Morphology-Controlled Synthesis of Hematite Nanocrystals and Their Optical, Magnetic and Electrochemical Performance
Abstract
:1. Introduction
2. Experimental Details
2.1. Materials
2.2. Methods
2.3. Characterization and Measurement
3. Results and Discussion
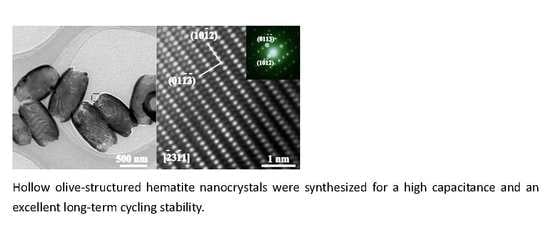
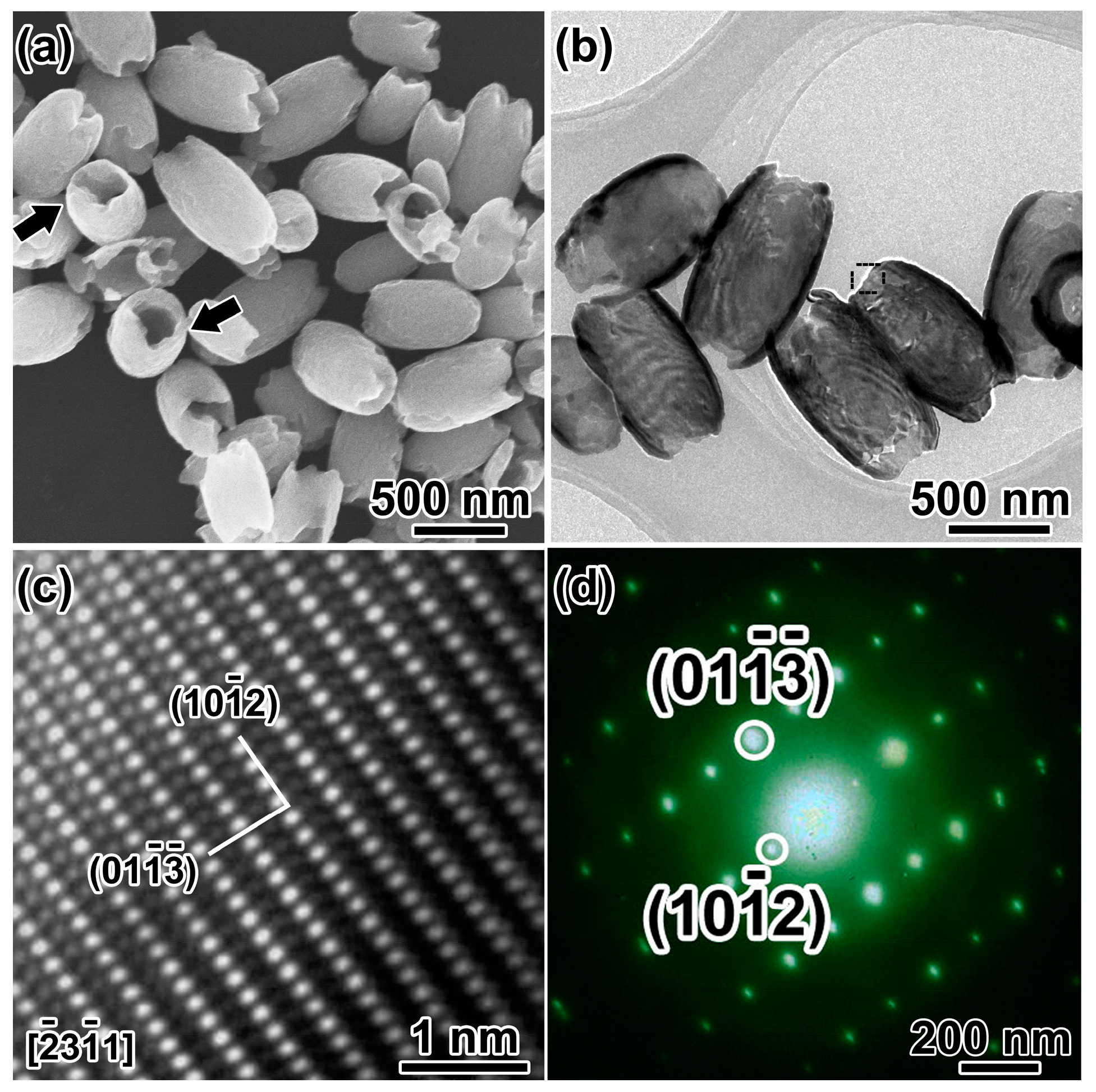
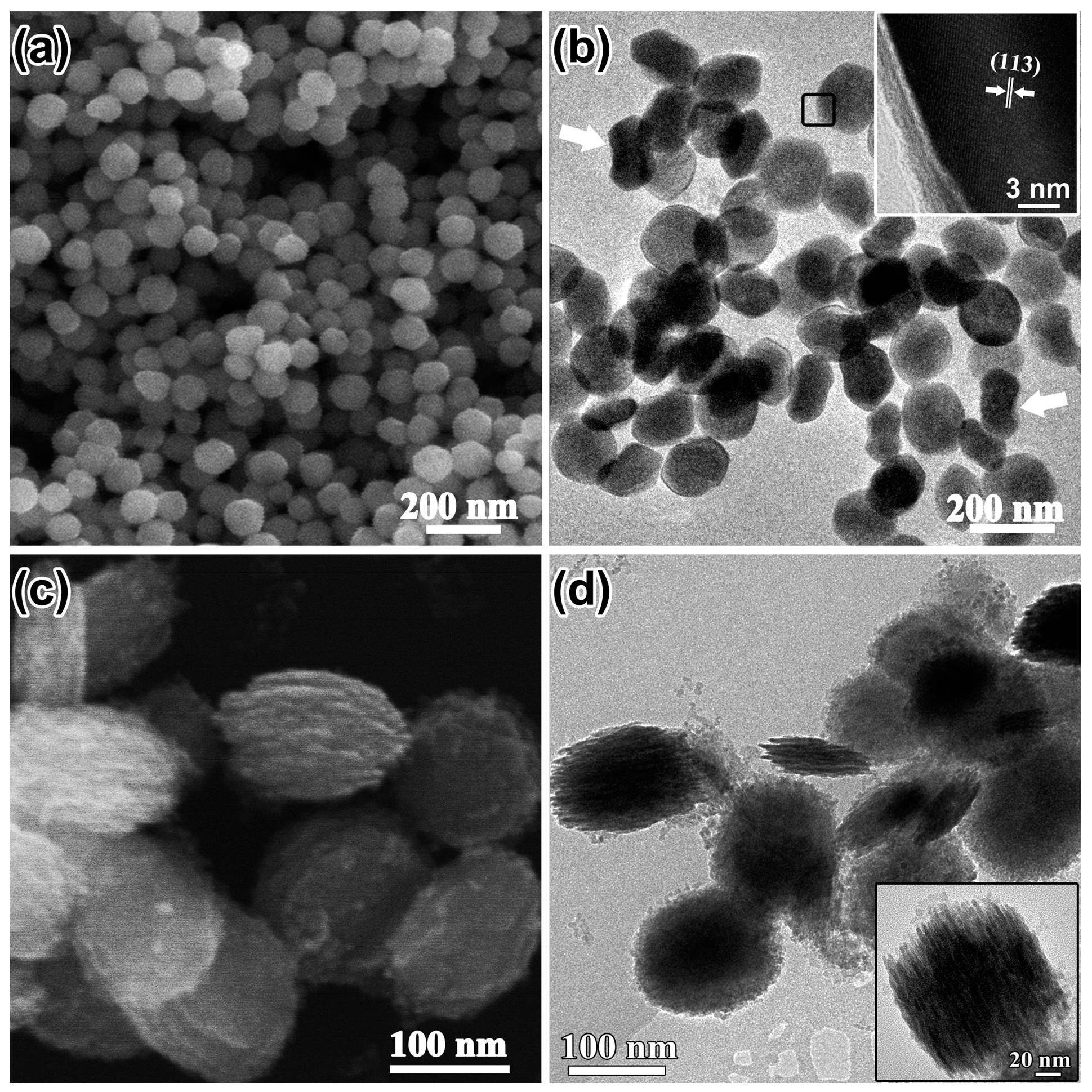
3.1. Morphological and Structural Analysis
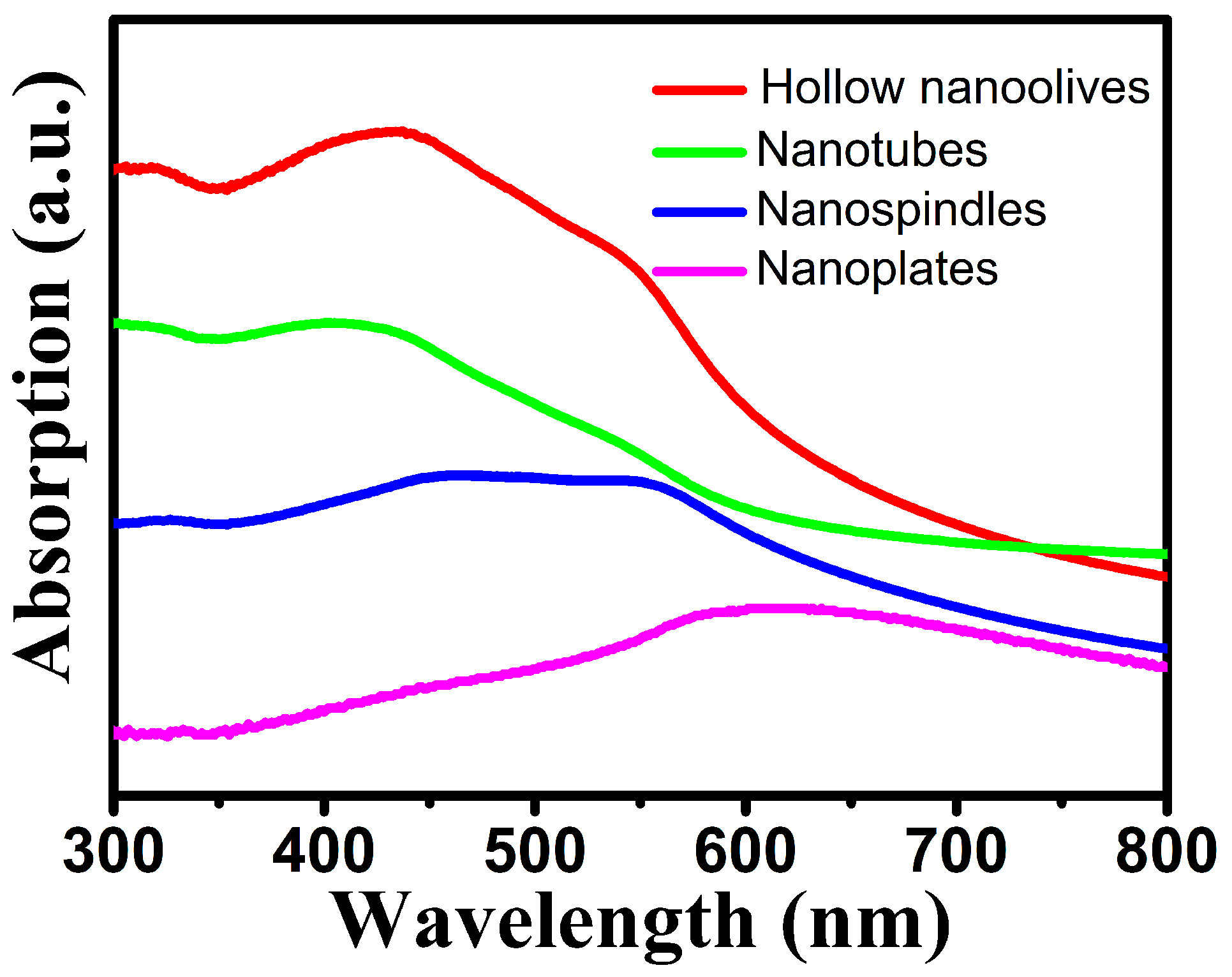
3.2. Optical Properties
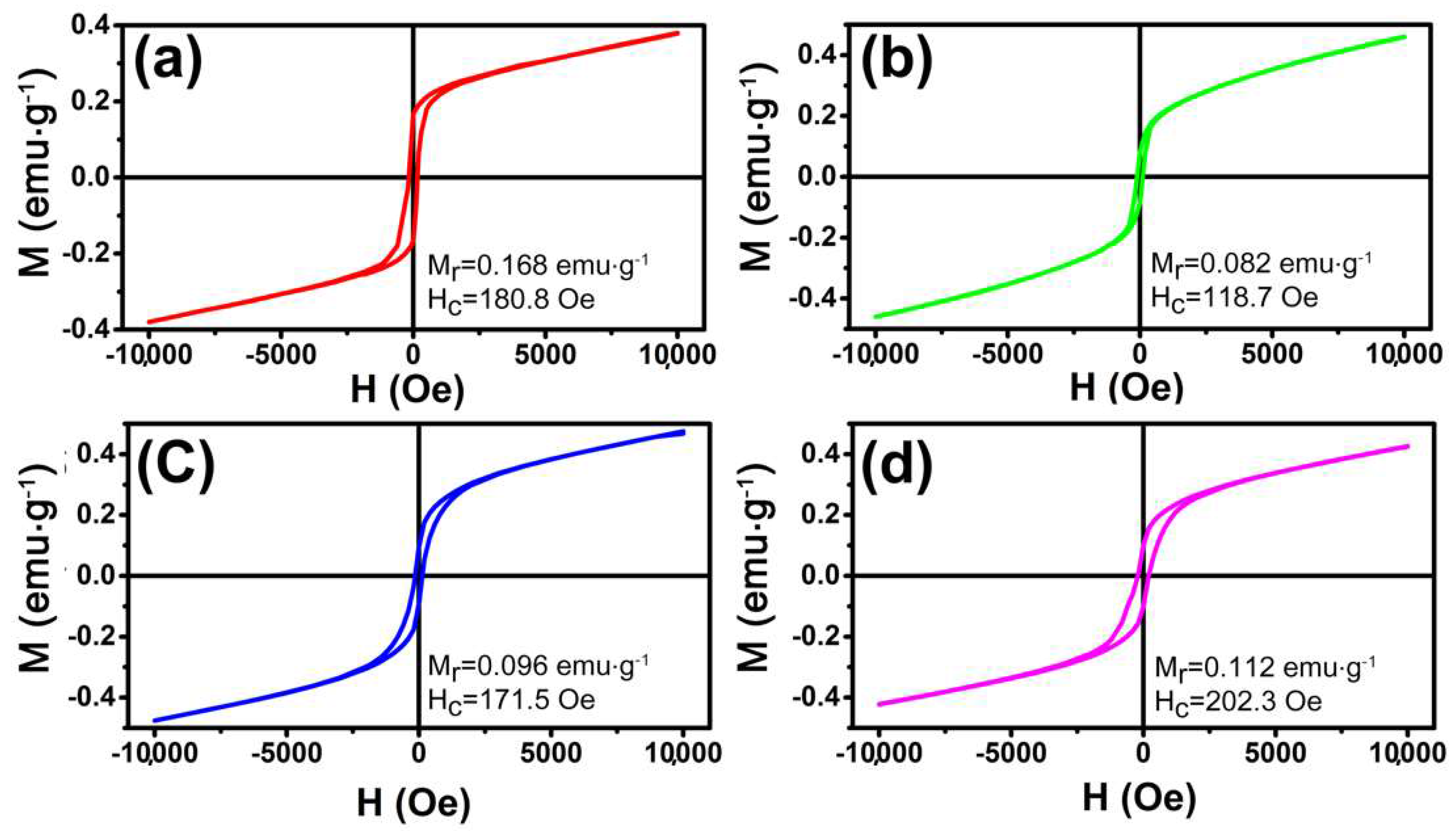
3.3. Magnetic Properties
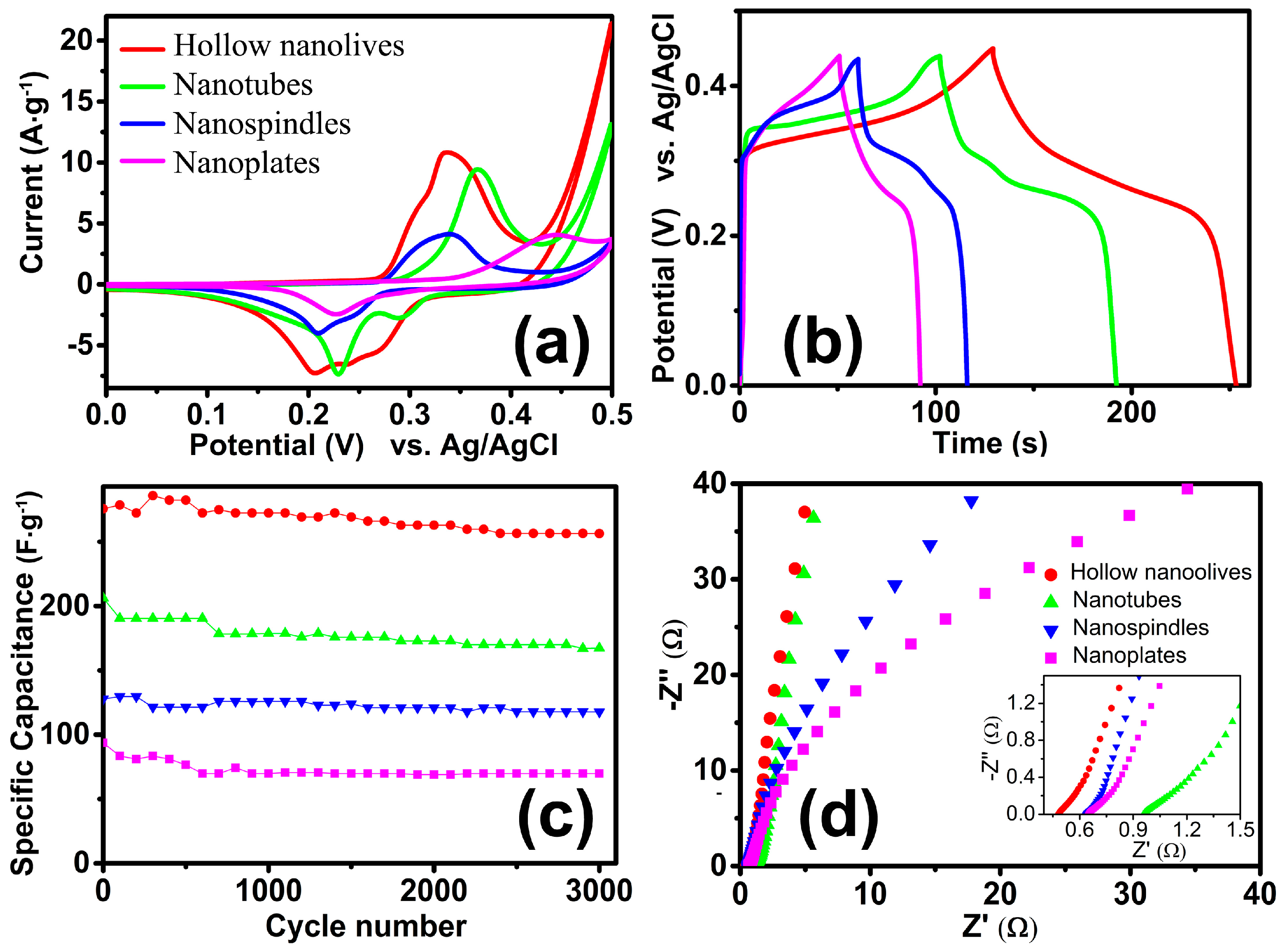
3.4. Electrochemical Properties
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yu, X.G.; Marks, T.J.; Facchetti, A. Metal oxides for optoelectronic applications. Nat. Mater. 2016, 15, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Saif, S.; Tahir, A.; Chen, Y. Green Synthesis of Iron Nanoparticles and Their Environmental Applications and Implications. Nanomaterials 2016, 6, 209. [Google Scholar] [CrossRef] [PubMed]
- Movlaee, K.; Ganjali, M.; Norouzi, P.; Neri, G. Iron-Based Nanomaterials/Graphene Composites for Advanced Electrochemical Sensors. Nanomaterials 2017, 7, 406. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Duan, S.B.; Fan, H.S.; Su, X.R.; Cui, Y.M.; Wang, R.M. Core@shell CoO@Co3O4 nanocrystals assembling mesoporous microspheres for high performance asymmetric supercapacitors. Chem. Eng. J. 2017, 327, 100–108. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Prakash, S.; Rao, C.R.K.; Vijayan, M. Organically Soluble Bifunctional Polyaniline-Magnetite Composites for Sensing and Supercapacitor Applications. Electrochem. Solid State Lett. 2009, 12, A84–A87. [Google Scholar] [CrossRef]
