Inihibition of Glycolysis by Using a Micro/Nano-Lipid Bromopyruvic Chitosan Carrier as a Promising Tool to Improve Treatment of Hepatocellular Carcinoma
Abstract
:1. Introduction
2. Results and Discussion
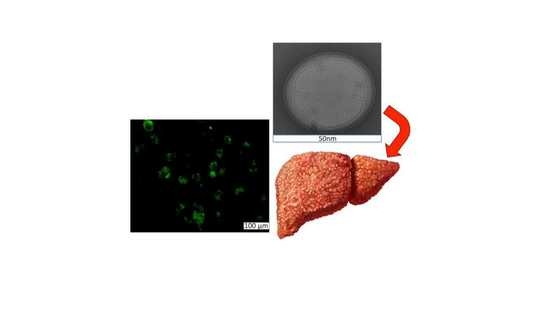
2.1. Characterization
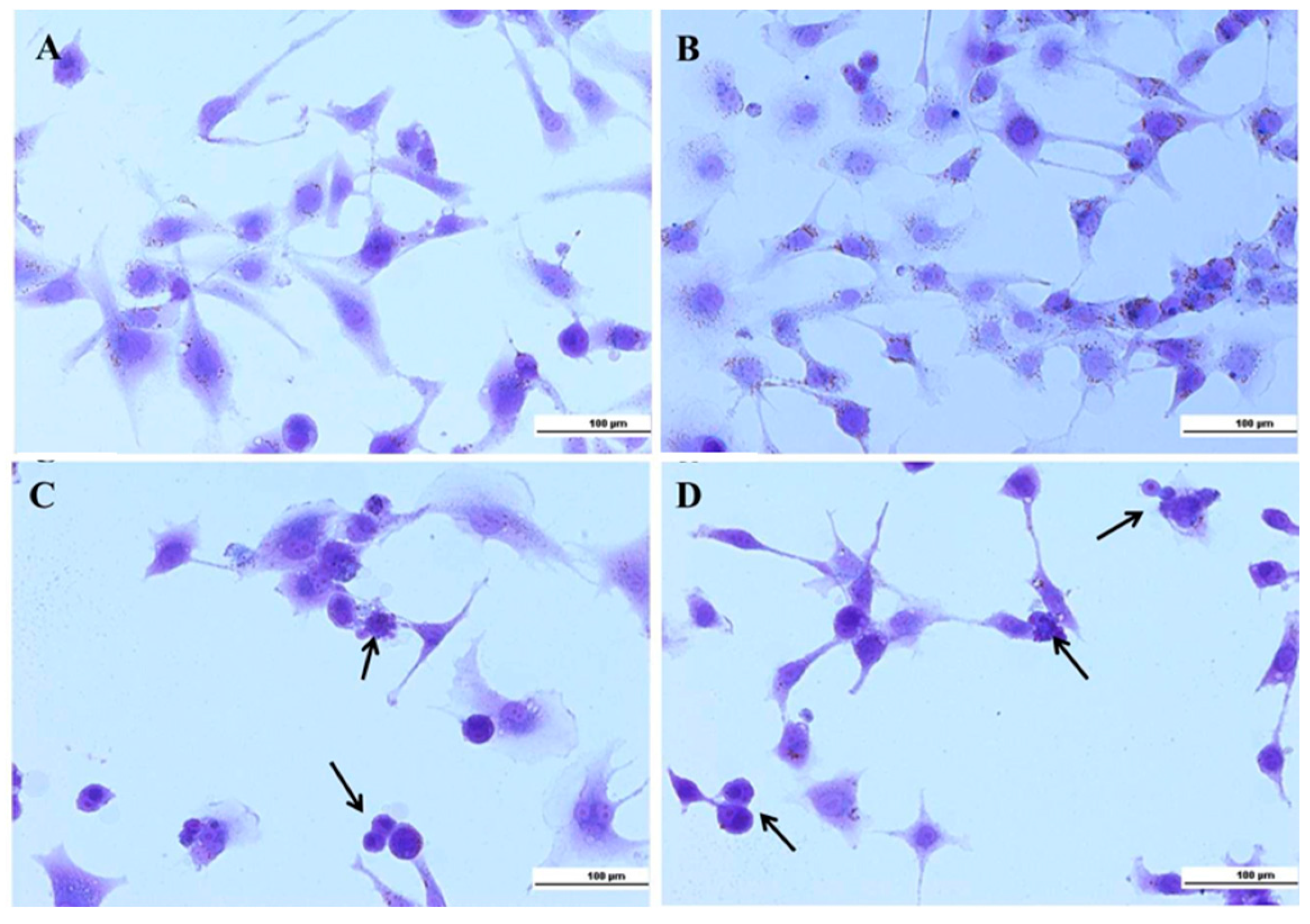
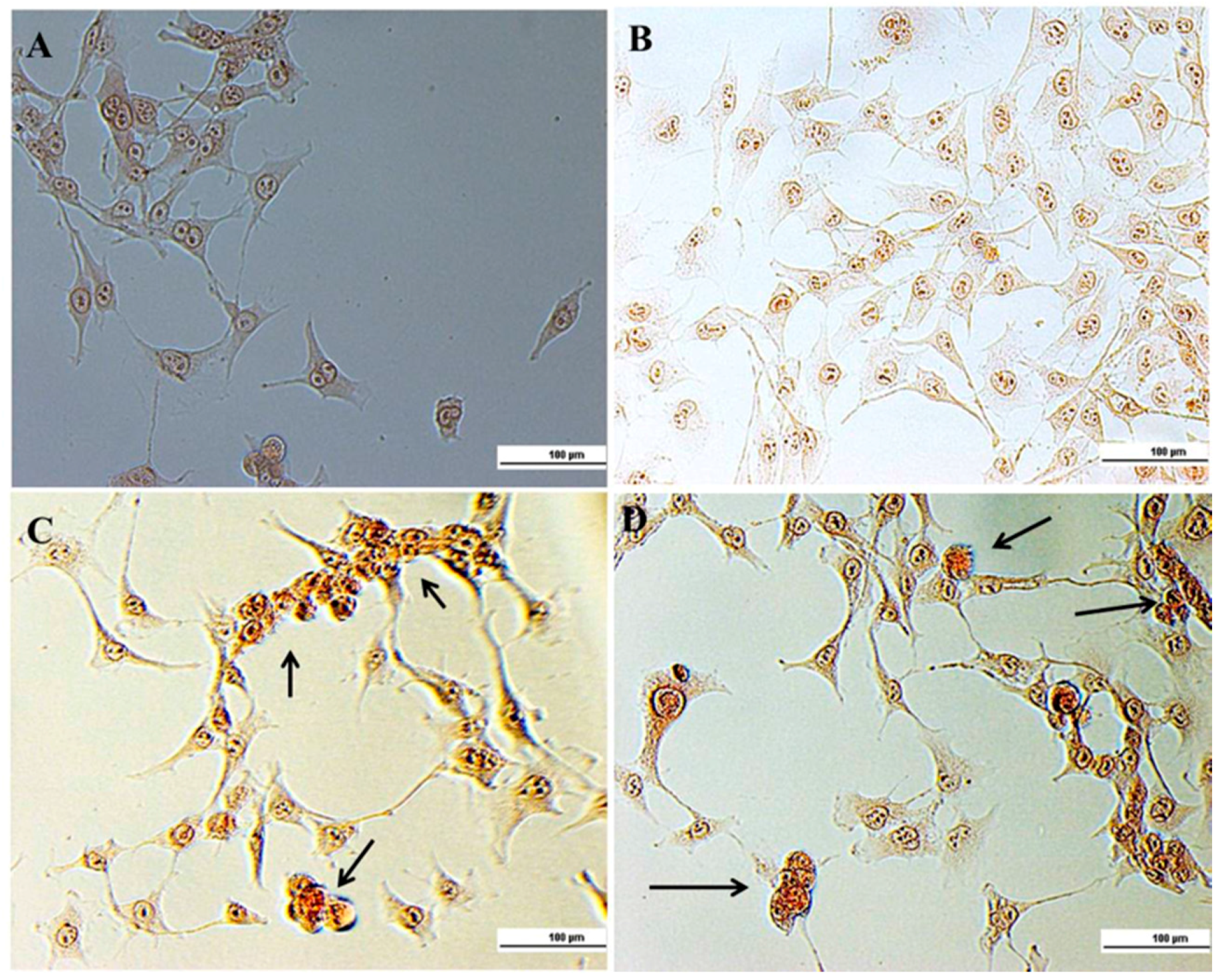
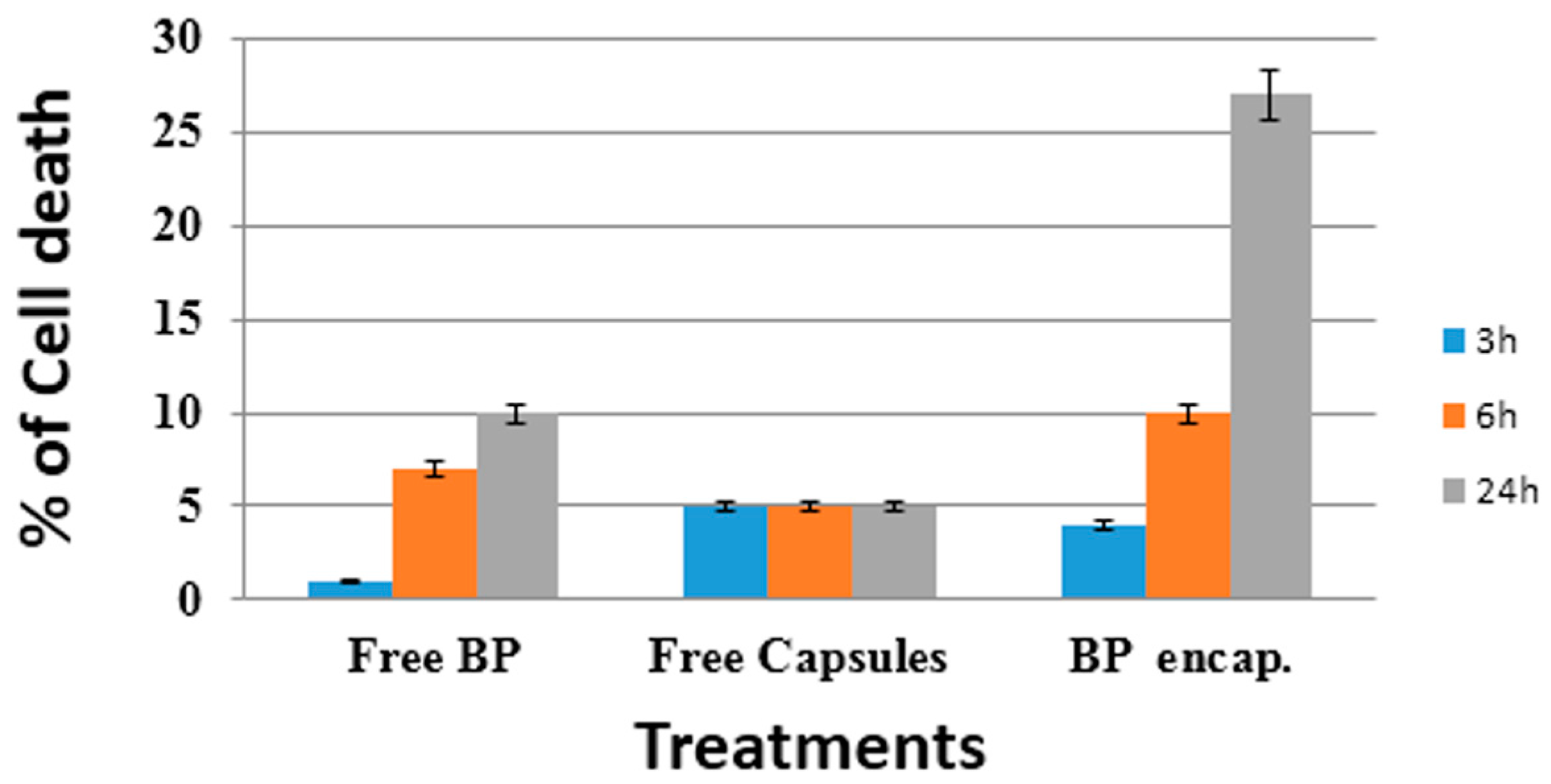
2.2. Cellular Experiments
3. Materials and Methods
3.1. Chemicals
3.2. Carrier Fabrication
3.3. Characterization
3.3.1. Transmission Electron Microscopy (TEM)
3.3.2. Fluorescence Spectrophotometry
3.3.3. Zeta Potential Measurements
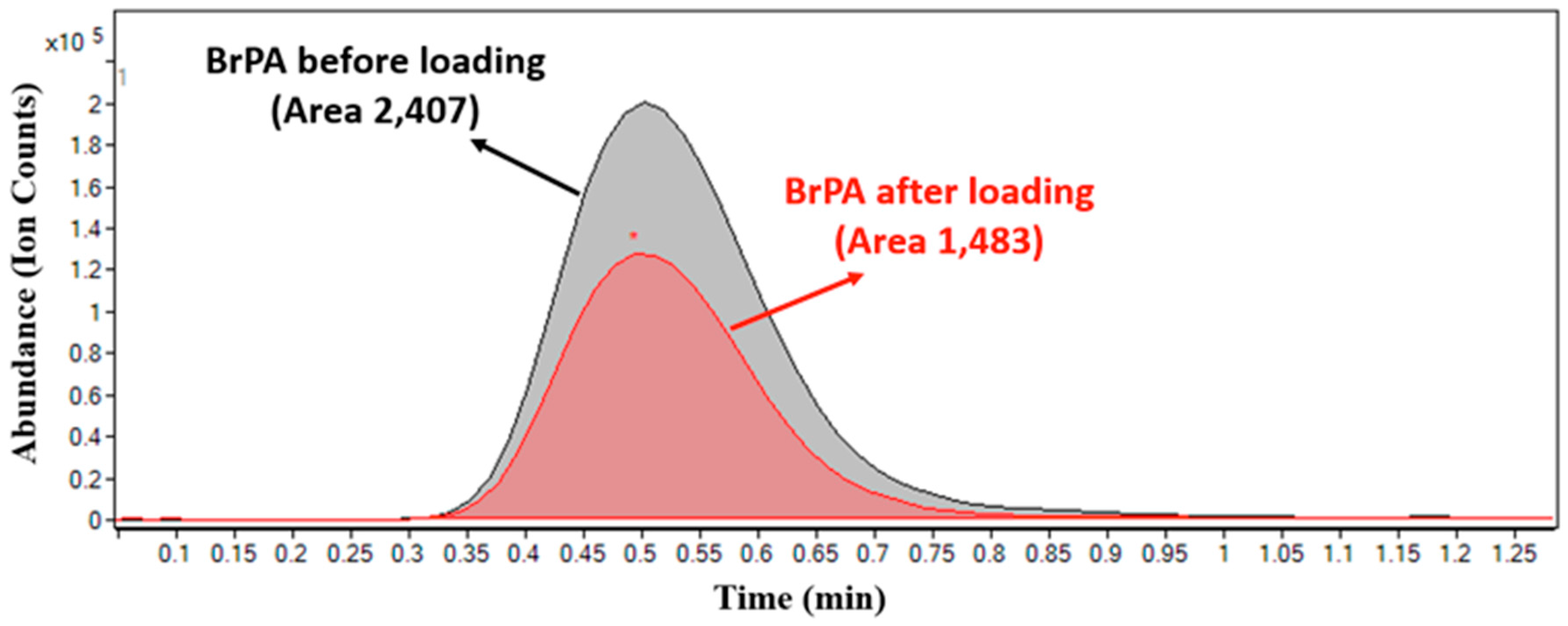
3.3.4. Quantification of BrPA loaded HPLPNCs by Using HPLC-Mass Spectrometry (HPLC-MS)
3.4. Cellular Experiments
3.4.1. Cellular Studies
3.4.2. Cellular Uptake
3.4.3. Crystal Violet
3.4.4. Ethidium Bromide (EB)
3.4.5. Trypan Blue
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, Z.; Zhang, H.; Lu, W.; Huang, P. Role of mitochondria-associated hexokinase II in cancer cell death induced by 3-bromopyruvate. Biochim. Biophys. Acta 2009, 1787, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Macchioni, L.; Davidescu, M.; Sciaccaluga, M.; Marchetti, C.; Migliorati, G.; Coaccioli, S.; Roberti, R.; Corazzi, L.; Castigli, E. Mitochondrial dysfunction and effect of antiglycolytic bromopyruvic acid in GL15 glioblastoma cells. J. Bioenerg. Biomembr. 2011, 43, 507–518. [Google Scholar] [CrossRef] [PubMed]
