Highly-Ordered PdIn Intermetallic Nanostructures Obtained from Heterobimetallic Acetate Complex: Formation and Catalytic Properties in Diphenylacetylene Hydrogenation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Catalyst Preparation
2.3. Catalyst Characterization
2.4. Catalytic Tests
3. Results and Discussion
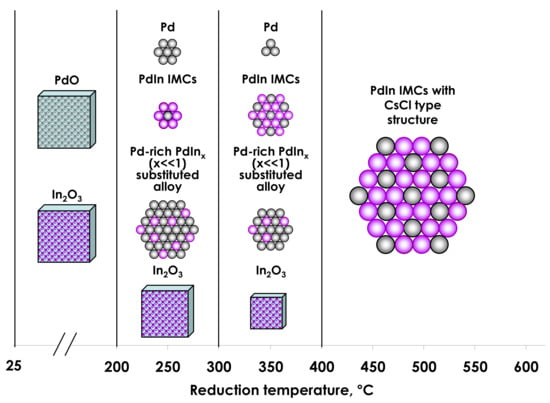
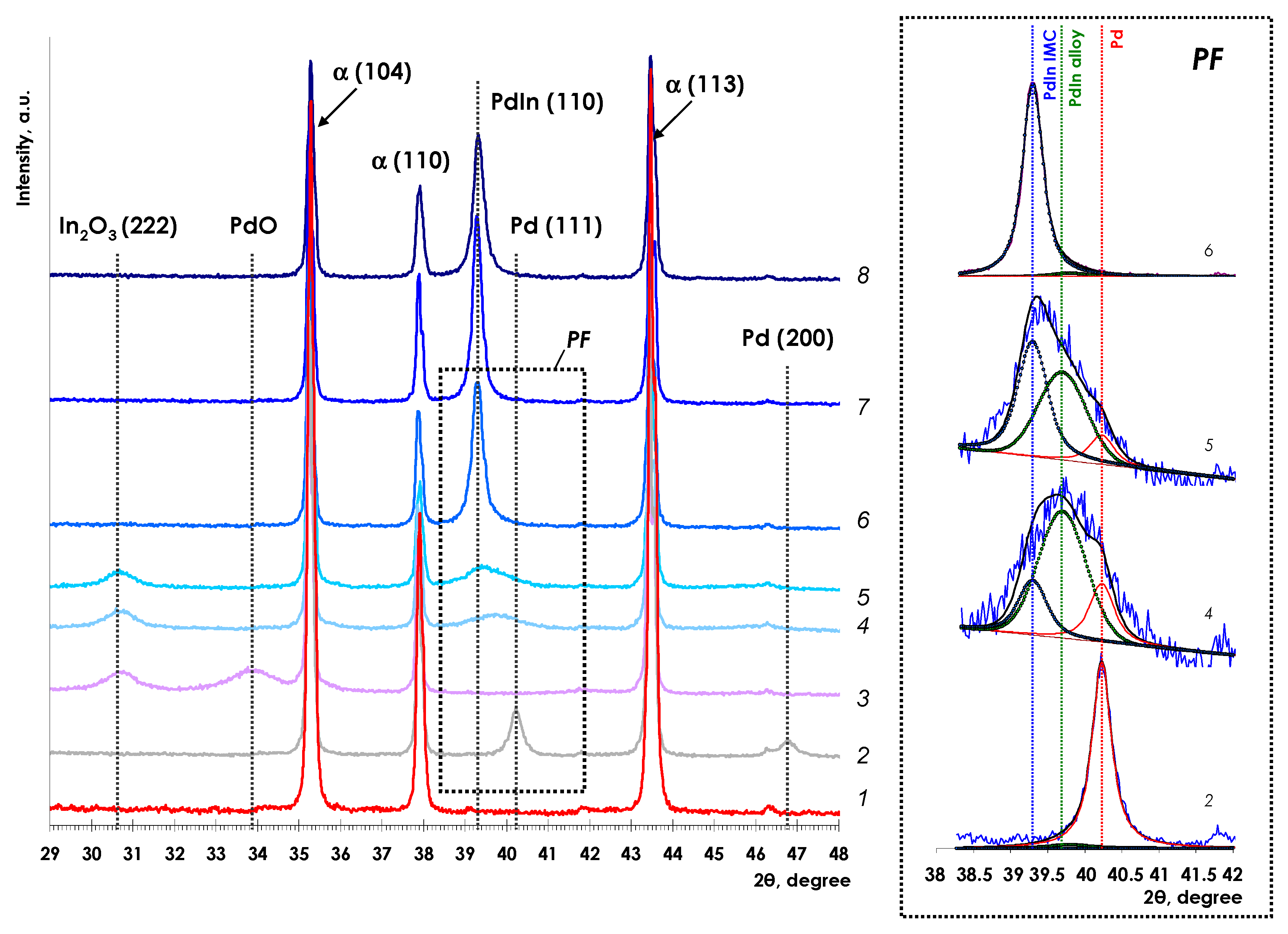
3.1. Catalyst Characterization
3.1.1. X-ray Diffraction
3.1.2. Transmitted Electron Microscopy
3.1.3. Temperature-Programmed Pd Hydride Decomposition
3.2. Catalytic Hydrogenation of DPA
3.2.1. Effect of Reduction Temperature on the Activity of PdIn Catalysts
3.2.2. Selectivity to Olefin Formation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Armbrüster, M. Intermetallic Compounds in Catalysis. In Encyclopedia of Catalysis; Horváth, I.T., Ed.; Wiley-VCH: Weinheim, Germany, 2011; ISBN 9780471227618. [Google Scholar]
- Furukawa, S.; Komatsu, T. Intermetallic Compounds: Promising Inorganic Materials for Well-Structured and Electronically Modified Reaction Environments for Efficient Catalysis. ACS Catal. 2017, 7, 735–765. [Google Scholar] [CrossRef]
- Zafeiratos, S.; Piccinin, S.; Teschner, D. Alloys in catalysis: Phase separation and surface segregation phenomena in response to the reactive environment. Catal. Sci. Technol. 2012, 2, 1787–1801. [Google Scholar] [CrossRef]
- Komatsu, T.; Furukawa, S. Intermetallic Compound Nanoparticles Dispersed on the Surface of Oxide Support as Active and Selective Catalysts. Mater. Trans. 2015, 56, 460–467. [Google Scholar] [CrossRef] [Green Version]
- Penner, S.; Armbrüster, M. Formation of Intermetallic Compounds by Reactive Metal–Support Interaction: A Frequently Encountered Phenomenon in Catalysis. ChemCatChem 2015, 7, 374–392. [Google Scholar] [CrossRef]
- Armbrüster, M.; Schlögl, R.; Grin, Y. Intermetallic compounds in heterogeneous catalysis—A quickly developing field. Sci. Technol. Adv. Mater. 2014, 15, 034803. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, H.; Rameshan, C.; Bielz, T.; Memmel, N.; Stadlmayr, W.; Mayr, L.; Zhao, Q.; Soisuwan, S.; Klötzer, B.; Penner, S. From Oxide-Supported Palladium to Intermetallic Palladium Phases: Consequences for Methanol Steam Reforming. ChemCatChem 2013, 5, 1273–1285. [Google Scholar] [CrossRef]
- Ananikov, V.P.; Eremin, D.B.; Yakukhnov, S.A.; Dilman, A.D.; Levin, V.V.; Egorov, M.P.; Karlov, S.S.; Kustov, L.M.; Tarasov, A.L.; Greish, A.A.; et al. Organic and hybrid systems: From science to practice. Mendeleev Commun. 2017, 27, 425–438. [Google Scholar] [CrossRef]
