, R.; Olariu, R.-I.; Arsene, C. Sources and sinks of hydroxyl radicals upon irradiation of natural water samples. Environ. Sci. Technol. 2006, 40, 3775–3781. [Google Scholar] [CrossRef] [PubMed]
- Piscopo, A.; Robert, D.; Weber, J.V. Influence of pH and chloride anion on the photocatalytic degradation of organic compounds: Part I. Effect on the benzamide and para-hydroxybenzoic acid in TiO2 aqueous solution. Appl. Catal. B 2001, 35, 117–124. [Google Scholar] [CrossRef]
- Okamoto, K.-I.; Yamamoto, Y.; Tanaka, H.; Itaya, A. Kinetics of heterogeneous photocatalytic decomposition of phenol over anatase TiO2 powder. Bull. Chem. Soc. Jpn. 1985, 58, 2023–2028. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (OH/O− in aqueous solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef]
- Pelaez, M.; Armah, A.; O’Shea, K.; Falaras, P.; Dionysiou, D.D. Effects of water parameters on the degradation of microcystin-LR under visible light-activated TiO2 photocatalyst. Water Res. 2011, 45, 3787–3796. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Mathur, N. Photocatalytic degradation of aniline at the interface of TiO2 suspensions containing carbonate ions. J. Colloid Interface Sci. 2006, 300, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Lair, A.; Ferronato, C.; Chovelon, J.-M.; Herrmann, J.-M. Naphthalene degradation in water by heterogeneous photocatalysis: An investigation of the influence of inorganic anions. J. Photochem. Photobiol. A 2008, 193, 193–203. [Google Scholar] [CrossRef]
- Hu, L.; Flanders, P.M.; Miller, P.L.; Strathmann, T.J. Oxidation of sulfamethoxazole and related antimicrobial agents by TiO2 photocatalysis. Water Res. 2007, 41, 2612–2626. [Google Scholar] [CrossRef] [PubMed]
- Hem, J.D. Study and Interpretation of the Chemical Characteristics of Natural Water; Department of the Interior, US Geological Survey: Reston, VA, USA, 1985; Volume 2254.
- Chakoumakos, C.; Russo, R.C.; Thurston, R.V. Toxicity of copper to cutthroat trout (Salmo clarki) under different conditions of alkalinity, pH, and hardness. Environ. Sci. Technol. 1979, 13, 213–219. [Google Scholar] [CrossRef]
- Jermann, D.; Pronk, W.; Meylan, S.; Boller, M. Interplay of different NOM fouling mechanisms during ultrafiltration for drinking water production. Water Res. 2007, 41, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- Nas, B.; Berktay, A. Groundwater quality mapping in urban groundwater using GIS. Environ. Monit. Assess. 2010, 160, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Serruya, C.; Edelstein, M.; Pollingher, U.; Serruya, S. Lake Kinneret sediments: Nutrient composition of the pore water and mud water exchanges. Limnol. Oceanogr. 1974, 19, 489–508. [Google Scholar] [CrossRef]
- Mamane, H.; Horovitz, I.; Lozzi, L.; Di Camillo, D.; Avisar, D. The role of physical and operational parameters in photocatalysis by N-doped TiO2 sol–gel thin films. Chem. Eng. J. 2014, 257, 159–169. [Google Scholar] [CrossRef]
- Wiegman, S.; Termeer, J.A.; Verheul, T.; Kraak, M.H.; de Voogt, P.; Laane, R.W.; Admiraal, W. UV absorbance dependent toxicity of acridine to the marine diatom Phaeodactylum tricornutum. Environ. Sci. Technol. 2002, 36, 908–913. [Google Scholar] [CrossRef] [PubMed]