- Pastorino, J.G.; Hoek, J.B.; Shulga, N. Activation of Glycogen Synthase Kinase 3β Disrupts the Binding of Hexokinase II to Mitochondria by Phosphorylating Voltage-Dependent Anion Channel and Potentiates Chemotherapy-Induced Cytotoxicity. Cancer Res. 2005, 65, 10545–10554. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Lu, W.; Garcia-Prieto, C.; Huang, P. The Warburg effect and its cancer therapeutic implications. J. Bioenerg. Biomembr. 2007, 39, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.H.; Pedersen, P.L.; Geschwind, J.F. Glucose catabolism in the rabbit VX2 tumor model for liver cancer: Characterization and targeting hexokinase. Cancer Lett. 2001, 173, 83–91. [Google Scholar] [CrossRef]
- Sadowska-Bartosz, I.; Soszynski, M.; Ulaszewski, S.; Ko, Y.; Bartosz, G. Transport of 3-bromopyurvate across the human erytherocyte membrane. Cell. Mol. Biol. Lett. 2014, 19, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Hanafy, N.A.N.; De Giorgi, M.L.; Nobile, C.; Cascione, M.F.; Rinaldi, R.; Leporatti, S. CaCO3 rods as chitosan-polygalacturonic acid carriers for bromopyruvic acid delivery. Sci. Adv. Mater. 2016, 8, 514–523. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Li, F.; Liu, W.G.; Yao, K.D. Preparation of oxidized glucose-crosslinked N-alkylated chitosan membrane and in vitro studies of pH-sensitive drug delivery behavior. Biomaterials 2002, 23, 343–347. [Google Scholar] [CrossRef]
- Martin, L.; Wilson, C.G.; Koosha, F.; Tetley, L.; Gray, A.I.; Senel, S.; Uchegbu, I.F. The release of model macromolecules may be controlled by the hydrophobicity of palmitoyl glycol chitosan hydrogels. J. Control. Release 2002, 80, 87–100. [Google Scholar] [CrossRef]
