Preparation of Magnetic Nanoparticles via a Chemically Induced Transition: Role of Treating Solution’s Temperature
Abstract
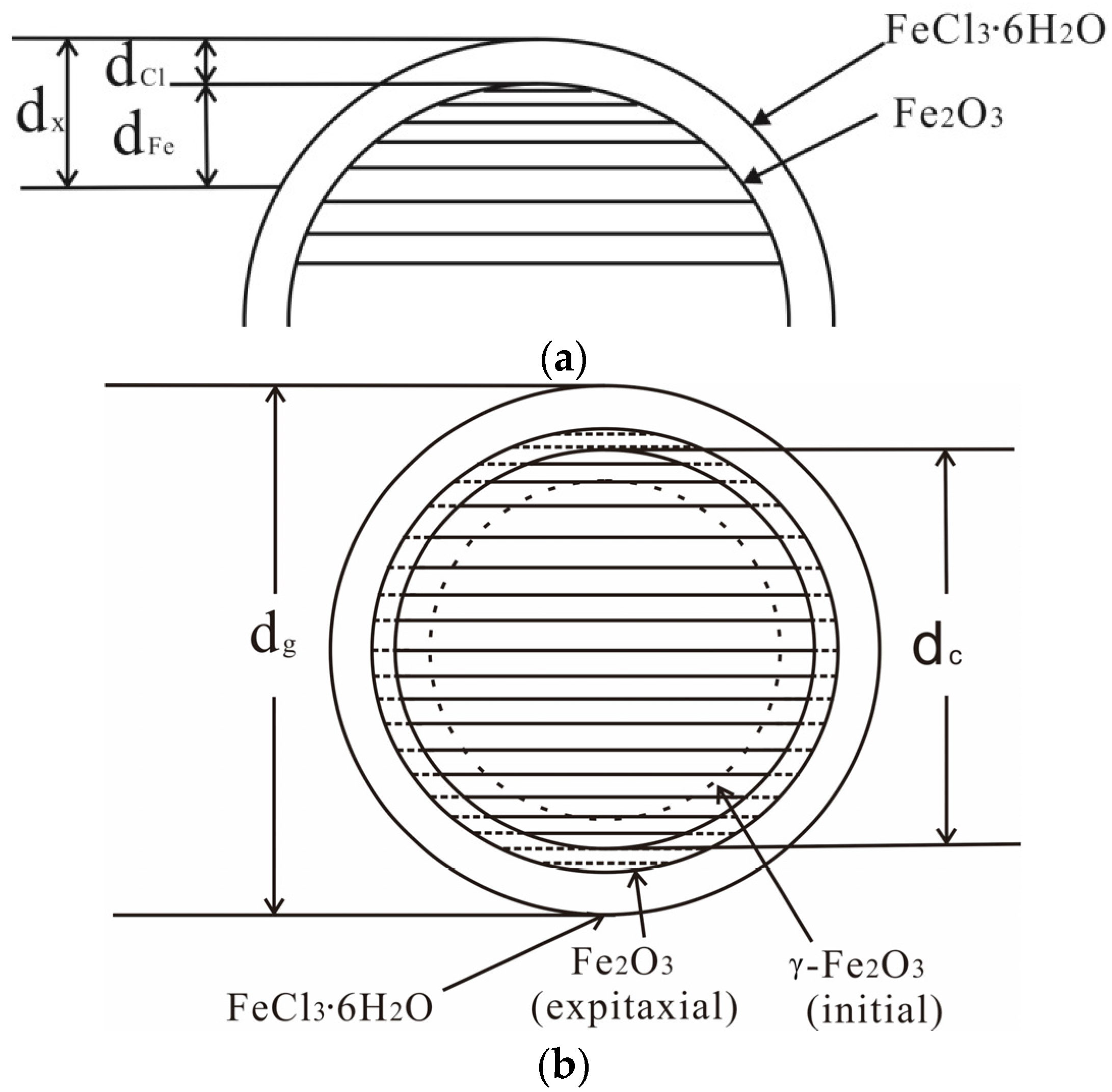
:1. Introduction
2. Experimental
2.1. Preparation Using Chemicals
2.2. Characterization
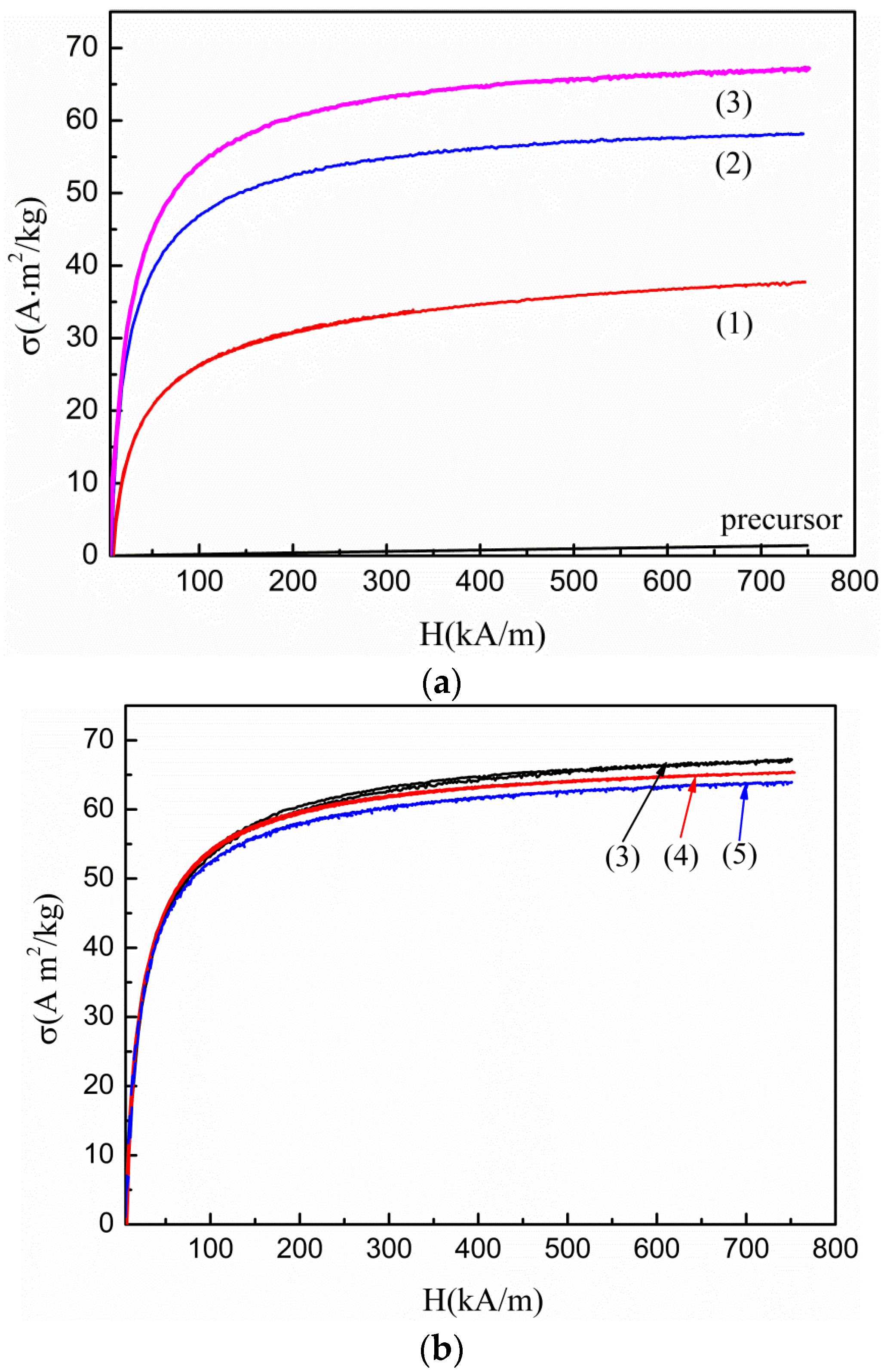
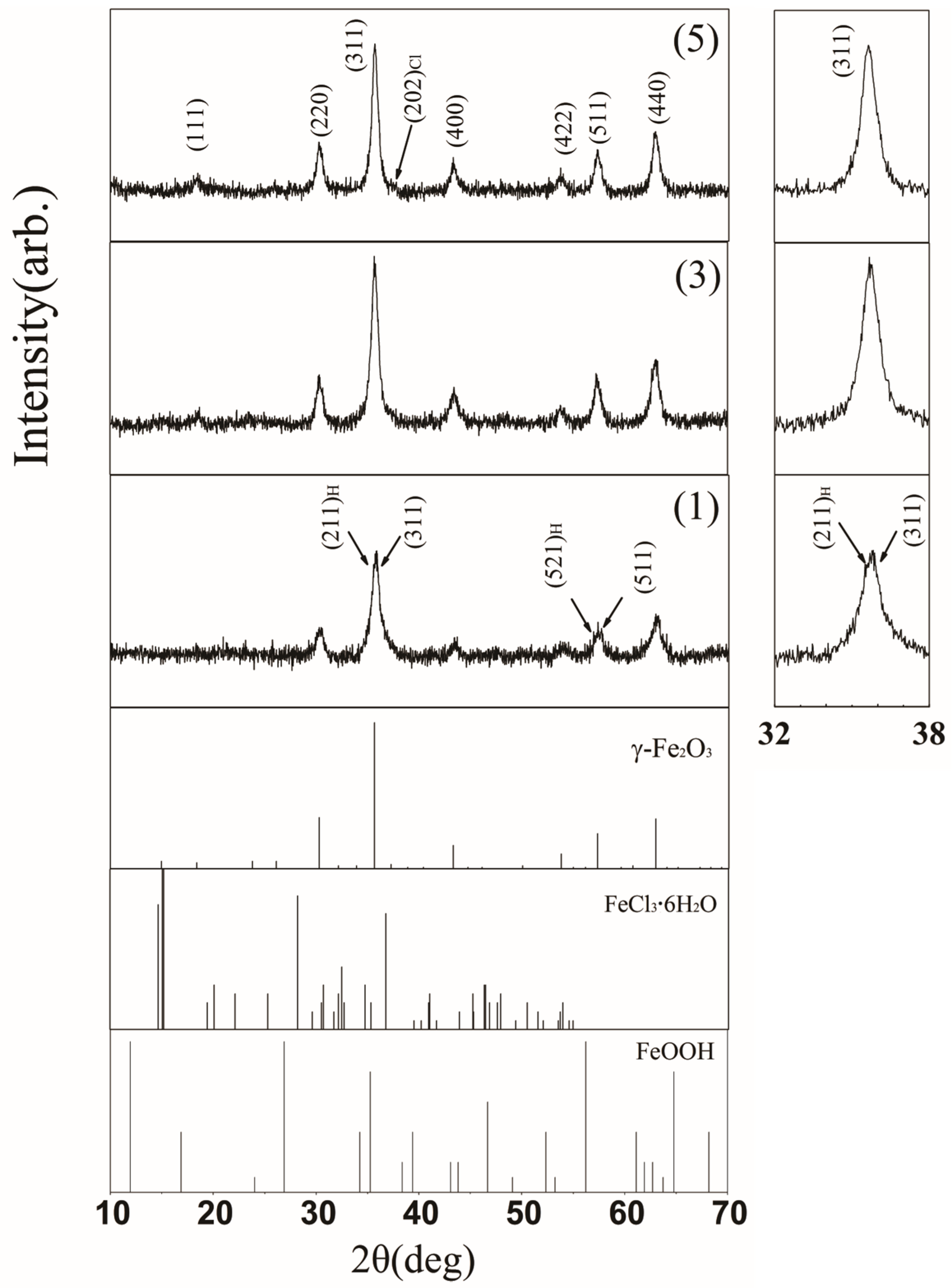
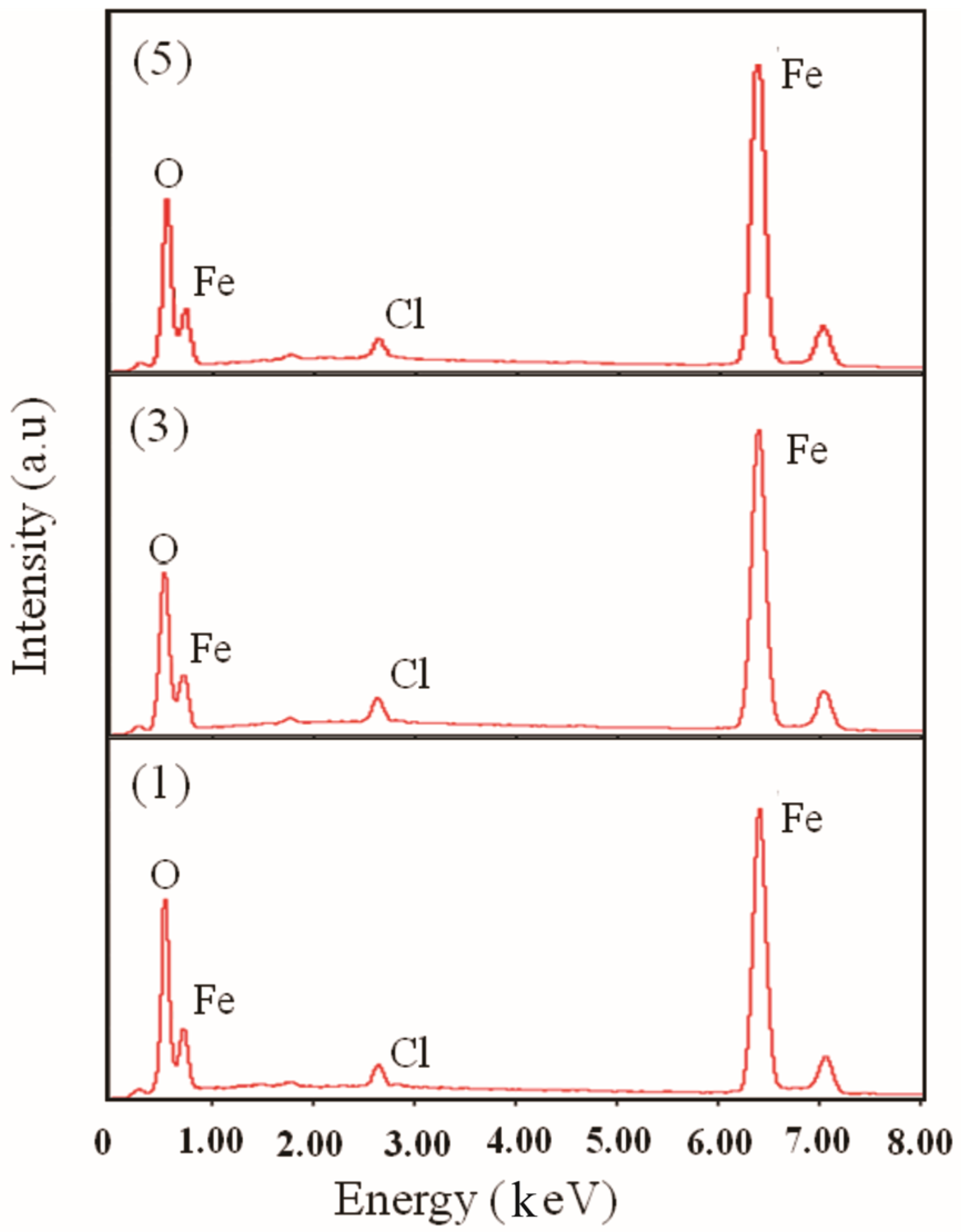
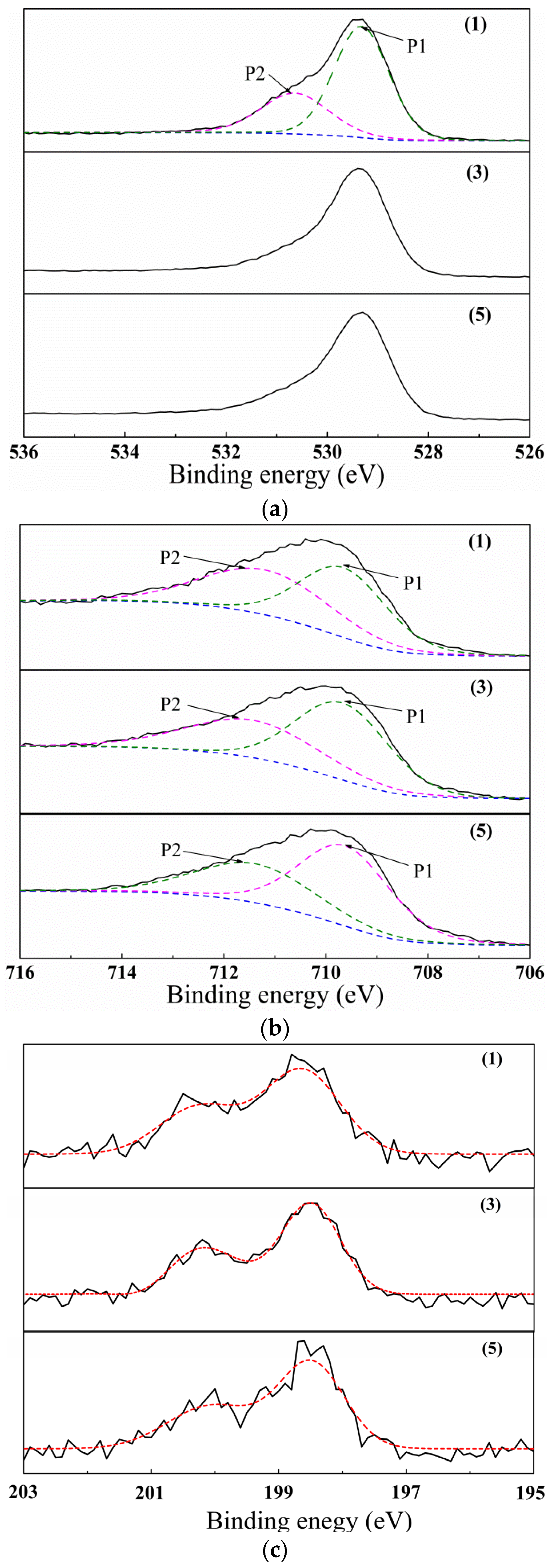
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mathew, D.S.; Juang, R.-S. An overview of the structure and magnetism of spinel ferrite nanoparticles and their synthesis in microemulsions. Chem. Eng. J. 2007, 129, 51–65. [Google Scholar] [CrossRef]
- Willard, M.A.; Kurihara, L.K.; Carpenter, E.E.; Calvin, S.; Harris, V.G. Chemically Prepared Magnetic Nanoparticles. Int. Mater. Rev. 2004, 36, 125–170. [Google Scholar] [CrossRef]
- Talapin, D.V.; Lee, J.-S.; Kovalenko, M.V.; Shevchenko, E.V. Prospects of colloidal nanocrystals for electronic and optoelectronic applications. Chem. Rev. 2010, 110, 389–458. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.H.; Hackett, M.J.; Pcok, J.; Hyeon, A.T. Synthesis, Characterization, and Application of Ultrasmall Nanoparticles. Chem. Mater. 2014, 26, 59–71. [Google Scholar] [CrossRef]
