Colloidal Synthesis and Thermoelectric Properties of CuFeSe2 Nanocrystals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of Selenium Precursor Solution
2.3. Synthesis of CuFeSe2 Nanocrystals
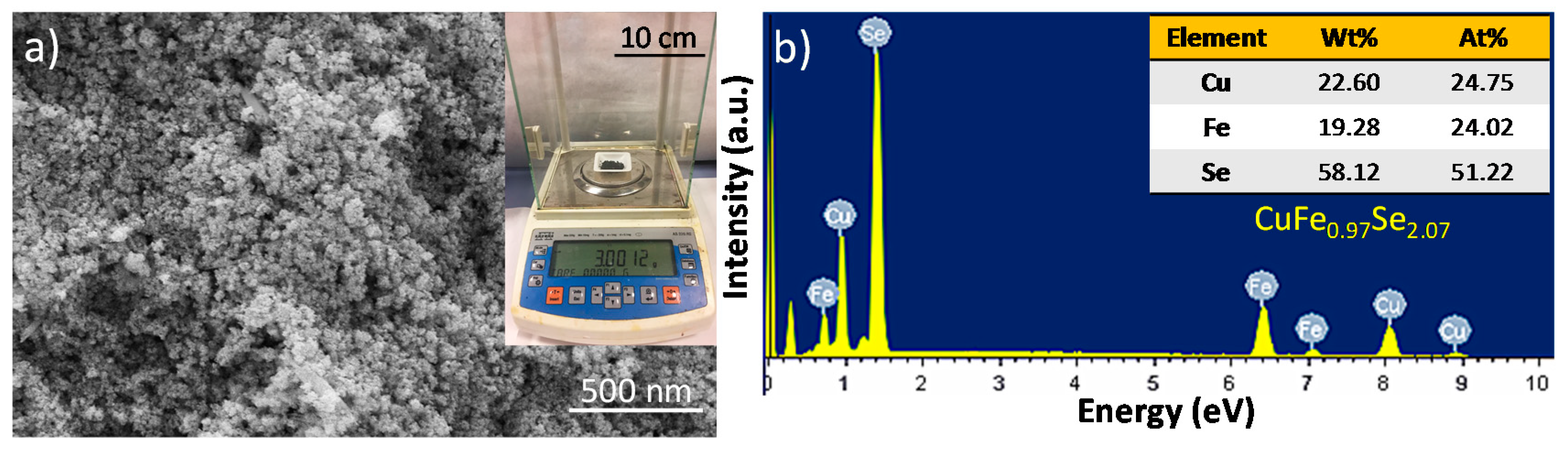
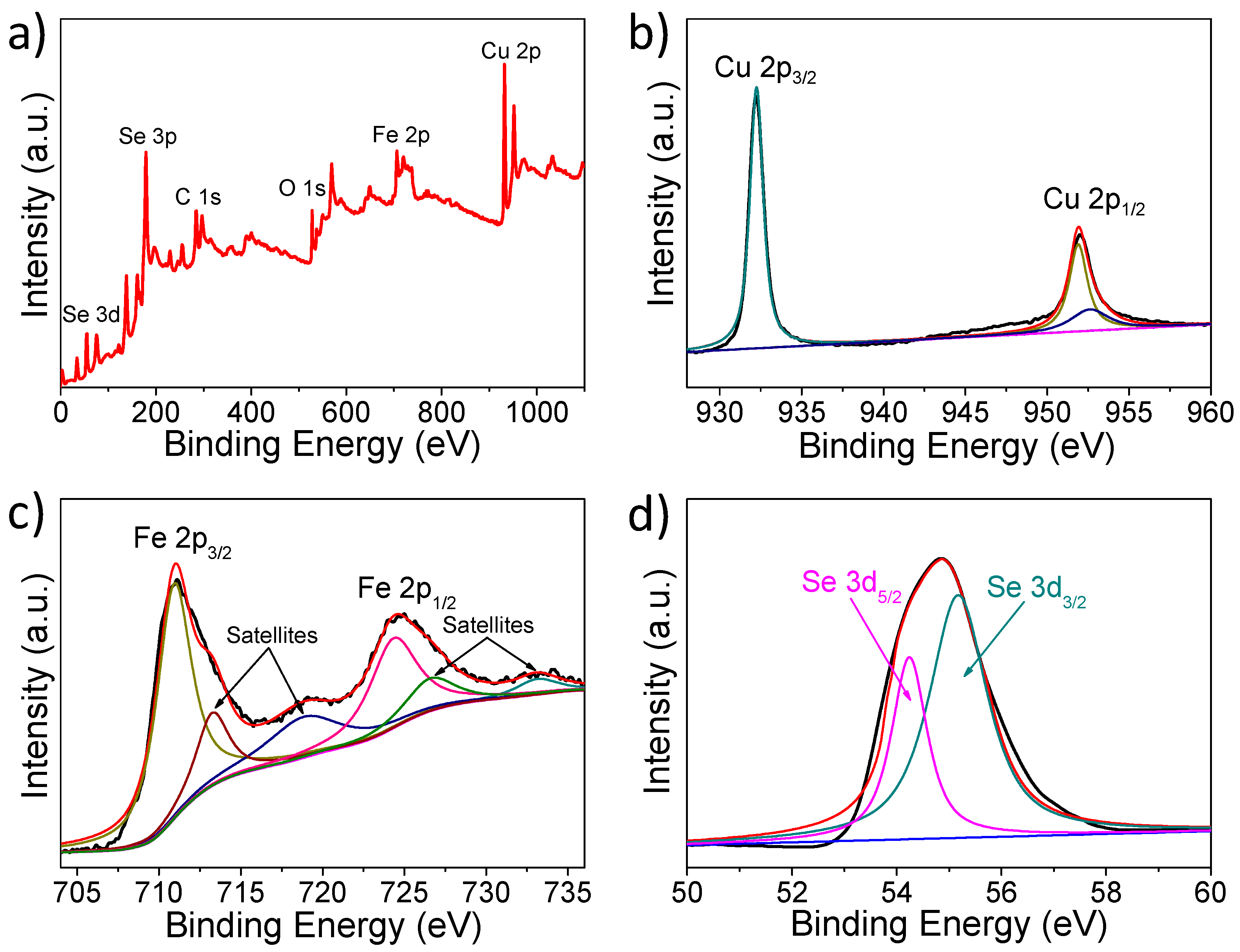
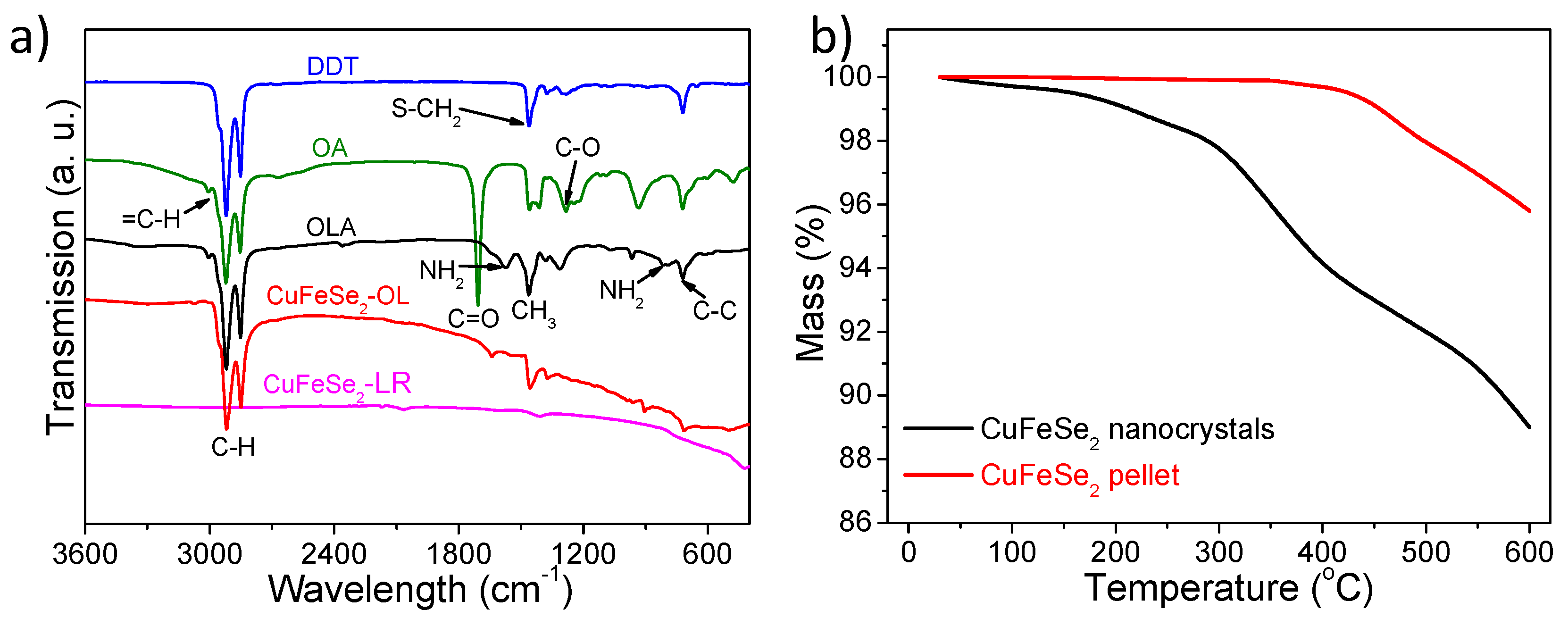
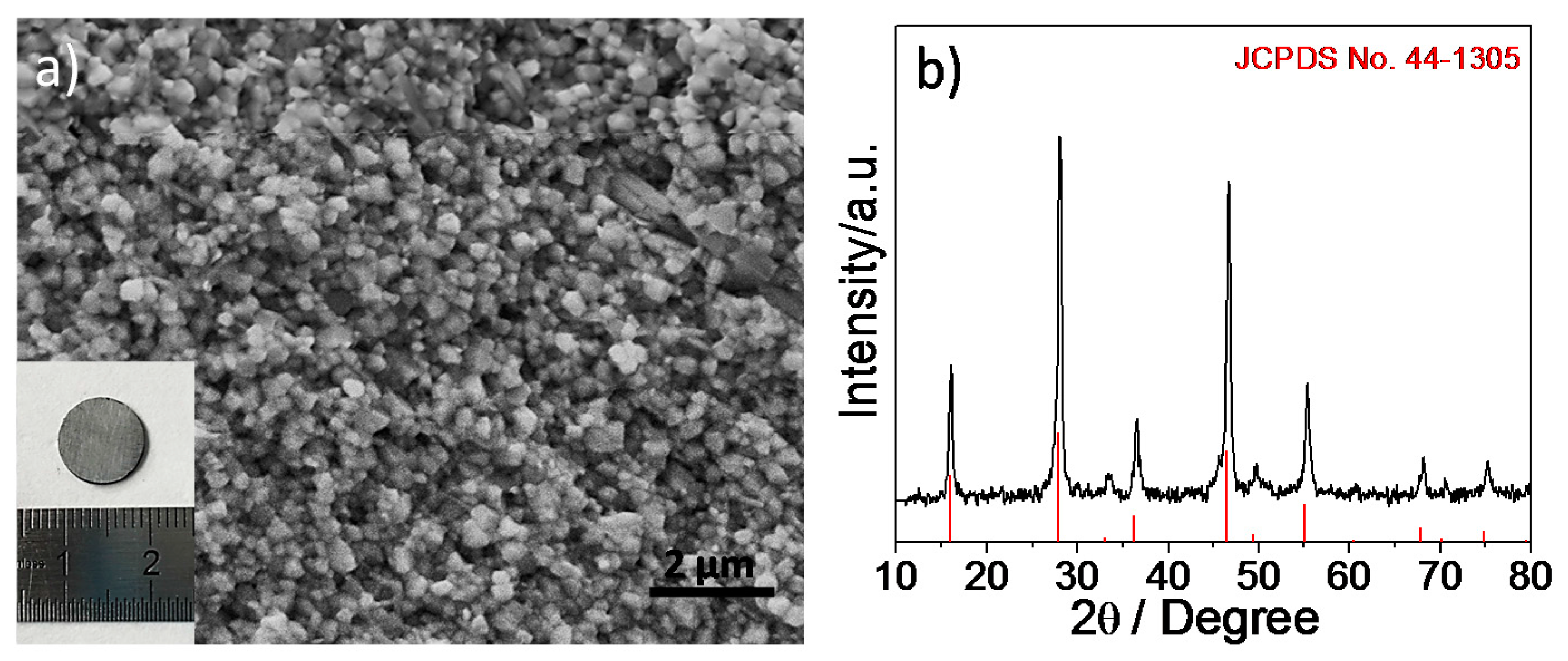
2.4. Structure and Characterization
2.5. Ligand Removal and Bulk Nanomaterial Fabrication
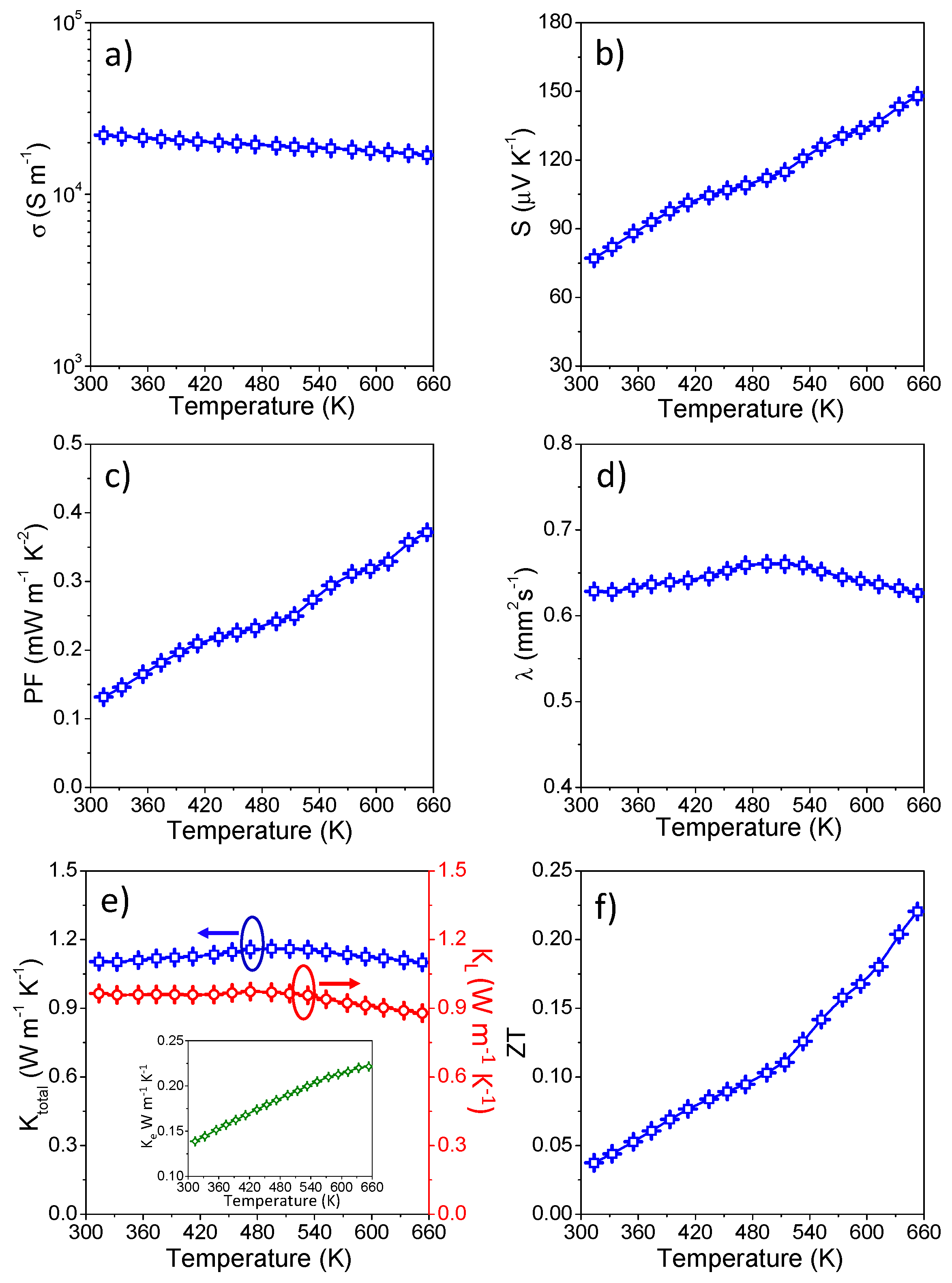
2.6. Thermoelectric Property Measurements
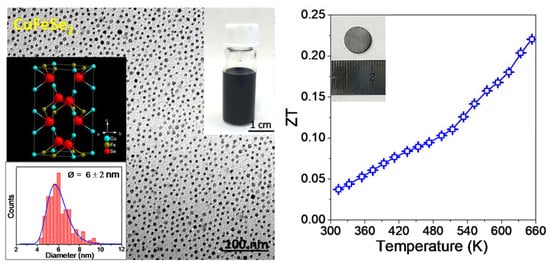
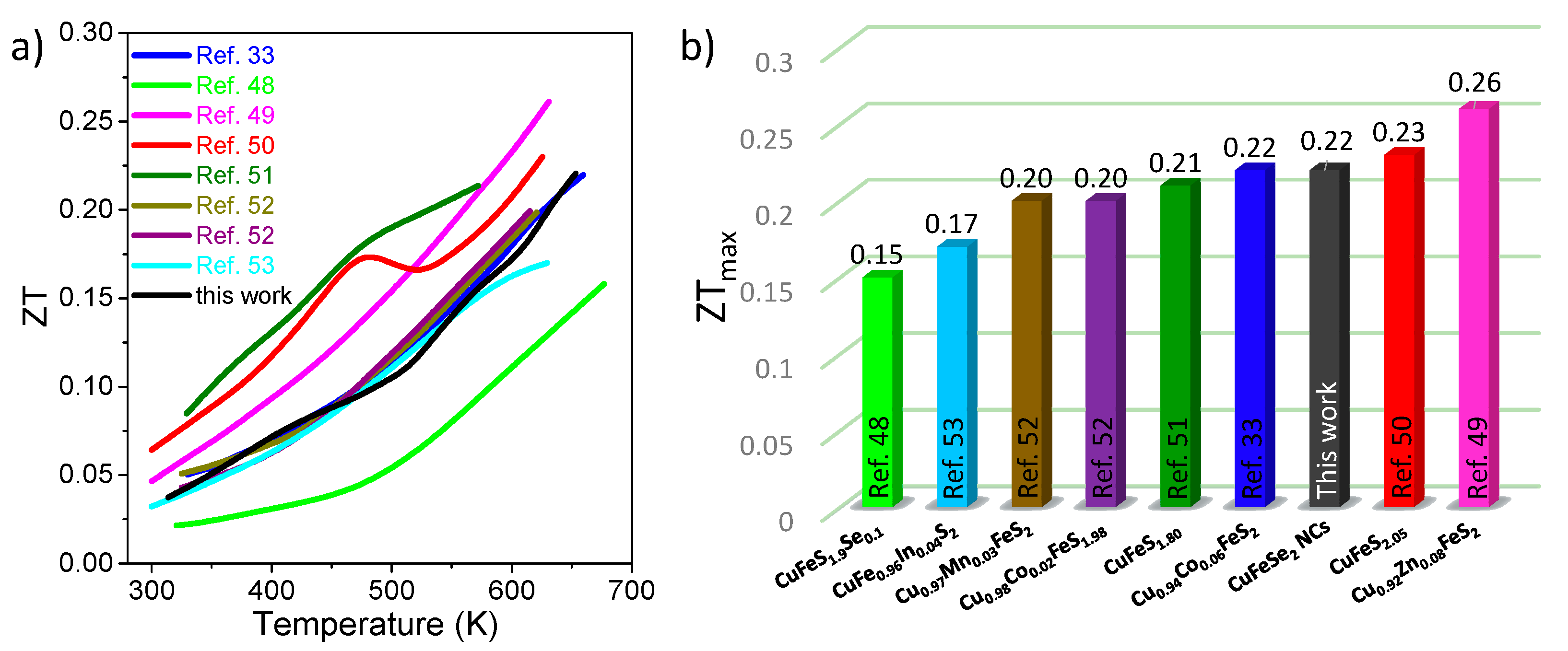
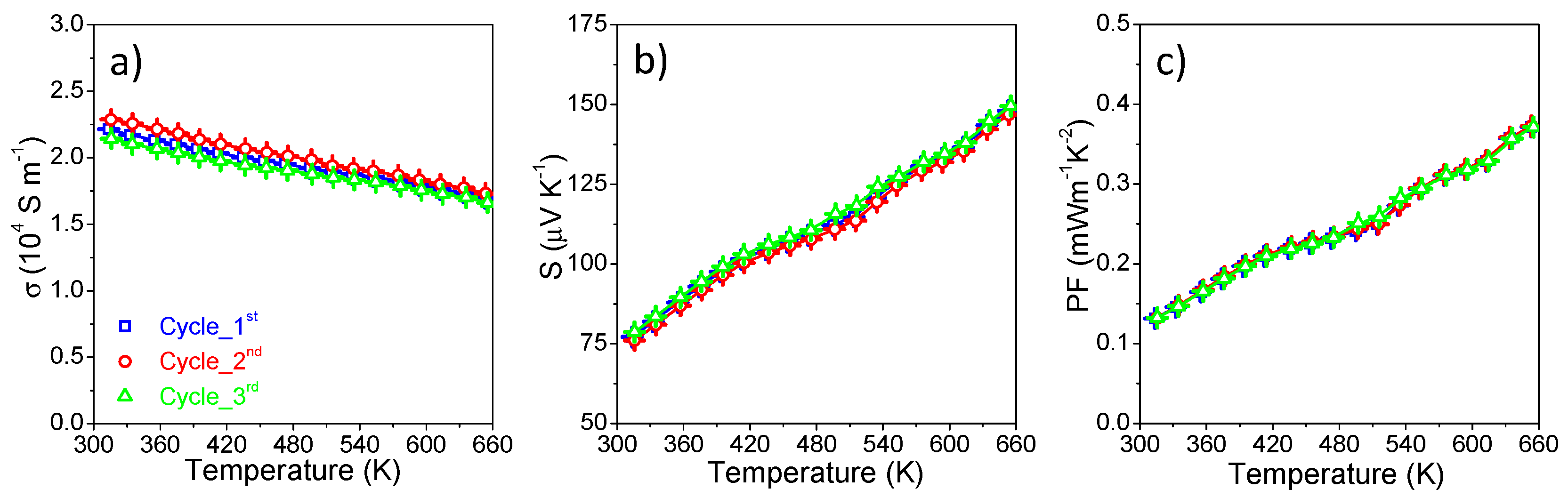
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Xie, W.J.; Weidenkaff, A.; Tang, X.F.; Zhang, Q.J.; Poon, J.; Tritt, T.M. Recent advances in nanostructured thermoelectric half-heuslercompounds. Nanomaterials 2012, 2, 379–412. [Google Scholar] [CrossRef] [PubMed]
- Sootsman, J.R.; Chung, D.Y.; Kanatzidis, M.G. New and old concepts in thermoelectric materials. Angew. Chem. Int. Ed. 2009, 48, 8616–8639. [Google Scholar] [CrossRef] [PubMed]
- Chieruzzi, M.; Pagano, S.; Moretti, S.; Pinna, R.; Milia, E.; Torre, L.; Eramo, S. Nanomaterials for tissue engineering in dentistry. Nanomaterials 2016, 6, 134. [Google Scholar] [CrossRef] [PubMed]