- Kim, Y.H.; Gihm, S.H.; Park, C.R.; Lee, K.Y.; Kim, T.W.; Kwon, I.C.; Chung, H.; Jeong, S.Y. Structural characteristics of size-controlled self-aggregates of deoxycholic acid-modified chitosan and their application as a DNA delivery carrier. Bioconjug. Chem. 2001, 12, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.Z.; Fang, Y.; Hu, D.D. Preparation and properties of chitosan-poly(N-isopropylacrylamide) full-IPN hydrogels. React. Funct. Polym. 2001, 48, 215–221. [Google Scholar] [CrossRef]
- Chen, X.G.; Lee, C.M.; Park, H.J. O/W emulsification for the selfaggregation and nanoparticle formation of linoleic acid-modified chitosan in the aqueous system. J. Agric. Food Chem. 2003, 51, 3135–3139. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.G.; Desai, K.G.H.; Chen, X.G.; Park, H.J. Linolenic acidmodified chitosan for formation of self assembled nanoparticles. J. Agric. Food Chem. 2005, 53, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Ping, Q.N.; Zhang, H.J.; Shen, J. Preparation of N-alkyl-O-sulfate chitosan derivatives and micellar solubilization of taxol. Carbohydr. Polym. 2003, 54, 137–141. [Google Scholar] [CrossRef]
- Wu, Y.; Zheng, Y.L.; Yang, W.L.; Wang, C.C.; Hu, J.H.; Fu, S.K. Synthesis and characterization of a novel amphiphilic chitosan-polylactide graft copolymer. Carbohydr. Polym. 2005, 59, 165–171. [Google Scholar] [CrossRef]
- Lee, D.W.; Powers, K.; Baney, R. Physicochemical properties and blood compatibility of acylated chitosan nanoparticles. Carbohydr. Polym. 2004, 58, 371–377. [Google Scholar] [CrossRef]
- Xiao, J.B.; Wu, M.X.; Kai, G.Y.; Wang, F.J.; Cao, H.; Yu, X.B. ZnO-ZnS QDs interfacial heterostructure for drug/food delivery application: Enhancement of the binding affinities of flavonoid aglycones to bovine serum albumin. Nanomedicine 2011, 7, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Uludag, H. Recent developments in nanoparticle-based drug delivery and targeting systems with emphasis on protein-based nanoparticles. Expert Opin. Drug Deliv. 