- Föttinger, K. PdZn based catalysts: Connecting electronic and geometric structure with catalytic performance. Catalysis 2013, 25, 77–117. [Google Scholar] [CrossRef]
- Kovnir, K.; Armbrüster, M.; Teschner, D.; Venkov, T.V.; Jentoft, F.C.; Knop-Gericke, A.; Grin, Y.; Schlögl, R. A new approach to well-defined, stable and site-isolated catalysts. Sci. Technol. Adv. Mater. 2007, 8, 420–427. [Google Scholar] [CrossRef] [Green Version]
- Arnold, H.; Döbert, F.; Gaube, J. Selective Hydrogenation of Hydrocarbons. In Handbook of Heterogeneous Catalysis, 2nd ed.; Ertl, G., Knözinger, H., Schüth, F., Weitkamp, J., Eds.; Wiley-VCH Verlag GmbH& Co. KGaA: Weinheim, Germany, 2008; pp. 3266–3284. ISBN 978-3-527-31241-2. [Google Scholar]
- Molnár, Á.; Sárkány, A.; Varga, M. Hydrogenation of carbon–carbon multiple bonds: Chemo-, regio- and stereo-selectivity. J. Mol. Catal. A Chem. 2001, 173, 185–221. [Google Scholar] [CrossRef]
- Stakheev, A.Y.; Smirnova, N.S.; Krivoruchenko, D.S.; Baeva, G.N.; Mashkovsky, I.S.; Yakushev, I.A.; Vargaftik, M.N. Single-atom Pd sites on the surface of Pd-In nanoparticles supported on γ-Al2O3: A CO-DRIFTS study. Mendeleev Commun. 2017, 27, 515–517. [Google Scholar] [CrossRef]
- Markov, P.V.; Bragina, G.O.; Baeva, G.N.; Mashkovskii, I.S.; Rassolov, A.V.; Yakushev, I.A.; Vargaftik, M.N.; Stakheev, A.Y. Supported Catalysts Based on Pd–In Nanoparticles for the Liquid-Phase Hydrogenation of Terminal and Internal Alkynes: 2. Catalytic Properties. Kinet. Catal. 2016, 57, 625–631. [Google Scholar] [CrossRef]
- Feng, Q.; Zhao, S.; Wang, Y.; Dong, J.; Chen, W.; He, D.; Wang, D.; Yang, J.; Zhu, Y.; Zhu, H.; et al. Isolated Single-Atom Pd Sites in Intermetallic Nanostructures: High Catalytic Selectivity for Semihydrogenation of Alkynes. J. Am. Chem. Soc. 2017, 139, 7294–7301. [Google Scholar] [CrossRef] [PubMed]
- Burueva, D.B.; Kovtunov, K.V.; Bukhtiyarov, A.V.; Barskiy, D.A.; Prosvirin, I.P.; Mashkovsky, I.S.; Baeva, G.N.; Bukhtiyarov, V.I.; Stakheev, A.Y.; Koptyug, I.V. Selective Single-Site Pd-In Hydrogenation Catalyst for Production of Enhanced Magnetic Resonance Signals using Parahydrogen. Chem. Eur. J. 2018, 24, 2547–2553. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, H.; Turner, S.; Lebedev, O.I.; van Tendeloo, G.; Klötzer, B.; Rameshan, C.; Pfaller, K.; Penner, S. Pd–In2O3 interaction due to reduction in hydrogen: Consequences for methanol steam reforming. Appl. Catal. A 2010, 374, 180–188. [Google Scholar] [CrossRef]