- Shen, S.; Lindley, S.A.; Chen, X.; Zhang, J.Z. Hematite heterostructures for photoelectrochemical water splitting: Rational materials design and charge carrier dynamics. Energy Environ. Sci. 2016, 9, 2744–2775. [Google Scholar] [CrossRef]
- Iandolo, B.; Wickman, B.; Zoric, I.; Hellman, A. The rise of hematite: Origin and strategies to reduce the high onset potential for the oxygen evolution reaction. J. Mater. Chem. A 2015, 3, 16896–16912. [Google Scholar] [CrossRef]
- Koukabi, N.; Kolvari, E.; Khazaei, A.; Zolfigol, M.A.; Shirmardi-Shaghasemi, B.; Khavasi, H.R. Hantzsch reaction on free nano-Fe2O3 catalyst: Excellent reactivity combined with facile catalyst recovery and recyclability. Chem. Commun. 2011, 47, 9230–9232. [Google Scholar] [CrossRef] [PubMed]
- Dahan, M.H.; Caspary Toroker, M. Water Oxidation Catalysis with Fe2O3 Constrained at the Nanoscale. J. Phys. Chem. C 2017, 121, 6120–6125. [Google Scholar] [CrossRef]
- Hao, Q.Y.; Liu, S.A.; Yin, X.M.; Du, Z.F.; Zhang, M.; Li, L.M.; Wang, Y.G.; Wang, T.H.; Li, Q.H. Flexible morphology-controlled synthesis of mesoporous hierarchical α-Fe2O3 architectures and their gas-sensing properties. Crystengcomm 2011, 13, 806–812. [Google Scholar] [CrossRef]
- Hua, M.; Zhang, S.; Pan, B.; Zhang, W.; Lv, L.; Zhang, Q. Heavy metal removal from water/wastewater by nanosized metal oxides: A review. J. Hazard. Mater. 2012, 211, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Yan, X.Q.; Sun, Y.H.; Yu, Y.S.; Zhang, G.J.; Shen, Y.W.; Liang, Q.J.; Liao, Q.L.; Zhang, Y. Temperature-dependent electrochemical capacitive performance of the α-Fe2O3 hollow nanoshuttles as supercapacitor electrodes. J. Colloid Interface Sci. 2016, 466, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Uchaker, E.; Candelaria, S.L.; Cao, G. Nanomaterials for energy conversion and storage. Chem. Soc. Rev. 2013, 42, 3127–3171. [Google Scholar] [CrossRef] [PubMed]