2008, 5, 499–515. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Deng, D.; Shan, L.; Wan, S.; Cao, J.; Tian, J.; Achilefu, S.; Gu, Y. A pH-sensitive doxorubicin prodrug based on folate-conjugated BSA for tumor-targeted drug delivery. Biomaterials 2013, 34, 3087–3097. [Google Scholar] [CrossRef] [PubMed]
- Gruner, B.A.; Weitman, S.D. The folate receptor as a potential therapeutic anticancer target. Investig. New Drugs 1999, 16, 205–219. [Google Scholar] [CrossRef]
- Sabharanjak, S.; Mayor, S. Folate receptor endocytosis and trafficking. Adv. Drug Deliv. Rev. 2004, 56, 1099–1109. [Google Scholar] [CrossRef] [PubMed]
- Gandham, S.K.; Talekar, M.; Amit Singh, A.; Amiji, M.M. Inhibition of hexokinase-2 with targeted liposomal 3-bromopyruvate in an ovarian tumor spheroid model of aerobic glycolysis. Int. J. Nanomed. 2015, 10, 4405–4423. [Google Scholar]
- Ballarín-González, B.; Dagnaes-Hansen, F.; Fenton, R.A.; Gao, S.; Hein, S.; Dong, M.; Kjems, J.; Howard, K.A. Protection and Systemic Translocation of siRNA Following Oral Administration of Chitosan/siRNA Nanoparticles. Mol. Ther. Nucleic Acids 2013, 5, e76. [Google Scholar] [CrossRef] [PubMed]
- Hanafy, N.A.N.; Quarta, A.; Di Corato, R.; Dini, L.; Nobile, C.; Tasco, V.; Carallo, S.; Cascione, M.F.; Malfettone, A.; Soukupova, J.; et al. Hybrid Polymeric-Protein Nano-Carriers (HPPNC) for Targeted Delivery of TGFβ Inhibitors to Hepatocellular Carcinoma Cells. J. Mater. Sci. Mater. Med. 2017, 28, 120. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shimizu, K.; Kondo, R. Anti-androgenic activity of fatty acids. Chem. Biodivers. 