- Alqasem, B.; Yahya, N.; Qureshi, S.; Irfan, M.; Rehman, Z.U.; Soleimani, H. The enhancement of the magnetic properties of α-Fe2O3 nanocatalyst using an external magnetic field for the production of green ammonia. Mater. Sci. Eng. B 2017, 217, 49–62. [Google Scholar] [CrossRef]
- Cushing, B.L.; Kolenichenko, V.L.; O’conor, C.J. Recent Advances in the Liquid-Phase Syntheses of Inorganic Nanoparticles. Chem. Rev. 2004, 104, 3893–3946. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-Y.; Harris, M.T. Surface modification of magnetic nanoparticles capped by oleic acids: Characterization and colloidal stability in polar solvents. J. Colloid Interface Sci. 2006, 293, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Restrepo, J.; Labaye, Y.; Greneche, J.M. Surface anisotropy in maghemite nanoparticles. Physica B 2006, 384, 221–223. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Elst, L.V.; Muller, R.N. Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Chow, C.M. Carboxyl group (–CO2H) functionalized ferrimagnetic iron oxide nanoparticles for potential bio-applications. J. Mater. Chem. 2004, 14, 2781–2786. [Google Scholar] [CrossRef]
- Szabó, D.V.; Vollath, D. Nanocomposites from coated nanopparticles. Adv. Mater. 1999, 11, 1313–1316. [Google Scholar] [CrossRef]
- Narasimhan, B.R.V.; Prabhakar, S.; Manohar, P.; Granam, F.D. Synthesis of gamma ferric oxide by direct thermal decomposition of ferrous carbonate. Mater. Lett. 2002, 52, 295–300. [Google Scholar] [CrossRef]