- Smirnova, N.S.; Shlyapin, D.A.; Mironenko, O.O.; Anoshkina, E.A.; Temerev, V.L.; Shitova, N.B.; Kochubey, D.I.; Tsyrul’nikov, P.G. EXAFS study of Pd/Ga2O3 model catalysts of selective liquid-phase hydrogenation of acetylene to ethylene. J. Mol. Catal. A Chem. 2012, 358, 152–158. [Google Scholar] [CrossRef]
- Vilé, G.; Dähler, P.; Vecchietti, J.; Baltanás, M.; Collins, S.; Calatayud, M.; Bonivardi, A.; Pérez-Ramírez, J. Promoted ceria catalysts for alkyne semi-hydrogenation. J. Catal. 2015, 324, 69–78. [Google Scholar] [CrossRef]
- Neumann, M.; Teschner, D.; Knop-Gericke, A.; Reschetilowski, W.; Armbrüster, M. Controlled synthesis and catalytic properties of supported In–Pd intermetallic compounds. J. Catal. 2016, 340, 49–59. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, X.; Li, L.; Liu, X.; Huang, Y.; Pan, X.; Wang, A.; Li, J.; Zhang, T. PdZn Intermetallic Nanostructure with Pd-Zn-Pd ensembles for Highly Active and Chemoselective Semi-hydrogenation of Acetylene. ACS Catal. 2016, 6, 1054–1061. [Google Scholar] [CrossRef]
- García-Trenco, A.; Regoutz, A.; White, E.R.; Payne, D.J.; Shaffer, M.S.P.; Williams, C.K. PdIn intermetallic nanoparticles for the Hydrogenation of CO2 to Methanol. Appl. Catal. B 2018, 220, 9–18. [Google Scholar] [CrossRef]
- Wu, Z.; Wegener, E.C.; Tseng, H.-T.; Gallagher, J.R.; Harris, J.W.; Diaz, R.E.; Ren, Y.; Ribeiro, F.H.; Miller, J.T. Pd–In intermetallic alloy nanoparticles: Highly selective ethane dehydrogenation catalysts. Catal. Sci. Technol. 2016, 6, 6965–6976. [Google Scholar] [CrossRef]
- Armbrüster, M.; Behrens, M.; Föttinger, K.; Friedrich, M.; Gaudry, É.; Matam, S.K.; Sharma, H.R. The Intermetallic Compound ZnPd and Its Role in Methanol Steam Reforming. Catal. Rev. Sci. Eng. 2013, 55, 289–367. [Google Scholar] [CrossRef]
- Predel, B. Phase Equilibria, Crystallographic and Thermodynamic Data of Binary Alloys. In Landolt-Börnstein, New Series IV/5G (1997), New Series IV/5G; Madelung, O., Ed.; Springer: Berlin/Heidelberg, Germany, 1997; p. 142. [Google Scholar]
- Okamoto, H. In–Pd (Indium–Palladium). J. Phase Equilib. 2003, 24, 481. [Google Scholar] [CrossRef]
- Penner, S.; Wang, D.; Su, D.S.; Rupprechter, G.; Podloucky, R.; Schlögl, R.; Hayek, K. Platinum nanocrystals supported by silica, alumina and ceria: Metal–support interaction due to high-temperature reduction in hydrogen. Surf. Sci. 2003, 532–535, 276–280. [Google Scholar] [CrossRef]