- Snyder, G.J.; Toberer, E.S. Complex thermoelectric materials. Nat. Mater. 2008, 7, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Ortega, S.; Ibáñez, M.; Liu, Y.; Zhang, Y.; Kovalenko, M.V.; Cadavid, D.; Cabot, A. Bottom-up engineering of thermoelectric nanomaterials and devices from solution-processed nanoparticle building blocks. Chem. Soc. Rev. 2017, 46, 3510–3528. [Google Scholar] [CrossRef] [PubMed]
- Biswas, K.; He, J.; Zhang, Q.; Wang, G.; Uher, C.; Dravid, V.P.; Kanatzidis, M.G. Strained endotaxial nanostructures with high thermoelectric figure of merit. Nat. Chem. 2011, 3, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, M.; Luo, Z.; Genç, A.; Piveteau, L.; Ortega, S.; Cadavid, D.; Dobrozhan, O.; Liu, Y.; Nachtegaal, M.; Zebarjadi, M. High-performance thermoelectric nanocomposites from nanocrystal building blocks. Nat. Commun. 2016, 7, 10766. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.J.; Zhang, Y.; Karthik, C.; Singh, B.; Siegel, R.W.; Borca-Tasciuc, T.; Ramanath, G. A new class of doped nanobulk high-figure-of-merit thermoelectrics by scalable bottom-up assembly. Nat. Mater. 2012, 11, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Heremans, J.P.; Jovovic, V.; Toberer, E.S.; Saramat, A.; Kurosaki, K.; Charoenphakdee, A.; Yamanaka, S.; Snyder, G.J. Enhancement of thermoelectric efficiency in PbTe by distortion of the electronic density of states. Science 2008, 321, 554–557. [Google Scholar] [CrossRef] [PubMed]