2009, 6, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Cheng, X.; Liu, C.; Xing, K.; Zhang, J.; Sun, G.; Li, X.; Chen, X. Preparation, characterization, and antibacterial activity of oleic acid-grafted chitosan oligosaccharide nanoparticles. Front. Biol. China 2009, 4, 321–327. [Google Scholar] [CrossRef]
- Marchetti, J.M.; Errazu, A.F. Esterification of free fatty acids using sulfuric acid as catalyst in the presence of triglycerides. Biomass Bioenergy 2008, 32, 892–895. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, X.G.; Huang, L.; Han, J.T.; Zhang, X.F. Self-assembled polymeric nanoparticles based on oleic acid-grafted chitosan oligosaccharide: Biocompatibility, protein adsorption and cellular uptake. J. Mater. Sci. Mater. Med. 2012, 23, 1775–1783. [Google Scholar] [CrossRef] [PubMed]
- Janes, K.A.; Fresneau, M.P.; Marazuela, A.; Fabra, A.; Alonso, M.J. Chitosan nanoparticles as delivery systems for doxorubicin. J. Control. Release 2001, 73, 255. [Google Scholar] [CrossRef]
- Uliasz, T.F.; Hewe, S.J. A microtiter trypan blue absorbance assay for the quantitative determination of excitotoxic neuronal injury in cell culture. J. Neurosci. Methods 2000, 100, 157–163. [Google Scholar] [CrossRef]
- Esquenet, C.; Terech, P.; Boue, F.; Buhler, E. Structural and rheological properties of hydrophobically modified polysaccharide associative networks. Langmuir 2004, 20, 3583–3592. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.M.; Zhang, L.; Yuet, K.P.; Liao, G.; Rhee, J.W.; Langer, R.; Farokhzad, O.C. PLGA-lecithin-PEG core-shell nanoparticles for controlled drug delivery. Biomaterials 2009, 30, 1627–1634. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Finnegan, C.E.; Rogers, K.M.; Purcell, J.W.; Trimble, A.; Johnston, P.G.; Boland, M.P. STAT1: A modulator of chemotherapy-induced apoptosis. Cancer Res. 2004, 64, 8357–8364. [Google Scholar] [CrossRef] [PubMed]
- Zivadinovic, D.; Gametchu, B.; Watson, C.S. Membrane estrogen receptor-alpha levels in MCF-7 breast cancer cells predict cAMP and proliferation responses. Breast Cancer Res 2005, 7, R101–R112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shukla, S.; Jadaun, A.; Arora, V.; Sinha, R.K.; Biyani, N.; Jain, V.K. In vitro toxicity assessment of chitosan oligosaccharidecoated iron oxide nanoparticles. Toxicol. Rep. 2015, 2, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Pappenheimer, A.J. Experimental studies upon lymphocytes: The reactions of lymphocytes under various experimental conditions. J. Exp. Med. 1917, 25, 25–31. [Google Scholar] [CrossRef]
- Patterson, M.K., Jr. Measurement of growth and viability of cells in culture. Methods Enzymol. 1979, 58, 141–152. [Google Scholar] [PubMed]
- Sahoo, S.K.; Ma, W.; Labhasetwar, V. Efficacy of transferrin-conjugated paclitaxel-loaded nanoparticles in a murine model of prostate. Int. J. Cancer 2004, 112, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.X.; Karnik, R.; Wang, A.Z.; Alexis, F.; Levy-Nissenbaum, E.; Hong, S.; Langer, R.S.; Farokhzad, O.C. Targeted nanoparticles for cancer therapy. Nano Today 2007, 2, 14–21. [Google Scholar] [CrossRef]
- Weiner, L.M.; Surana, R.; Wang, S. Monoclonal antibodies: Versatile platforms for cancer immunotherapy. Nat. Rev. Immunol. 2010, 10, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Pasqualini, R.; Ruoslahti, E. Organ targeting in vivo using phage display peptide libraries. Nature 1996, 380, 364–366. [Google Scholar] [CrossRef] [PubMed]