- Stolarov, I.P.; Yakushev, I.A.; Churakov, A.V.; Cherkashina, N.V.; Smirnova, N.S.; Khramov, E.V.; Zubavichus, Y.V.; Khrustalev, V.N.; Markov, A.A.; Klyagina, A.P.; et al. Heterometallic Palladium(II)−Indium(III) and −Gallium(III) Acetate-Bridged Complexes: Synthesis, Structure, and Catalytic Performance in Homogeneous Alkyne and Alkene Hydrogenation. Inorg. Chem. 2018, 5, 11482–11491. [Google Scholar] [CrossRef] [PubMed]
- Markov, P.V.; Bragina, G.O.; Rassolov, A.V.; Baeva, G.N.; Mashkovsky, I.S.; Murzin, V.Y.; Zubavichus, Y.V.; Stakheev, A.Y. Pd–Cu catalyst prepared from heterobimetallic PdCu2(OAc)6: An XRD-EXAFS study and activity/selectivity in the liquid-phase hydrogenation of a C≡C bond. Mendeleev Commun. 2016, 26, 502–504. [Google Scholar] [CrossRef]
- Rassolov, A.V.; Markov, P.V.; Bragina, G.O.; Baeva, G.N.; Mashkovskii, I.S.; Yakushev, I.A.; Vargaftik, M.N.; Stakheev, A.Y. Catalytic Properties of Nanostructured Pd–Ag Catalysts in the Liquid-Phase Hydrogenation of Terminal and Internal Alkynes. Kinet. Catal. 2016, 57, 853–858. [Google Scholar] [CrossRef]
- Rassolov, A.V.; Markov, P.V.; Bragina, G.O.; Baeva, G.N.; Krivoruchenko, D.S.; Mashkovskii, I.S.; Yakushev, I.A.; Vargaftik, M.N.; Stakheev, A.Y. Formation of Pd–Ag Nanoparticles in Supported Catalysts Based on the Heterobimetallic Complex PdAg2(OAc)4(HOAc)4. Kinet. Catal. 2016, 57, 859–865. [Google Scholar] [CrossRef]
- Rassolov, A.V.; Krivoruchenko, D.S.; Medvedev, M.G.; Mashkovsky, I.S.; Stakheev, A.Y.; Svitanko, I.V. Diphenylacetylene hydrogenation on a PdAg/Al2O3 single-atom catalyst: An experimental and DFT study. Mendeleev Commun. 2017, 27, 615–617. [Google Scholar] [CrossRef]
- Ichimura, K. Photoalignment of Liquid-Crystal Systems. Chem. Rev. 2000, 100, 1847–1873. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Yokoyama, A.; Komatsu, T. Efficient Catalytic System for Synthesis of trans-Stilbene from Diphenylacetylene Using Rh-Based Intermetallic Compounds. ACS Catal. 2014, 4, 3581–3585. [Google Scholar] [CrossRef]
- Halim, M.; Samuel, I.D.W.; Pillow, J.N.G.; Monkman, A.P.; Burn, P.L. Control of Colour and Charge Injection in Conjugated Dendrimer/Polypyridine Bilayer LEDs. Synth. Met. 1999, 102, 1571–1574. [Google Scholar] [CrossRef]
- Blaser, H.-U.; Schnyder, A.; Steiner, H.; Rossler, F.; Baumeister, P. Selective Hydrogenation of Functionalized Hydrocarbons. In Handbook of Heterogeneous Catalysis, 2nd ed.; Ertl, G., Knözinger, H., Schüth, F., Weitkamp, J., Eds.; Wiley-VCH Verlag GmbH& Co. KGaA: Weinheim, Germany, 2008; pp. 3284–3308. ISBN 978-3-527-31241-2. [Google Scholar]