- Sootsman, J.R.; Kong, H.; Uher, C.; D’Angelo, J.J.; Wu, C.I.; Hogan, T.P.; Caillat, T.; Kanatzidis, M.G. Large enhancements in the thermoelectric power factor of bulk PbTe at high temperature by synergistic nanostructuring. Angew. Chem.-Int. Ed. 2008, 47, 8618–8622. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, M.; Zamani, R.; Gorsse, S.; Fan, J.; Ortega, S.; Cadavid, D.; Morante, J.R.; Arbiol, J.; Cabot, A. Core-shell nanoparticles as building blocks for the bottom-up production of functional nanocomposites: PbTe-PbSthermoelectric properties. ACS Nano 2013, 7, 2573–2586. [Google Scholar] [CrossRef] [PubMed]
- Coughlan, C.; Ibáñez, M.; Dobrozhan, O.; Singh, A.; Cabot, A.; Ryan, K.M. Compound copper chalcogenide nanocrystals. Chem. Rev. 2017, 117, 5865–6109. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.-J.; Yu, B.; Wang, Y.-X.; Zhu, Y.-L.; Liu, X.-J.; Yu, S.-H.; Ren, Z. Colloidal Synthesis of Cu2CdSnSe4 Nanocrystals and Hot-Pressing to Enhance the Thermoelectric Figure-of-Merit. J. Am. Chem. Soc. 2011, 133, 15910–15913. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.-L.; Chen, I.-W.; Huang, F.-Q.; Chen, L.-D. Improved thermoelectric properties of Cu-doped quaternary chalcogenides of Cu2CdSnSe4. Adv. Mater. 2009, 21, 3808–3812. [Google Scholar] [CrossRef]