- Hyeon, T.; Lee, S.S.; Park, J.; Chung, Y.; Na, H.B. Synthesis of highly crystalline and monodisperse maghemite nanocrystallites without a size-selection process. J. Am. Chem. Soc. 2001, 123, 12798–12801. [Google Scholar] [CrossRef] [PubMed]
- Shafi, K.V.P.M.; Ulman, A.; Yan, X.; Yang, N.L.; Estournès, C.; White, H.; Rafailovich, M. Sonochemical Synthesis of Functionalized Amorphous Iron Oxide Nanoparticles. Langmuir 2001, 17, 5093–5097. [Google Scholar] [CrossRef]
- Nakanishi, T.; Iida, H.; Osaka, T. Preparation of Iron Oxide Nanoparticles via Successive Reduction–Oxidation in Reverse Micelles. Chem. Lett. 2003, 32, 1166–1167. [Google Scholar] [CrossRef]
- Sreeja, V.; Joy, P.A. Microwave–hydrothermal synthesis of γ-Fe2O3 nanoparticles and their magnetic properties. Mater. Res. Bull. 2007, 42, 1570–1576. [Google Scholar] [CrossRef]
- Rouanet, A.; Solmon, H.; Pichelin, G.; Roucau, C.; Sibieude, F.; Monty, C. Synthesis by vaporization-condensation and characterization of γ-Fe2O3, In2O3, SnO2, ZnO and Zr1-xYxO2-nanophases. Nanostruct. Mater. 1995, 6, 283–286. [Google Scholar] [CrossRef]
- Casas, L.; Roig, A.; Molins, E.; Grenèche, J.M.; Asenjo, J.; Tejata, J. Iron oxide nanoparticles hosted in silica aerogels. Appl. Phys. A 2002, 74, 591–597. [Google Scholar] [CrossRef]
