Charge Transfer Effect on Raman and Surface Enhanced Raman Spectroscopy of Furfural Molecules
Abstract
:1. Introduction
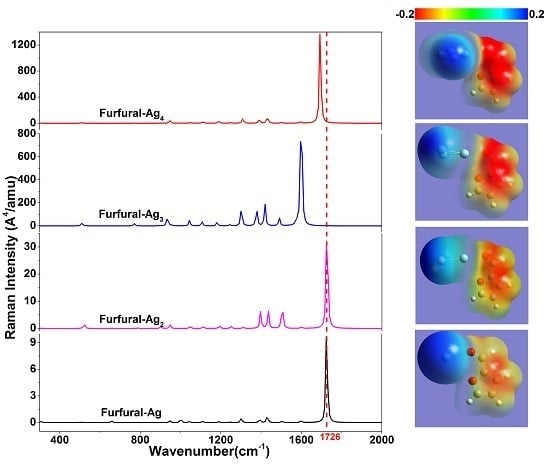
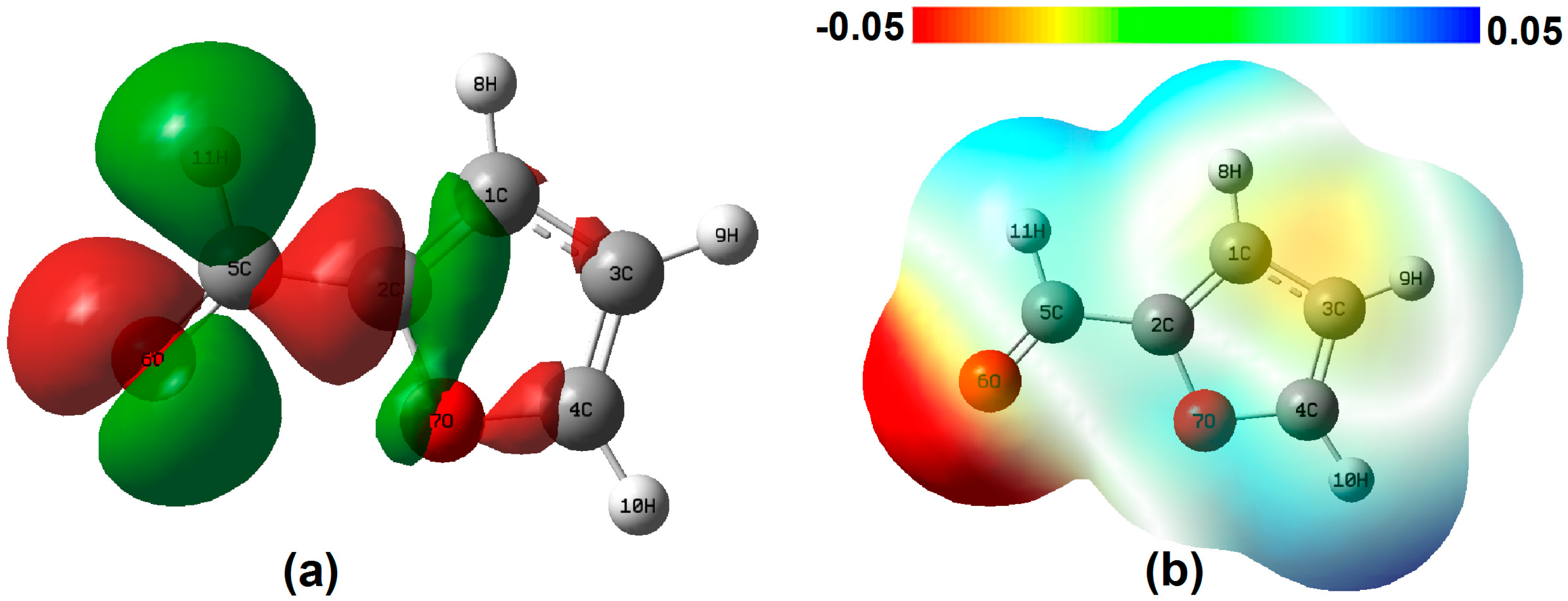
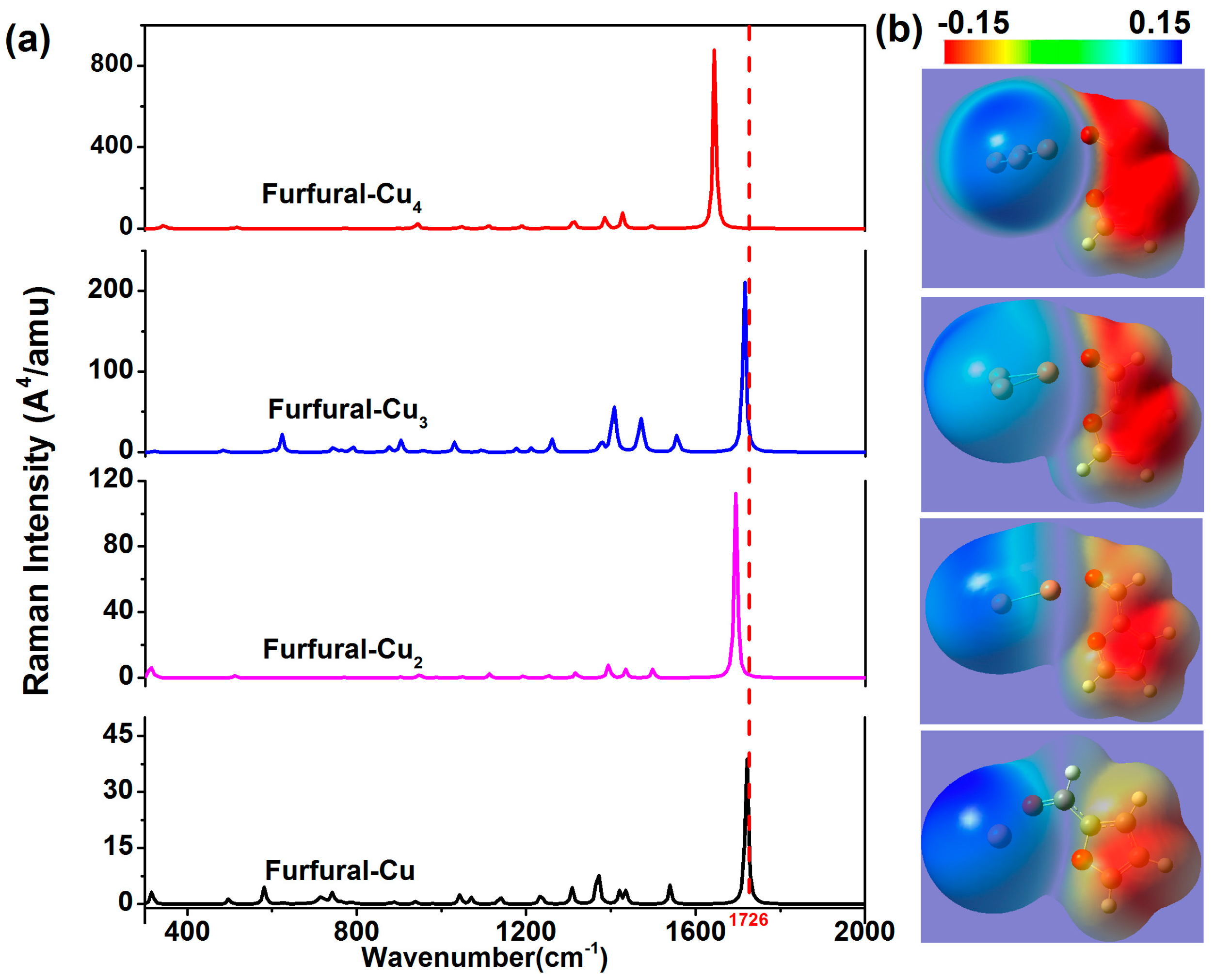
2. Results and Discussion
3. Theoretical Section & Experimental Part
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fleischmann, M.; Hendra, P.J.; Mc Quillan, A.J. Raman Spectra of Pyridine Adsorbed at Silver Electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Lord, R.C. Introduction to Infrared and Raman Spectroscopy. J. Am. Chem. Soc. 1990, 87, 1155–1156. [Google Scholar] [CrossRef]
- Schopf, J.W.; Kudryavtsev, A.B. Confocal Laser Scanning Microscopy and Raman (and Fluorescence) Spectroscopic Imagery of Permineralized Cambrian and Neoproterozoic Fossils; Springer: Dordrecht, The Netherlands, 2011; pp. 241–270. [Google Scholar]
- Pelletier, M.J. Analytical Applications of Raman Spectroscopy; Blackwell Science: Malden, MA, USA, 1999. [Google Scholar]
- Wilkinson, G.R. Raman effect. Nature 1979, 478, 144–151. [Google Scholar] [CrossRef]
- Moskovits, M. Surface-enhanced spectroscopy. Rev. Mod. Phys. 1985, 57, 783–826. [Google Scholar] [CrossRef]
- Yang, Y.; Wan, F.; Wang, P.; Zeng, X.; Jia, Y.; Shi, H.; Chen, W.; Huang, Y. Nanoparticle assisted Raman information acquisition from metal encapsulated sandwich structure. J. Raman Spectrosc. 2017, 48, 443–447. [Google Scholar] [CrossRef]
- Huang, Y.; Fang, Y.; Zhang, Z.; Zhu, L.; Sun, M. Nanowire-supported Plasmonic waveguide for remote excitation of surface-enhanced Raman scattering. Light Sci. Appl. 2014, 3, e199. [Google Scholar] [CrossRef]
- Hao, J.; Liu, T.; Huang, Y.; Chen, G.; Liu, A.; Wang, S.; Wen, W. Metal Nanoparticle–Nanowire Assisted SERS on Film. J. Phys. Chem. C 2015, 119, 19376–19381. [Google Scholar] [CrossRef]