- Ibáñez, M.; Zamani, R.; LaLonde, A.; Cadavid, D.; Li, W.; Shavel, A.; Arbiol, J.; Morante, J.R.; Gorsse, S.; Snyder, G.J. Cu2ZnGeSe4 nanocrystals: Synthesis and thermoelectric properties. J. Am. Chem. Soc. 2012, 134, 4060–4063. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Huang, F.; Wu, L.; Xu, K. New stannite-like p-type thermoelectric material Cu3SbSe4. J. Phys. D Appl. Phys. 2011, 44, 295404. [Google Scholar] [CrossRef]
- Wei, T.-R.; Wang, H.; Gibbs, Z.M.; Wu, C.-F.; Snyder, G.J.; Li, J.-F. Thermoelectric properties of Sn-doped p-type Cu3SbSe4: A compound with large effective mass and small band gap. J. Mater. Chem. A 2014, 2, 13527–13533. [Google Scholar] [CrossRef]
- Liu, Y.; García, G.; Ortega, S.; Cadavid, D.; Palacios, P.; Lu, J.; Ibáñez, M.; Xi, L.; De Roo, J.; López, A.M.; et al. Solution-based synthesis and processing of Sn- and Bi-doped Cu3SbSe4 nanocrystals, nanomaterials and ring-shaped thermoelectric generators. J. Mater. Chem. A 2017, 5, 2592–2602. [Google Scholar] [CrossRef]
- Song, J.-M.; Liu, Y.; Niu, H.-L.; Mao, C.-J.; Cheng, L.-J.; Zhang, S.-Y.; Shen, Y.-H. Hot-injection synthesis and characterization of monodispersed ternary Cu2SnSe3 nanocrystals for thermoelectric applications. J. Alloys Compd. 2013, 581, 646–652. [Google Scholar] [CrossRef]
- Ibáñez, M.; Cadavid, D.; Anselmi-Tamburini, U.; Zamani, R.; Gorsse, S.; Li, W.; López, A.M.; Morante, J.R.; Arbiol, J.; Cabot, A. Colloidal synthesis and thermoelectric properties of Cu2SnSe3 nanocrystals. J. Mater. Chem. A 2013, 1, 1421–1426. [Google Scholar] [CrossRef]