- Kosjek, T.; Andersen, H.R.; Kompare, B.; Ledin, A.; Heath, E. Fate of carbamazepine during water treatment. Environ. Sci. Technol. 2009, 43, 6256–6261. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.V.S.; Subrahmanyam, M.; Boule, P. Immobilized TiO2 photocatalyst during long-term use: Decrease of its activity. Appl. Catal. B 2004, 49, 239–249. [Google Scholar] [CrossRef]
- Nakano, K.; Obuchi, E.; Takagi, S.; Yamamoto, R.; Tanizaki, T.; Taketomi, M.; Eguchi, M.; Ichida, K.; Suzuki, M.; Hashimoto, A. Photocatalytic treatment of water containing dinitrophenol and city water over TiO2/SiO2. Sep. Purif. Technol. 2004, 34, 67–72. [Google Scholar] [CrossRef]
- Miranda-García, N.; Suárez, S.; Maldonado, M.I.; Malato, S.; Sánchez, B. Regeneration approaches for TiO2 immobilized photocatalyst used in the elimination of emerging contaminants in water. Catal. Today 2014, 230, 27–34. [Google Scholar] [CrossRef]
- Baker, M.; Fakhouri, H.; Grilli, R.; Pulpytel, J.; Smith, W.; Arefi-Khonsari, F. Effect of total gas pressure and O2/N2 flow rate on the nanostructure of N-doped TiO2 thin films deposited by reactive sputtering. Thin Solid Films 2014, 552, 10–17. [Google Scholar] [CrossRef]
- Di Valentin, C.; Pacchioni, G.; Selloni, A.; Livraghi, S.; Giamello, E. Characterization of paramagnetic species in N-doped TiO2 powders by EPR spectroscopy and DFT calculations. J. Phys. Chem. B 2005, 109, 11414–11419. [Google Scholar] [CrossRef] [PubMed]
- Asahi, R.; Morikawa, T. Nitrogen complex species and its chemical nature in TiO2 for visible-light sensitized photocatalysis. Chem. Phys. 2007, 339, 57–63. [Google Scholar] [CrossRef]
- Sun, J.; Qiao, L.; Sun, S.; Wang, G. Photocatalytic degradation of Orange G on nitrogen-doped TiO2 catalysts under visible light and sunlight irradiation. J. Hazard. Mater. 2008, 155, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Sathish, M.; Viswanathan, B.; Viswanath, R.; Gopinath, C.S. Synthesis, characterization, electronic structure, and photocatalytic activity of nitrogen-doped TiO2 nanocatalyst. Chem. Mater. 2005, 17, 6349–6353. [Google Scholar] [CrossRef]
- Andreozzi, R.; Marotta, R.; Pinto, G.; Pollio, A. Carbamazepine in water: Persistence in the environment, ozonation treatment and preliminary assessment on algal toxicity. Water Res. 2002, 36, 2869–2877. [Google Scholar] [CrossRef]
- Martínez, C.; Fernández, M.; Santaballa, J.; Faria, J. Kinetics and mechanism of aqueous degradation of carbamazepine by heterogeneous photocatalysis using nanocrystalline TiO2, ZnO and multi-walled carbon nanotubes–anatase composites. Appl. Catal. B 2011, 102, 563–571. [Google Scholar] [CrossRef]