- Jeanmaire, D.L.; Duyne, R.P.V. Surface Raman spectra electrochemistry: Part I. Heterocyclic, aromatic, and aliphatic amines adsorbed on the anodized silver electrode. J. Electroanal. Chem. Interfac. Electrochem. 1977, 84, 1–20. [Google Scholar] [CrossRef]
- Creighton, J.A.; Blatchford, C.G.; Albrecht, M.G. Plasma resonance enhancement of Raman scattering by pyridine adsorbed on silver or gold sol particles of size comparable to the excitation wavelength. J. Chem. Soc. Faraday Trans. 1979, 75, 790–798. [Google Scholar] [CrossRef]
- Campion, A.; Iii, J.E.I.; Child, C.M.; Foster, M. On the Mechanism of Chemical Enhancement in Surface-Enhanced Raman Scattering. J. Am. Chem. Soc. 1995, 117, 11807–11808. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, S.; Zhang, Z.; Huang, Y.; Chen, G.; Wei, H.; Wen, W. Selective plasmon driven surface catalysis in metal triangular nanoplate-molecule-film sandwich structure. Chem. Phys. Lett. 2015, 639, 47–51. [Google Scholar] [CrossRef]
- Wang, S.; Wang, H.; Liu, T.; Huang, Y.; Chen, G.; Wei, H.; Su, X.; Zeng, X.; Xia, Z.; Wen, W.; et al. Ascertaining Plasmonic Hot Electrons Generation from Plasmon Decay in Hybrid Plasmonic Modes. Plasmonics 2016, 11, 909–915. [Google Scholar] [CrossRef]
- Huang, Y.; Fang, Y.; Yang, Z.; Sun, M. Can p,p′-Dimercaptoazobisbenzene Be Produced from p-Aminothiophenol by Surface Photochemistry Reaction in the Junctions of a Ag Nanoparticle–Molecule–Ag (or Au) Film. J. Phys. Chem. C 2010, 114, 18263–18269. [Google Scholar] [CrossRef]
- Pan, L.; Huang, Y.; Yang, Y.; Xiong, W.; Chen, G.; Su, X.; Wei, H.; Wang, S.; Wen, W. Electromagnetic field redistribution induced selective plasmon driven surface catalysis in metal nanowire-film systems. Sci. Rep. 2015, 5, 17223. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhao, X.; Li, Y.; Chen, M.; Sun, M. DFT study of adsorption site effect on surface-enhanced Raman scattering of neutral and charged pyridine–Ag4 complexes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 73, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Chen, M. DFT study on the influence of electric field on surface-enhanced Raman scattering from pyridine–metal complex. J. Raman Spectrosc. 2014, 45, 62–67. [Google Scholar] [CrossRef]
- Sun, M.; Huang, Y.; Xia, L.; Chen, X.; Xu, H. The pH-Controlled Plasmon-Assisted Surface Photo catalysis Reaction of 4-Aminothiophenol to P,P′-Dimercaptoazobenzene on Au, Ag, and Cu Colloids. J. Phys. Chem. C 2011, 115, 9629–9636. [Google Scholar] [CrossRef]
- Lange, J.P.; van der Heide, E.; van Buijtenen, J.; Price, R. Furfural—A promising platform for lignocellulosic biofuels. ChemSusChem 2012, 5, 150–166. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Gu, Z.; Zou, J.; Wan, F.; Xiang, Y. Analysis of furfural dissolved in transformer oil based on confocal laser Raman spectroscopy. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 915–921. [Google Scholar] [CrossRef]
- Yuan, Z.; Chen, M.; Lei, H.; Lin, C. Make Use of Water and Furfural to Carry Out Transformer Aging Estimation. In Proceedings of the Power and Energy Engineering Conference, Chengdu, China, 28–31 March 2010; pp. 1–3. [Google Scholar]
- Liu, H.; Hu, H.; Jahan, M.S.; Ni, Y. Furfural formation from the pre-hydrolysis liquor of a hardwood kraft-based dissolving pulp production process. Bioresour. Technol. 2013, 131, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Vila, C.; Santos, V.; Parajó, J.C. Recovery of lignin and furfural from acetic acid-water-HCl pulping liquors. Bioresour. Technol. 2003, 90, 339–344. [Google Scholar] [CrossRef]