- Shi, X.; Xi, L.; Fan, J.; Zhang, W.; Chen, L. Cu-Se bond network and thermoelectric compounds with complex diamondlike structure. Chem. Mater. 2010, 22, 6029–6031. [Google Scholar] [CrossRef]
- Tapley, A.; Vaccarello, D.; Hedges, J.; Jia, F.; Love, D.A.; Ding, Z. Preparation and characterization of CuInS2 nanocrystals for photovoltaic materials. Phys. Chem. Chem. Phys. 2013, 15, 1431–1436. [Google Scholar] [CrossRef] [PubMed]
- Gromova, M.; Lefrançois, A.; Vaure, L.; Agnese, F.; Aldakov, D.; Maurice, A.; Djurado, D.; Lebrun, C.; de Geyer, A.; Schulli, T.U.; et al. Growth Mechanism and Surface State of CuInS2 Nanocrystals Synthesized with Dodecanethiol. J. Am. Chem. Soc. 2017, 139, 15748–15759. [Google Scholar] [CrossRef] [PubMed]
- Sandroni, M.; Wegner, K.D.; Aldakov, D.; Reiss, P. Prospects of Chalcopyrite-Type Nanocrystals for Energy Applications. ACS Energy Lett. 2017, 2, 1076–1088. [Google Scholar] [CrossRef]
- Reifsnyder, D.C.; Ye, X.; Gordon, T.R.; Song, C.; Murray, C.B. Three-dimensional self-assembly of chalcopyrite copper indium diselenide nanocrystals into oriented films. ACS Nano 2013, 7, 4307–4315. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.-C.; Wang, R.-Z.; Liu, L.-M.; Chen, Y.-P.; Wei, X.-L.; Yan, H.; Lau, W.-M. Wurtzite-type CuInSe2 for high-performance solar cell absorber: Ab initio exploration of the new phase structure. J. Mater. Chem. 2012, 22, 21662–21666. [Google Scholar] [CrossRef]