- Ruzicka, K.; Zessner, M.; Blaschke, A.P.; Fenz, R.; Clara, M.; Kroiss, H. Evaluating the success of sewer reconstruction by using carbamazepine as anthropogenic marker in groundwater. Water Sci. Technol. 2011, 63, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Geißen, S.-U.; Gal, C. Carbamazepine and diclofenac: removal in wastewater treatment plants and occurrence in water bodies. Chemosphere 2008, 73, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Margot, J.; Kienle, C.; Magnet, A.; Weil, M.; Rossi, L.; De Alencastro, L.F.; Abegglen, C.; Thonney, D.; Chèvre, N.; Schärer, M. Treatment of micropollutants in municipal wastewater: Ozone or powdered activated carbon? Sci. Total Environ. 2013, 461, 480–498. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Knox, C.; Guo, A.C.; Shrivastava, S.; Hassanali, M.; Stothard, P.; Chang, Z.; Woolsey, J. DrugBank: A comprehensive resource for in silico drug discovery and exploration. Nucleic Acids Res. 2006, 34, D668–D672. [Google Scholar] [CrossRef] [PubMed]
- Grilli, R.; Di Camillo, D.; Lozzi, L.; Horovitz, I.; Mamane, H.; Avisar, D.; Baker, M. Surface characterisation and photocatalytic performance of N-doped TiO2 thin films deposited onto 200 nm pore size alumina membranes by sol–gel methods. Mater. Chem. Phys. 2015, 159, 25–37. [Google Scholar] [CrossRef] [Green Version]



| Phase of Experiment | Ca2+ 120 mg/L | Mg2+ 120 mg/L | |
|---|---|---|---|
| pH = 7 | pH = 8.5 | pH = 8.5 | |
| Initial | 118.8 ± 4 | 118.8 ± 4 | 121 ± 2 |
| Final | 97.1 ± 0.7 | 96.4 ± 1 | 117.8 ± 0.5 |
| Rate Constant Change with DOM | 4 mg/L SRNOM | 8 mg/L SRNOM | 8 mg/L SRHA | 8 mg/L SRFA |
|---|---|---|---|---|
| % decrease in k | 11 | 24 | 40 | 37 |
| [Cl−] | No Additive | 100 mg/L | 250 mg/L | 500 mg/L |
|---|---|---|---|---|
| k (min−1) | 0.0082 ± 0.00019 | 0.0088 ± 0.0006 | 0.0082 ± 0.00005 | 0.0086 ± 0.0011 |
| Water Type | pH | Alkalinity (mg/L as CaCO3) | Hardness (mg/L as CaCO3) | Ca2+ (mg/L) | Mg2+ (mg/L) | Chlorides (mg/L Cl−) | DOC (mg/L) | % Increase/Decrease in k |
|---|---|---|---|---|---|---|---|---|
| Lake Kinneret | 8.5 | 84 | 305 | 48 | 41 | 310 | 4.2 | −44 |
| DI + Magnesium | 8.5 | - | - | - | 120 | - | - | −2.3 |
| DI + Calcium | 8.5 | - | 300 | 120 | - | - | - | - |
| DI + Calcium | 7 | - | 300 | 120 | - | - | - | 30.5 |
| DI + Chloride | 7 | - | - | - | - | 100 | - | 8.4 |
| 7 | - | - | - | - | 250 | - | 1.6 | |
| 7 | - | - | - | - | 500 | - | 7.2 | |
| DI + Alkalinity | 7 | 50 | - | - | - | - | - | −1.5 |
| 7 | 100 | - | - | - | - | - | −14.4 | |
| 7 | 200 | - | - | - | - | - | −33.8 | |
| 7 | 300 | - | - | - | - | - | −48 | |
| DI + SRNOM | 7 | - | - | - | - | - | 4 | −11 |
| 7 | - | - | - | - | - | 8 | −24 | |
| DI + SRFA | 7 | - | - | - | - | - | 8 | −37 |
| DI + SRHA | 7 | - | - | - | - | - | 8 | −40 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luster, E.; Avisar, D.; Horovitz, I.; Lozzi, L.; Baker, M.A.; Grilli, R.; Mamane, H. N-Doped TiO2-Coated Ceramic Membrane for Carbamazepine Degradation in Different Water Qualities. Nanomaterials 2017, 7, 206. https://doi.org/10.3390/nano7080206
Luster E, Avisar D, Horovitz I, Lozzi L, Baker MA, Grilli R, Mamane H. N-Doped TiO2-Coated Ceramic Membrane for Carbamazepine Degradation in Different Water Qualities. Nanomaterials. 2017; 7(8):206. https://doi.org/10.3390/nano7080206
Chicago/Turabian StyleLuster, Enbal, Dror Avisar, Inna Horovitz, Luca Lozzi, Mark A. Baker, Rossana Grilli, and Hadas Mamane. 2017. "N-Doped TiO2-Coated Ceramic Membrane for Carbamazepine Degradation in Different Water Qualities" Nanomaterials 7, no. 8: 206. https://doi.org/10.3390/nano7080206