- Sun, M.; Liu, S.; Chen, M.; Xu, H. Direct visual evidence for the chemical mechanism of surface-enhanced resonance Raman scattering via charge transfer: (II) Binding-site and quantum-size effects. J. Raman Spectrosc. 2009, 40, 1172–1177. [Google Scholar] [CrossRef]
- Wu, D.; Duan, S.; Ren, B.; Tian, Z. Density functional theory study of surface-enhanced Raman scattering spectra of pyridine adsorbed on noble and transition metal surfaces. J. Raman Spectrosc. 2005, 36, 533–540. [Google Scholar] [CrossRef]
- Hakonen, A.; Svedendahl, M.; Ogier, R.; Yang, Z.J.; Lodewijks, K.; Verre, R.; Shegai, T.; Andersson, P.O.; Kall, M. Dimer-on-mirror SERS substrates with attogram sensitivity fabricated by colloidal lithography. Nanoscale 2015, 7, 9405–9410. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.Y.; Hayashi, M.; Lin, S.H.; Tian, Z. Theoretical differential Raman scattering cross-sections of totally-symmetric vibrational modes of free pyridine and pyridine-metal cluster complexes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2004, 60, 137–146. [Google Scholar] [CrossRef]
- Sun, M.; Xu, H. Direct visualization of the chemical mechanism in SERRS of 4-aminothiophenol/metal complexes and metal/4-aminothiophenol/metal junctions. ChemPhysChem Eur. J. Chem. Phys. Phys. Chem. 2009, 10, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Fang, Y.; Yang, Z.; Xu, H. Chemical and electromagnetic mechanisms of tip-enhanced Raman scattering. Phys. Chem. Chem. Phys. 2009, 11, 9412–9419. [Google Scholar] [CrossRef] [PubMed]
- Devipriya, B.; Kumaradhas, P. Charge density distribution and the electrostatic moments of CTPB in the active site of p300 enzyme: A DFT and charge density study. J. Theor. Biol. 2013, 335, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Dunn, D.A.; Self, S.A. Static Theory of Density and Potential Distribution in a Beam-Generated Plasma. J. Appl. Phys. 1964, 35, 113–122. [Google Scholar] [CrossRef]