- Kim, S.; Kang, M.; Kim, S.; Heo, J.-H.; Noh, J.H.; Im, S.H.; Seok, S.I.; Kim, S.-W. Fabrication of CuInTe2 and CuInTe2−xSex ternary gradient quantum dots and their application to solar cells. ACS Nano 2013, 7, 4756–4763. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhou, B.; Yang, C.; Liao, S.; Zhang, W.-H.; Li, C. CuFeS2 colloidal nanocrystals as an efficient electrocatalyst for dye sensitized solar cells. Chem. Commun. 2016, 52, 11488–11491. [Google Scholar] [CrossRef] [PubMed]
- Gabka, G.; Bujak, P.; Ostrowski, A.; Tomaszewski, W.; Lisowski, W.; Sobczak, J.W.; Pron, A. Cu–Fe–S Nanocrystals Exhibiting Tunable Localized Surface Plasmon Resonance in the Visible to NIR Spectral Ranges. Inorg. Chem. 2016, 55, 6660–6669. [Google Scholar] [CrossRef] [PubMed]
- Żukrowski, J.; Błachowski, A.; Komędera, K.; Ruebenbauer, K.; Gabka, G.; Bujak, P.; Pron, A.; Przybylski, M. Dynamics of Ternary Cu–Fe–S2 Nanoparticles Stabilized by Organic Ligands. J. Phys. Chem. C 2017, 121, 6977–6985. [Google Scholar] [CrossRef]
- Hamdadou, N.; Morsli, M.; Khelil, A.; Bernede, J. Fabrication of n- and p-type doped CuFeSe2 thin films achieved by selenization of metal precursors. J. Phys. D Appl. Phys. 2006, 39, 1042. [Google Scholar] [CrossRef]
- Joint Committee on Powder Diffraction Standards ASTM. Powder Diffraction File; Joint Committee on Powder Diffraction Standards ASTM: Philadelphia, PA, USA, 1967; pp. 9–185. [Google Scholar]