- Julien, C.M.; Mauger, A. Nanostructured MnO2 as Electrode Materials for Energy Storage. Nanomaterials 2017, 7, 396. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, S.; Kim, H.Y.; Kim, B.S. Expeditious and eco-friendly fabrication of highly uniform microflower superstructures and their applications in highly durable methanol oxidation and high-performance supercapacitors. J. Mater. Chem. A 2016, 4, 12253–12262. [Google Scholar] [CrossRef]
- Cheng, M.; Duan, S.B.; Fan, H.S.; Wang, R.M. From channeled to hollow CoO octahedra: Controlled growth, structural evolution and energetic applications. Crystengcomm 2016, 18, 6849–6859. [Google Scholar] [CrossRef]
- Nithya, V.D.; Arul, N.S. Review on α-Fe2O3 based negative electrode for high performance supercapacitors. J. Power Sources 2016, 327, 297–318. [Google Scholar] [CrossRef]
- Shivakumara, S.; Penki, T.R.; Munichandraiah, N. Synthesis and Characterization of Porous Flowerlike α-Fe2O3 Nanostructures for Supercapacitor Application. ECS Electrochem. Lett. 2013, 2, A60–A62. [Google Scholar] [CrossRef]
- Chaudhari, S.; Bhattacharjya, D.; Yu, J.-S. 1-Dimensional porous α-Fe2O3 nanorods as high performance electrode material for supercapacitors. RSC Adv. 2013, 3, 25120–25128. [Google Scholar] [CrossRef]
- Zheng, X.; Han, Z.C.; Chai, F.; Qu, F.Y.; Xia, H.; Wu, X. Flexible heterostructured supercapacitor electrodes based on α-Fe2O3 nanosheets with excellent electrochemical performances. Dalton Trans. 2016, 45, 12862–12870. [Google Scholar] [CrossRef] [PubMed]