- Maeda, Y.; Manabe, T.; Nagai, K.; Yoshimura, F.; Hirono, S. High Coercive SiOx- adsorbed γ-Fe2O3 particles. In Ferrites; Proc. Inter. Con. Jpn.: Tokyo, Japan, 1982; pp. 541–544. [Google Scholar]
- Hsu, J.H.; Chang, C.R.; Kuo, P.C.; Huang, J.H. Annealing effect on magnetic properties of Si-modified γ-Fe2O3 particles. J. Magn. Magn. Mater. 1990, 89, 167–172. [Google Scholar] [CrossRef]
- Wen, B.C.; Li, J.; Lin, Y.Q.; Liu, X.D.; Fu, J.; Miao, H.; Zhang, Q.M. A novel preparation method for γ-Fe2O3 nanoparticles and their characterization. Mater. Chem. Phys. 2011, 128, 35–38. [Google Scholar] [CrossRef]
- Fu, J.; Lin, L.H.; Li, J. A Novel Preparation Method for Magnetic Nanoparticles and Their Characterization; FMTIF: Chongqing, China, 2011; pp. 46–50. [Google Scholar]
- Chen, Y.S.; Chen, Q.; Mao, H.; Zhang, T.; Qiu, X.Y.; Lin, Y.Q.; Li, J. Preparation of magnetic nanoparticles via chemically induced transition: Dependence of components and magnetization on the concentration of treating solution used. Nanomater. Nanotechnol. 2017, 7, 1–9. [Google Scholar] [CrossRef]
- Arulmurugan, R.; Vaidyanathan, G.; Sendhilnathan, S.; Jeyadevan, B. Co–Zn ferrite nanoparticles for ferrofluid preparation: Study on magnetic properties. Physica B 2005, 363, 225–231. [Google Scholar] [CrossRef]