- Berthebaud, D.; Lebedev, O.; Maignan, A. Thermoelectric properties of n-type cobalt doped chalcopyrite Cu1−xCoxFeS2 and p-type eskebornite CuFeSe2. J. Materiomics 2015, 1, 68–74. [Google Scholar] [CrossRef]
- Lee, P.C.; Ou, M.N.; Luo, J.Y.; Wu, M.K.; Chen, Y.Y. Cross-plane Seebeck coefficient and thermal conductivity of CuFeSe2 thin film. AIP Conf. Proc. 2012, 1449, 405–408. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, S.; Ren, F.; Chen, L.; Zeng, J.; Zhu, M.; Cheng, Z.; Gao, M.; Li, Z. Ultrasmall Magnetic CuFeSe2 Ternary Nanocrystals for Multimodal Imaging Guided Photothermal Therapy of Cancer. ACS Nano 2017, 11, 5633–5645. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.-K.; Lin, Y.-G.; Chen, Y.-C. One-pot synthesis of CuFeSe2 cuboid nanoparticles. Mater. Res. Bull. 2011, 46, 2117–2119. [Google Scholar] [CrossRef]
- Wang, W.; Jiang, J.; Ding, T.; Wang, C.; Zuo, J.; Yang, Q. Alternative synthesis of CuFeSe2 nanocrystals with magnetic and photoelectric properties. ACS Appl. Mater. Interfaces 2015, 7, 2235–2241. [Google Scholar] [CrossRef] [PubMed]
- Henaoa, J.; Delgado, J.; Quintero, M. X-ray powder diffraction data for CuFeSe2. Powder Diffr. 1994, 9, 108–110. [Google Scholar] [CrossRef]
- NIST-XPS Database, Version 3.5. Available online: http://srdata.nist.gov/xps/ (accessed on 30 November 2011).