- Rettie, A.J.E.; Chemelewski, W.D.; Wygant, B.R.; Lindemuth, J.; Lin, J.-F.; Eisenberg, D.; Brauer, C.S.; Johnson, T.J.; Beiswenger, T.N.; Ash, R.D.; et al. Synthesis, electronic transport and optical properties of Si: α-Fe2O3 single crystals. J. Mater. Chem. C 2016, 4, 559–567. [Google Scholar] [CrossRef]
- Rafi, M.M.; Ahmed, K.S.Z.; Nazeer, K.P.; Kumar, D.S.; Thamilselvan, M. Synthesis, characterization and magnetic properties of hematite (α-Fe2O3) nanoparticles on polysaccharide templates and their antibacterial activity. Appl. Nanosci. 2015, 5, 515–520. [Google Scholar] [CrossRef]
- Obaidat, I.; Nayek, C.; Manna, K.; Bhattacharjee, G.; Al-Omari, I.; Gismelseed, A. Investigating Exchange Bias and Coercivity in Fe3O4–γ-Fe2O3 Core–Shell Nanoparticles of Fixed Core Diameter and Variable Shell Thicknesses. Nanomaterials 2017, 7, 415. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Meng, X.; He, Z.; Lin, Y.; Liu, X.; Li, D.; Li, J.; Qiu, X. Preparation of Magnetic Nanoparticles via a Chemically Induced Transition: Role of Treating Solution’s Temperature. Nanomaterials 2017, 7, 220. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Zhang, Y.; Munyemana, J.C.; Wu, T.; Yang, Z.; Chen, H.; Qu, W.; Xiao, J. Tuning the hierarchical nanostructure of hematite mesocrystals via collagen-templated biomineralization. J. Mater. Chem. B 2017, 5, 1423–1429. [Google Scholar] [CrossRef]
- Wang, C.W.; Yang, S.; Fang, W.Q.; Liu, P.R.; Zhao, H.J.; Yang, H.G. Engineered Hematite Mesoporous Single Crystals Drive Drastic Enhancement in Solar Water Splitting. Nano Lett. 2016, 16, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Patra, A.K.; Kundu, S.K.; Bhaumik, A.; Kim, D. Morphology evolution of single-crystalline