- Grangvist, C.G.; Bhrman, R.A. Ultrafine metal particles. J. Appl. Phys. 1976, 47, 2200–2219. [Google Scholar] [CrossRef]
- Sato, T.; Iijima, T.; Seki, M.; Inagaki, N. Magnetic properties of ultrafine ferrite particles. J. Magn. Magn. Mater. 1987, 65, 252–256. [Google Scholar] [CrossRef]
- Blanco-Mantecón, M.; O’Grady, K. Interaction and size effects in magnetic nanoparticles. J. Magn. Magn. Mater. 2006, 296, 124–133. [Google Scholar] [CrossRef]
- Cong, C.J.; Hong, J.H.; Liu, Q.Y.; Liao, L.; Zhang, K.L. Synthesis, Structure and ferromagnetic properties of Ni-dopeed ZnO nanoparticles. Solid State Commun. 2006, 138, 511–515. [Google Scholar] [CrossRef]
- Frison, R.; Cernuto, G.; Cervellino, A.; Zaharko, O.; Colonna, G.M.; Guagliardi, A.; Masciocchi, N. Magnetite–Maghemite Nanoparticles in the 5–15 nm Range: Correlating the Core–Shell Composition and the Surface Structure to the Magnetic Properties. A Total Scattering Study. Chem. Mater. 2013, 25, 4820–4827. [Google Scholar] [CrossRef]
- Li, J.; Qiu, X.Y.; Lin, Y.Q.; Liu, X.D.; Gao, R.L.; Wang, A.R. A study of modified Fe3O4 nanoparticles for the synthesis of ionic ferrofluids. Appl. Surf. Sci. 2010, 256, 6977–6981. [Google Scholar] [CrossRef]
- Srnová-Sloufová, I.; Vlcková, B.; Bastl, Z.; Hasslett, T.L. Bimetallic (Ag)Au nanoparticles prepared by the seed growth method: Two-dimensional assembling, characterization by energy dispersive X-ray analysis, X-ray photoelectron spectroscopy, and surface enhanced Raman spectroscopy, and proposed mechanism of growth. Langmuir 2004, 20, 3407–3415. [Google Scholar] [PubMed]