- Gavrikov, A.V.; Koroteev, P.S.; Dobrokhotova, Z.V.; Ilyukhin, A.B.; Efimov, N.N.; Kirdyankin, D.I.; Bykov, M.A.; Ryumin, M.A.; Novotortsev, V.M. Novel heterometallic polymeric lanthanide acetylacetonates with bridging cymantrenecarboxylate groups—Synthesis, magnetism and thermolysis. Polyhedron 2015, 102, 48–59. [Google Scholar] [CrossRef]
- Markov, P.V.; Bragina, G.O.; Baeva, G.N.; Tkachenko, O.P.; Mashkovskii, I.S.; Yakushev, I.A.; Kozitsyna, N.Y.; Vargaftik, M.N.; Stakheev, A.Y. Pd–Cu Catalysts from Acetate Complexes in Liquid-Phase Diphenylacetylene Hydrogenation. Kinet. Catal. 2015, 56, 591–597. [Google Scholar] [CrossRef]
- Mashkovsky, I.S.; Markov, P.V.; Bragina, G.O.; Rassolov, A.V.; Baeva, G.N.; Stakheev, A.Y. Intermetallic Pd1–Zn1 Nanoparticles in the Selective Liquid-Phase Hydrogenation of Substituted Alkynes. Kinet. Catal. 2017, 58, 480–491. [Google Scholar] [CrossRef]
- Chauruka, S.R.; Hassanpour, A.; Brydson, R.; Roberts, K.J.; Ghadiri, M.; Stitt, H. Effect of mill type on the size reduction and phase transformation of gamma alumina. Chem. Eng. Sci. 2015, 134, 774–783. [Google Scholar] [CrossRef]
- Matori, K.A.; Wah, L.C.; Hashim, M.; Ismail, I.; Mohd Zaid, M.H. Phase Transformations of α-Alumina Made from Waste Aluminum via a Precipitation Technique. Int. J. Mol. Sci. 2012, 13, 16812–16821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Wu, K.; Cao, J.; Wang, Y. Controlled synthesis of α-Al2O3 via the hydrothermalpyrolysis method. IOP Conf. Ser. Mater. Sci. Eng. 2017, 207, 012004. [Google Scholar] [CrossRef]
- Akhtar, M.J.; Ahamed, M.; Kumar, S.; Majeed Khan, M.A.; Ahmad, J.; Alrokayan, S.A. Zinc oxide nanoparticles selectively induce apoptosis in human cancer cells through reactive oxygen species. Int. J. Nanomed. 2012, 7, 845–857. [Google Scholar] [CrossRef]
- Mashkovsky, I.S.; Markov, P.V.; Bragina, G.O.; Baeva, G.N.; Bukhtiyarov, A.V.; Prosvirin, I.P.; Bukhtiyarov, V.I.; Stakheev, A.Y. Formation of Supported Intermetallic Nanoparticles in the Pd–Zn/α-Al2O3 Catalyst. Kinet. Catal. 2017, 58, 471–479. [Google Scholar] [CrossRef]
- Mashkovsky, I.S.; Markov, P.V.; Bragina, G.O.; Baeva, G.N.; Rassolov, A.V.; Bukhtiyarov, A.V.; Prosvirin, I.P.; Bukhtiyarov, V.I.; Stakheev, A.Y. PdZn/α-Al2O3 catalyst for liquid-phase alkyne hydrogenation: Details of «solid-state alloy—Intermetallics» transformation. Mendeleev Commun. 2018, 28, 152–154. [Google Scholar] [CrossRef]