hematite nanocrystals: Magnetically recoverable nanocatalysts for enhanced facet-driven photoredox activity. Nanoscale 2016, 8, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Mo, Q.L.; Wei, J.X.; Jiang, K.Y.; Zhuang, Z.Y.; Yu, Y. Hollow α-Fe2O3 Nanoboxes Derived from Metal Organic Frameworks and Their Superior Ability for Fast Extraction and Magnetic Separation of Trace Pb2+. ACS Sustain. Chem. Eng. 2017, 5, 1476–1484. [Google Scholar] [CrossRef]
- Fan, H.S.; Cheng, M.; Wang, Z.L.; Wang, R.M. Layer-controlled Pt-Ni porous nanobowls with enhanced electrocatalytic performance. Nano Res. 2017, 10, 187–198. [Google Scholar] [CrossRef]
- Shan, A.X.; Chen, Z.C.; Li, B.Q.; Chen, C.P.; Wang, R.M. Monodispersed, ultrathin NiPt hollow nanospheres with tunable diameter and composition via a green chemical synthesis. J. Mater. Chem. A 2015, 3, 1031–1036. [Google Scholar] [CrossRef]
- Su, C.H.; Wang, H. Capsule-like α-Fe2O3 nanoparticles: Synthesis, characterization, and growth mechanism. Cryst. Res. Technol. 2012, 47, 896–902. [Google Scholar] [CrossRef]
- Manukyan, K.V.; Chen, Y.S.; Rouvimov, S.; Li, P.; Li, X.; Dong, S.N.; Liu, X.Y.; Furdyna, J.K.; Orlov, A.; Bernstein, G.H.; et al. Ultrasmall α-Fe2O3 Superparamagnetic Nanoparticles with High Magnetization Prepared by Template-Assisted Combustion Process. J. Phys. Chem. C 2014, 118, 16264–16271. [Google Scholar] [CrossRef]
- Almeida, T.P.; Fay, M.W.; Zhu, Y.; Brown, P.D. Hydrothermal growth mechanism of α-Fe2O3 nanorods derived by near in situ analysis. Nanoscale 2010, 2, 2390–2399. [Google Scholar] [CrossRef] [PubMed]
- Lv, B.L.; Xu, Y.; Wu, D.; Sun, Y.H. Morphology evolution of α-Fe2O3 nanoparticles: The effect of dihydrogen phosphate anions. Crystengcomm 2011, 13, 7293–7298. [Google Scholar] [CrossRef]