- Tanuma, S.; Powell, C.J.; Penn, D.R. Calculations of electron inelastic mean free paths. II. Data for 27 elements over the 50–2000 eV range. Surf. Interface Anal. 1991, 17, 911–926. [Google Scholar] [CrossRef]
- Li, J.; Lin, Y.; Liu, X.; Zhang, Q.; Miao, H.; Zhang, T.; Wen, B. The study of transition on NiFe2O4 nanoparticles prepared by co-precipitation/calcination. Phase Trans. 2011, 84, 49–57. [Google Scholar] [CrossRef]
- Zhang, Q.M.; Li, J.; Mao, H.; Fu, J. Preparation of γ-Fe2O3/Ni2O3/FeCl3(FeCl2) composite nanoparticles by hydrothermal process useful for ferrofluids. Smart Mater. Res. 2011, 2011. [Google Scholar] [CrossRef]
- Larcher, D.; Bonnin, D.; Cortes, R.; Rivals, I.; Personnaz, L.; Tarascon, J.-M. Combined XRD, EXAFS, and Mössbauer Studies of the Reduction by Lithium of α-Fe2O3 with Various Particle Size. J. Elecreochem. Soc. 2003, 150, A1643–A1650. [Google Scholar] [CrossRef]
- Tronc, E.; Ezzir, A.; Cherkaoui, R.; Chanéac, C.; Noguès, M.; Kachkachi, H.; Fiorani, D.; Testa, A.M.; Grenèche, J.M.; Jolivet, J.P. Surface-related properties of γ-Fe2O3 nanoparticles. J. Magn. Magn. Mater. 2000, 221, 63–79. [Google Scholar] [CrossRef]