- Baylet, A.; Marécot, P.; Duprez, D.; Castellazzi, P.; Groppi, G.; Forzatti, P. In situ Raman and in situ XRD analysis of PdO reduction and Pd0 oxidation supported on γ-Al2O3 catalyst under different atmospheres. Phys. Chem. Chem. Phys. 2011, 13, 4607–4613. [Google Scholar] [CrossRef] [PubMed]
- Chitturi, K.L.; Yaramma, A.; Merugu, R.; Dachepalli, R.; Kandhadi, J. Synthesis and Characterisation of In2O3 Nanoparticles from Astragalus gummifer. Adv. Nanopart. 2016, 5, 114–122. [Google Scholar] [CrossRef]
- Choi, Y.I.; Kim, S.K.; Lee, S.W.; Sohn, Y. Metallic indium spheres by the anaerobic ethanol oxidation of indium oxide. J. Alloys Compd. 2016, 687, 611–615. [Google Scholar] [CrossRef]
- Bukhtiyarov, V.I.; Zaikovskii, V.I.; Kashin, A.S.; Ananikov, V.P. Modern electron microscopy in the study of chemical systems at the boundary of organic synthesis and catalysis. Russ. Chem. Rev. 2016, 85, 1198–1214. [Google Scholar] [CrossRef]
- Armbrüster, M.; Behrens, M.; Cinquini, F.; Föttinger, K.; Grin, Y.; Haghofer, A.; Klötzer, B.; Knop-Gericke, A.; Lorenz, H.; Ota, A.; et al. How to Control the Selectivity of Palladium-based Catalysts in Hydrogenation Reactions: The Role of Subsurface Chemistry. ChemCatChem 2012, 4, 1048–1063. [Google Scholar] [CrossRef]
- Markov, P.V.; Bragina, G.O.; Baeva, G.N.; Tkachenko, O.P.; Mashkovsky, I.S.; Yakushev, I.A.; Vargaftik, M.N.; Stakheev, A.Y. Supported Catalysts Based on Pd–In Nanoparticles for the Liquid-Phase Hydrogenation of Terminal and Internal Alkynes: 1. Formation and Structure. Kinet. Catal. 2016, 57, 617–624. [Google Scholar] [CrossRef]
- Neri, G.; Musolino, M.G.; Milone, C.; Pietropaolo, D.; Galvagno, S. Particle size effect in the catalytic hydrogenation of 2,4-dinitrotoluene over Pd/C catalysts. Appl. Catal. A Gen. 2001, 208, 307–316. [Google Scholar] [CrossRef]
- Aduriz, H.R.; Bodnariuk, P.; Coq, B.; Figueras, F. Alumina-Supported Bimetallics of Palladium Alloyed with Germanium, Tin, Lead, or Antimony from Organometallic Precursors. I. Preparation and characterization. J. Catal. 1989, 119, 97–107. [Google Scholar] [CrossRef]
- Cao, Y.; Sui, Z.J.; Zhu, Y.; Zhou, X.; Chen, D. Selective Hydrogenation of Acetylene over Pd-In/Al2O3 Catalyst: Promotional Effect of Indium and Composition-dependent Performance. ASC Catal. 2017, 7, 7835–7846. [Google Scholar] [CrossRef]