- Lian, J.; Duan, X.; Ma, J.; Peng, P.; Kim, T.; Zheng, W. Hematite (α-Fe2O3) with Various Morphologies: Ionic Liquid-Assisted Synthesis, Formation Mechanism, and Properties. ACS Nano 2009, 3, 3749–3761. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, V.; Kumar, S. Formation of hierarchical structures of Fe2O3 by the liquid-liquid interface technique. Crystengcomm 2014, 16, 11122–11126. [Google Scholar] [CrossRef]
- Xi, L.; Chiam, S.Y.; Mak, W.F.; Tran, P.D.; Barber, J.; Loo, S.C.J.; Wong, L.H. A novel strategy for surface treatment on hematite photoanode for efficient water oxidation. Chem. Sci. 2013, 4, 164–169. [Google Scholar] [CrossRef]
- Huo, Y.; Zhu, Y.G.; Xie, J.; Cao, G.S.; Zhu, T.J.; Zhao, X.B.; Zhang, S.C. Controllable synthesis of hollow α-Fe2O3 nanostructures, their growth mechanism, and the morphology-reserved conversion to magnetic Fe3O4/C nanocomposites. RSC Adv. 2013, 3, 19097–19103. [Google Scholar] [CrossRef]
- Wu, W.; Xiao, X.H.; Zhang, S.F.; Zhou, J.A.; Fan, L.X.; Ren, F.; Jiang, C.Z. Large-Scale and Controlled Synthesis of Iron Oxide Magnetic Short Nanotubes: Shape Evolution, Growth Mechanism, and Magnetic Properties. J. Phys. Chem. C 2010, 114, 16092–16103. [Google Scholar] [CrossRef]
- Zhou, H.S.; Mito, A.; Kundu, D.; Honma, I. Nonlinear optical susceptibility of Fe2O3 thin film synthesized by a modified sol-gel method. J. Sol-Gel Sci. Technol. 2000, 19, 539–541. [Google Scholar] [CrossRef]
- He, Y.P.; Miao, Y.M.; Li, C.R.; Wang, S.Q.; Cao, L.; Xie, S.S.; Yang, G.Z.; Zou, B.S.; Burda, C. Size and structure effect on optical transitions of iron oxide nanocrystals. Phys. Rev. B 2005, 71, 125411. [Google Scholar] [CrossRef]