- Evarestov, R.A.; Bandura, A.V. HF and DFT calculations of MgO surface energy and electrostatic potential using two- and three-periodic models. Int. J. Quantum Chem. 2004, 100, 452–459. [Google Scholar] [CrossRef]
- Liu, T.; Gu, J.; Tan, X.; Zhu, W.; Luo, X.; Jiang, H.; Ji, R.; Chen, X.; Silman, I.; Suddman, J. The Relationship between Binding Models of TMA with Furan and Imidazole and the Molecular Electrostatic Potentials: DFT and MP2 Computational Studies. J. Phys. Chem. A 2013, 106, 157–164. [Google Scholar] [CrossRef]
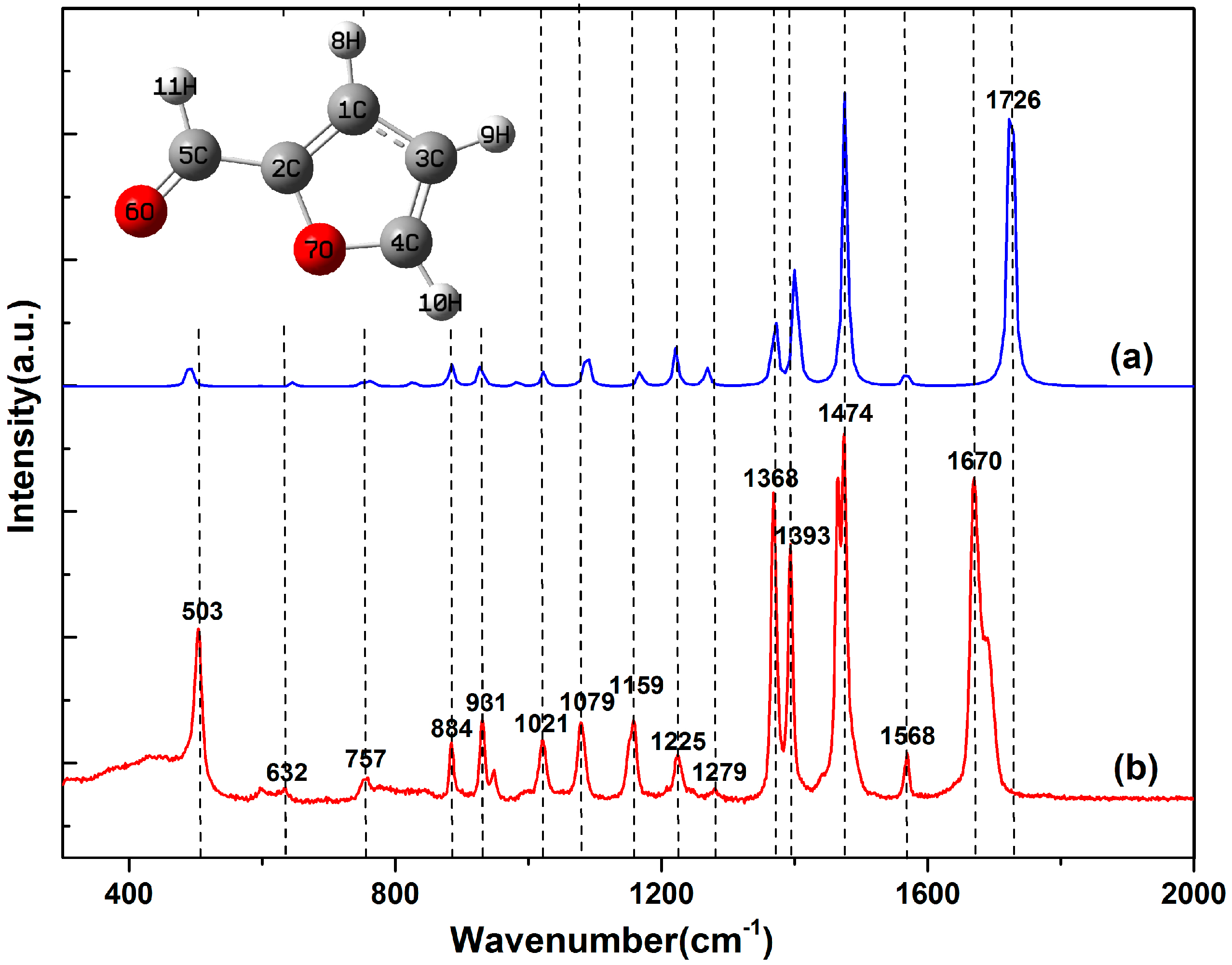


| Simulation (cm−1) | Experiment (cm−1) | Difference | Vibrational Plane | Vibrational Mode | |
|---|---|---|---|---|---|
| 1 | 494 | 503 | −9 | In-plane | C3=C8, C8–C9 symmetric bend |
| 2 | 643 | 632 | 9 | Out-plane | C3=C8, C8–O4 symmetric wag |
| 3 | 761 | 757 | 4 | In-plane | C9=O11 sway; C8–C9 stretch |
| 4 | 884 | 884 | 0 | Out-plane | C1–H5, C3–H7 synchronous sway; C2–H6 asynchronous sway |
| 5 | 926 | 931 | −5 | In-plane | C3=C8, C8–O4 symmetric bend |
| 6 | 1021 | 1021 | 0 | In-plane | C2–H6, C3–H7 symmetric bend; C2–C3 stretch |
| 7 | 1084 | 1079 | 5 | In-plane | C1–H5, C2–H6 symmetric bend; C1–O4 stretch |
| 8 | 1166 | 1159 | 7 | In-plane | C1–H5 sway; C1–O4 stretch |
| 9 | 1221 | 1225 | −4 | In-plane | C3–H7, C1–H5 asynchronous sway; C2–C3 stretch |
| 10 | 1271 | 1279 | −8 | In-plane | C8–C9, C8–O4 asynchronous stretch; C2–C3 stretch |
| 11 | 1372 | 1368 | 4 | In-plane | C9–H10, C1–H5 synchronous sway |
| 12 | 1398 | 1393 | 5 | In-plane | C8–C9, C8–O4 asymmetric stretch; C2–C3 stretch; C9–H10 sway; C2–H6 sway |
| 13 | 1474 | 1474 | 0 | In-plane | C1–O4, C8–O4 symmetric bend; C1=C2, C3=C8 synchronous stretch; C9–H10, C1–H5 asynchronous sway |
| 14 | 1567 | 1568 | −1 | In-plane | C1–O4, C8–O4 asymmetric bend; C1=C2, C3=C8 asynchronous stretch; C2–H6, C3–H7 synchronous sway |