- Kachkachi, H.; Ezzir, A.; Noguès, M.; Tronc, E. Surface effects in nanoparticles: Application to maghemite. Eur. Phys. J. B 2000, 14, 681–689. [Google Scholar] [CrossRef]
- Kodama, R.H.; Berkowitz, A.E.; McNiff, E.J.; Foner, S. Surface Spin Disorder in NiFe2O4 Nanoparticles. Phys. Rev. Lett. 1996, 7, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Alvarez, G.; Sort, J.; Uheida, A.; Muhammed, M.; Suriñach, S.; Baró, M.D.; Nogués, J. Reversible post-synthesis tuning of the superparamagnetic blocking temperature of γ-Fe2O3 nanoparticles by adsorption and desorption of Co(II) ions. J. Mater. Chem. 2007, 17, 322–328. [Google Scholar] [CrossRef]
- Coey, J.M.D.; Khalafalla, D. Superparamagnetic γ-Fe2O3. Phys. Status Solidi (A) 1972, 11, 229–241. [Google Scholar] [CrossRef]

| Samples | dg (nm) | lnσg | dc (nm) |
|---|---|---|---|
| (3) | 11.2 | 0.3 | 9.0 |
| (5) | 12.0 | 0.3 | 9.0 |
| Samples | EDS | XPS | ||||
|---|---|---|---|---|---|---|
| O | Fe | Cl | O | Fe | Cl | |
| (1) | 40.43 | 51.58 | 1.99 | 64.06 | 31.99 | 3.96 |
| (3) | 45.16 | 52.56 | 2.28 | 64.15 | 35.98 | 3.87 |
| (5) | 45.31 | 52.92 | 1.77 | 58.02 | 32.89 | 5.17 |
| Samples | O ls | Fe 2p3/2 | Cl 2p3/2 | ||
|---|---|---|---|---|---|
| (1) | 529.32(P1) | 530.46(P2) | 709.66(P1) | 711.19(P2) | 198.7 |
| (3) | 529.38 | 709.67(P1) | 711.44(P2) | 198.5 | |