- Kant, R.; Kumar, N.; Dutta, V. Fabrication of micro/nanostructured α-Fe2O3 hollow spheres: Effect of electric field on the morphological, magnetic and photocatalytic properties. RSC Adv. 2016, 6, 65789–65798. [Google Scholar] [CrossRef]
- Wheeler, D.A.; Wang, G.M.; Ling, Y.C.; Li, Y.; Zhang, J.Z. Nanostructured hematite: Synthesis, characterization, charge carrier dynamics, and photoelectrochemical properties. Energy Environ. Sci. 2012, 5, 6682–6702. [Google Scholar] [CrossRef]
- Yang, S.; Xu, Y.Y.; Sun, Y.Q.; Zhang, G.Y.; Gao, D.Z. Size-controlled synthesis, magnetic property, and photocatalytic property of uniform α-Fe2O3 nanoparticles via a facile additive-free hydrothermal route. Crystengcomm 2012, 14, 7915–7921. [Google Scholar] [CrossRef]
- Zhang, X.; Niu, Y.; Li, Y.; Hou, X.; Wang, Y.; Bai, R.; Zhao, J. Synthesis, optical and magnetic properties of α-Fe2O3 nanoparticles with various shapes. Mater. Lett. 2013, 99, 111–114. [Google Scholar] [CrossRef]
- Guan, B.Y.; Yu, L.; Wang, X.; Song, S.; Lou, X.W. Formation of Onion-Like NiCo2S4 Particles via Sequential Ion-Exchange for Hybrid Supercapacitors. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Binitha, G.; Soumya, M.S.; Madhavan, A.A.; Praveen, P.; Balakrishnan, A.; Subramanian, K.R.V.; Reddy, M.V.; Nair, S.V.; Nair, A.S.; Sivakumar, N. Electrospun α-Fe2O3 nanostructures for supercapacitor applications. J. Mater. Chem. A 2013, 1, 11698–11704. [Google Scholar] [CrossRef]
- Sarkar, D.; Mandal, M.; Mandal, K. Design and Synthesis of High Performance Multifunctional Ultrathin Hematite Nanoribbons. ACS Appl. Mater. Interfaces 2013, 5, 11995–12004. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.M.; Jiang, Z.X.; Liu, Q.; Zhu, X.D.; Liu, L.; Ni, L.; Shen, C.C. Novel red blood cell shaped α-Fe2O3 microstructures and FeO(OH) nanorods as high capacity supercapacitors. RSC Adv. 2015, 5, 91127–91133. [Google Scholar] [CrossRef]