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer Corporation: Eden Prairie, MN, USA, 1992; p. 87. [Google Scholar]
- Bernede, J.; Hamdadou, N.; Khelil, A. X-ray photoelectron spectroscopy study of CuFeSe2 thin films. J. Electron Spectrosc. Relat. Phenom. 2004, 141, 61–66. [Google Scholar] [CrossRef]
- Guo, D.; An, Y.; Cui, W.; Zhi, Y.; Zhao, X.; Lei, M.; Li, L.; Li, P.; Wu, Z.; Tang, W. Epitaxial growth and magnetic properties of ultraviolet transparent Ga2O3/(Ga1−xFex)2O3 multilayer thin films. Sci. Rep. 2016, 6, 25166. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Zhang, S.-S.; Song, J.-M.; Niu, H.-L.; Mao, C.-J.; Zhang, S.-Y.; Shen, Y.-H. Facile synthesis of antimony selenide with lamellar nanostructures and their efficient catalysis for the hydrogenation of p-nitrophenol. J. Alloys Compd. 2014, 585, 40–47. [Google Scholar] [CrossRef]
- Song, J.M.; Zhang, S.S.; Yu, S.H. Multifunctional Co0.85Se-Fe3O4 nanocomposites: Controlled synthesis and their enhanced performances for efficient hydrogenation of p-nitrophenol and adsorbents. Small 2014, 10, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-K.; Kim, J.K.; Kang, Y.C. Metal–organic framework-derived CoSe2/(NiCo)Se2 box-in-box hollow nanocubes with enhanced electrochemical properties for sodium-ion storage and hydrogen evolution. J. Mater. Chem. A 2017, 5, 18823–18830. [Google Scholar] [CrossRef]
- Liu, Y.; Cadavid, D.; Ibánez, M.; De Roo, J.; Ortega, S.; Dobrozhan, O.; Kovalenko, M.V.; Cabot, A. Colloidal AgSbSe2 nanocrystals: Surface analysis, electronic doping and processing into thermoelectric nanomaterials. J. Mater. Chem. C 2016, 4, 4756–4762. [Google Scholar] [CrossRef] [Green Version]
- Carr, W.D.; Morelli, D.T. The thermoelectric properties and solubility limit of CuFeS2(1−x)Se2x. J. Electron. Mater. 2016, 45, 1346–1350. [Google Scholar] [CrossRef]
- Xie, H.; Su, X.; Zheng, G.; Zhu, T.; Yin, K.; Yan, Y.; Uher, C.; Kanatzidis, M.G.; Tang, X. The Role of Zn in chalcopyrite CuFeS2: Enhanced thermoelectric properties of Cu1−xZnxFeS2 with in situ nanoprecipitates. Adv. Energy Mater. 2017, 7, 1601299. [Google Scholar] [CrossRef]
- Xie, H.; Su, X.; Yan, Y.; Liu, W.; Chen, L.; Fu, J.; Yang, J.; Uher, C.; Tang, X. Thermoelectric performance of CuFeS2+2x composites prepared by rapid thermal explosion. NPG Asia Mater. 2017, 9, e390. [Google Scholar] [CrossRef]
- Li, J.; Tan, Q.; Li, J.-F. Synthesis and property evaluation of CuFeS2−x as earth-abundant and environmentally-friendly thermoelectric materials. J. Alloys Compd. 2013, 551, 143–149. [Google Scholar] [CrossRef]
- Lefèvre, R.; Berthebaud, D.; Mychinko, M.Y.; Lebedev, O.I.; Mori, T.; Gascoin, F.; Maignan, A. Thermoelectric properties of the chalcopyrite Cu1−xMxFeS2−y series (M = Mn, Co., Ni). RSC Adv. 2016, 6, 55117–55124. [Google Scholar] [CrossRef]
- Xie, H.; Su, X.; Zheng, G.; Yan, Y.; Liu, W.; Tang, H.; Kanatzidis, M.G.; Uher, C.; Tang, X. Nonmagnetic in Substituted CuFe1−xInxS2 solid solution thermoelectric. J. Phys. Chem. C 2016, 120, 27895–27902. [Google Scholar] [CrossRef]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.-Q.; Liu, Y.; Zuo, Y.; Chen, J.-S.; Song, J.-M.; Niu, H.-L.; Mao, C.-J. Colloidal Synthesis and Thermoelectric Properties of CuFeSe2 Nanocrystals. Nanomaterials 2018, 8, 8. https://doi.org/10.3390/nano8010008
Zhang B-Q, Liu Y, Zuo Y, Chen J-S, Song J-M, Niu H-L, Mao C-J. Colloidal Synthesis and Thermoelectric Properties of CuFeSe2 Nanocrystals. Nanomaterials. 2018; 8(1):8. https://doi.org/10.3390/nano8010008
Chicago/Turabian StyleZhang, Bing-Qian, Yu Liu, Yong Zuo, Jing-Shuai Chen, Ji-Ming Song, He-Lin Niu, and Chang-Jie Mao. 2018. "Colloidal Synthesis and Thermoelectric Properties of CuFeSe2 Nanocrystals" Nanomaterials 8, no. 1: 8. https://doi.org/10.3390/nano8010008