- Giannelli, G.; Bergamini, C.; Fransvea, E.; Marinosci, F.; Quaranta, V.; Antonaci, S. Human hepatocellular carcinoma (HCC) cells require both α3β1 integrin and matrix metalloproteinases activity for migration and invasion. Lab. Investig. 2001, 81, 613–627. [Google Scholar] [CrossRef] [PubMed]
- Hanafy, N.A.; Ferraro, M.M.; Gaballo, A.; Dini, L.; Tasco, V.; Nobile, C.; De Giorgi, M.L.; Carallo, S.; Rinaldi, R.; Leporatti, S. Fabrication and characterization of ALK1fc-loaded fluoro-magnetic nanoparticles for inhibiting TGF β 1 in hepatocellular carcinoma. RSC Adv. 2016, 6, 48834–48842. [Google Scholar] [CrossRef]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanafy, N.A.; Dini, L.; Citti, C.; Cannazza, G.; Leporatti, S. Inihibition of Glycolysis by Using a Micro/Nano-Lipid Bromopyruvic Chitosan Carrier as a Promising Tool to Improve Treatment of Hepatocellular Carcinoma. Nanomaterials 2018, 8, 34. https://doi.org/10.3390/nano8010034
Hanafy NA, Dini L, Citti C, Cannazza G, Leporatti S. Inihibition of Glycolysis by Using a Micro/Nano-Lipid Bromopyruvic Chitosan Carrier as a Promising Tool to Improve Treatment of Hepatocellular Carcinoma. Nanomaterials. 2018; 8(1):34. https://doi.org/10.3390/nano8010034
Chicago/Turabian StyleHanafy, Nemany A., Luciana Dini, Cinzia Citti, Giuseppe Cannazza, and Stefano Leporatti. 2018. "Inihibition of Glycolysis by Using a Micro/Nano-Lipid Bromopyruvic Chitosan Carrier as a Promising Tool to Improve Treatment of Hepatocellular Carcinoma" Nanomaterials 8, no. 1: 34. https://doi.org/10.3390/nano8010034