| (5) | 529.36 | 709.63(P1) | 711.40(P2) | 198.5 | |
| Fe2O3 | 529.5 | 709.9 | |||
| FeCl3 | 711.3 | 199.0 | |||
| FeOOH | 530.1 | 711.5 | |||
| Samples | yFe | yCl | zFe | zCl | ϕFe | ϕCl | ϕCl/ϕFe |
|---|---|---|---|---|---|---|---|
| a | |||||||
| (3) | 97.14 | 2.85 | 95.27 | 4.73 | 88.41 | 11.59 | 0.13 |
| (5) | 97.80 | 2.20 | 96.33 | 3.67 | 90.88 | 9.12 | 0.10 |
| b | |||||||
| (3) | 93.08 | 6.92 | 88.82 | 11.78 | 74.29 | 25.91 | 0.35 |
| (5) | 90.03 | 9.97 | 84.22 | 15.78 | 66.94 | 33.06 | 0.49 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Meng, X.; He, Z.; Lin, Y.; Liu, X.; Li, D.; Li, J.; Qiu, X. Preparation of Magnetic Nanoparticles via a Chemically Induced Transition: Role of Treating Solution’s Temperature. Nanomaterials 2017, 7, 220. https://doi.org/10.3390/nano7080220
Zhang T, Meng X, He Z, Lin Y, Liu X, Li D, Li J, Qiu X. Preparation of Magnetic Nanoparticles via a Chemically Induced Transition: Role of Treating Solution’s Temperature. Nanomaterials. 2017; 7(8):220. https://doi.org/10.3390/nano7080220
Chicago/Turabian StyleZhang, Ting, Xiangshen Meng, Zhenghong He, Yueqiang Lin, Xiaodong Liu, Decai Li, Jian Li, and Xiaoyan Qiu. 2017. "Preparation of Magnetic Nanoparticles via a Chemically Induced Transition: Role of Treating Solution’s Temperature" Nanomaterials 7, no. 8: 220. https://doi.org/10.3390/nano7080220