| 15 | 1726 | 1670 | 56 | In-plane | C9–H10, C8–C9 synchronous stretch; C9=O11 asynchronous stretch |
| Atom Number (Ag) | Q (Furfural-Ag)/e | R (C=O)/Å | αxx/au | αyy/au | αzz/au | α/au |
|---|---|---|---|---|---|---|
| 0 | / | 1.2137 | 95.731 | 65.934 | 38.447 | 66.704 |
| 1 | 0.034 | 1.2136 | 144.041 | 129.828 | 87.600 | 120.490 |
| 2 | 0.0428 | 1.2232 | 227.029 | 166.383 | 107.592 | 167.001 |
| 3 | 0.249 | 1.2390 | 377.191 | 238.700 | 180.260 | 265.384 |
| 4 | 0.284 | 1.2369 | 331.198 | 359.486 | 196.830 | 295.838 |
| Au | Cu | |||
|---|---|---|---|---|
| Atom Number | Q (Furfural-Au)/e | R (C=O)/Å | Q (Furfural-Cu)/e | R (C=O)/Å |
| 1 | 0.157 | 1.219 | 0.139 | 1.218 |
| 2 | 0.218 | 1.230 | 0.171 | 1.230 |
| 3 | 0.269 | 1.235 | 0.274 | 1.228 |
| 4 | 0.309 | 1.232 | 0.317 | 1.237 |
| Atom Number | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Ag | 1.74 | 5.68 | 132.24 | 246.46 |
| Au | 1.69 | 15.71 | 23.39 | 39.09 |
| Cu | 6.98 | 20.27 | 38.06 | 158.23 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, F.; Shi, H.; Chen, W.; Gu, Z.; Du, L.; Wang, P.; Wang, J.; Huang, Y. Charge Transfer Effect on Raman and Surface Enhanced Raman Spectroscopy of Furfural Molecules. Nanomaterials 2017, 7, 210. https://doi.org/10.3390/nano7080210
Wan F, Shi H, Chen W, Gu Z, Du L, Wang P, Wang J, Huang Y. Charge Transfer Effect on Raman and Surface Enhanced Raman Spectroscopy of Furfural Molecules. Nanomaterials. 2017; 7(8):210. https://doi.org/10.3390/nano7080210
Chicago/Turabian StyleWan, Fu, Haiyang Shi, Weigen Chen, Zhaoliang Gu, Lingling Du, Pinyi Wang, Jianxin Wang, and Yingzhou Huang. 2017. "Charge Transfer Effect on Raman and Surface Enhanced Raman Spectroscopy of Furfural Molecules" Nanomaterials 7, no. 8: 210. https://doi.org/10.3390/nano7080210