- Zhong, Y.; Liu, J.; Lu, Z.; Xia, H. Hierarchical FeS2 nanosheet@Fe2O3 nanosphere heterostructure as promising electrode material for supercapacitors. Mater. Lett. 2016, 166, 223–226. [Google Scholar] [CrossRef]
- Liu, L.; Lang, J.; Zhang, P.; Hu, B.; Yan, X. Facile Synthesis of Fe2O3 Nano-Dots@Nitrogen-Doped Graphene for Supercapacitor Electrode with Ultralong Cycle Life in KOH Electrolyte. ACS Appl. Mat. Interfaces 2016, 8, 9335–9344. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.B.; Wang, R.M. Au/Ni12P5 core/shell nanocrystals from bimetallic heterostructures: In situ synthesis, evolution and supercapacitor properties. NPG Asia Mater. 2015, 6, 1–7. [Google Scholar] [CrossRef]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Sun, Q.; Fan, H.; Cheng, M.; Shan, A.; Cui, Y.; Wang, R. Morphology-Controlled Synthesis of Hematite Nanocrystals and Their Optical, Magnetic and Electrochemical Performance. Nanomaterials 2018, 8, 41. https://doi.org/10.3390/nano8010041
Li B, Sun Q, Fan H, Cheng M, Shan A, Cui Y, Wang R. Morphology-Controlled Synthesis of Hematite Nanocrystals and Their Optical, Magnetic and Electrochemical Performance. Nanomaterials. 2018; 8(1):41. https://doi.org/10.3390/nano8010041
Chicago/Turabian StyleLi, Bangquan, Qian Sun, Hongsheng Fan, Ming Cheng, Aixian Shan, Yimin Cui, and Rongming Wang. 2018. "Morphology-Controlled Synthesis of Hematite Nanocrystals and Their Optical, Magnetic and Electrochemical Performance" Nanomaterials 8, no. 1: 41. https://doi.org/10.3390/nano8010041