- Bond, G.C. Metal-Catalysed Reactions of Hydrocarbons; Springer: New York, NY, USA, 2005. [Google Scholar]
- Markov, P.V.; Bragina, G.O.; Rassolov, A.V.; Mashkovsky, I.S.; Baeva, G.N.; Tkachenko, O.P.; Yakushev, I.A.; Vargaftik, M.N.; Stakheev, A.Y. Performance of the bimetallic Pd-In catalyst in the selective liquid-phase hydrogenation of internal and terminal alkynes. Mendeleev Commun. 2016, 26, 494–496. [Google Scholar] [CrossRef]
- Choudary, B.M.; Lakshmi Kantam, M.; Mahender Reddy, N.; Koteswara Rao, K.; Haritha, Y.; Bhaskar, V.; Figueras, F.; Tuel, A. Hydrogenation of acetylenics by Pd-exchanged mesoporous materials. Appl. Catal. A Gen. 1999, 181, 139–144. [Google Scholar] [CrossRef]
- Marín-Astorga, N.; Alvez-Manoli, G.; Reyes, P. Stereoselective hydrogenation of phenyl alkyl acetylenes on pillared clays supported palladium catalysts. J. Mol. Catal. A Chem. 2005, 226, 81–88. [Google Scholar] [CrossRef]
- Ota, A.; Armbrüster, M.; Behrens, M.; Rosenthal, D.; Friedrich, M.; Kasatkin, I.; Girgsdies, F.; Zhang, W.; Wagner, R.; Schlögl, R. Intermetallic Compound Pd2Ga as a Selective Catalyst for the Semi-Hydrogenation of Acetylene: From Model to High Performance Systems. J. Phys. Chem. C 2011, 115, 1368–1374. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, Y.; Liu, C.; Wang, X.; Cao, C.; Song, W. Excellent Selectivity with High Conversion in the Semihydrogenation of Alkynes using Palladium-Based Bimetallic Catalysts. ChemCatChem 2017, 9, 4053–4057. [Google Scholar] [CrossRef]
- Wencka, M.; Hahne, M.; Kocjan, A.; Vrtnik, S.; Koželj, P.; Korže, D.; Jagličić, Z.; Sornić, M.; Popčević, P.; Ivkov, J.; et al. Physical properties of the InPd intermetallic catalyst. Intermetallics 2014, 55, 56–65. [Google Scholar] [CrossRef]








| Catalyst | r1 | r2 | r1/r2 |
|---|---|---|---|
| mmol/(gcat min) | |||
| Pd | 4.39 | 0.621 | 7.1 |
| PdIn-200 | 4.02 | 0.194 | 20.7 |
| PdIn-300 | 1.40 | 0.040 | 35.0 |
| PdIn-400 | 0.46 | 0.0103 | 44.7 |
| PdIn-500 | 0.42 | 0.0088 | 47.7 |
| PdIn-600 | 0.45 | 0.0095 | 47.4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mashkovsky, I.S.; Markov, P.V.; Bragina, G.O.; Baeva, G.N.; Rassolov, A.V.; Yakushev, I.A.; Vargaftik, M.N.; Stakheev, A.Y. Highly-Ordered PdIn Intermetallic Nanostructures Obtained from Heterobimetallic Acetate Complex: Formation and Catalytic Properties in Diphenylacetylene Hydrogenation. Nanomaterials 2018, 8, 769. https://doi.org/10.3390/nano8100769
Mashkovsky IS, Markov PV, Bragina GO, Baeva GN, Rassolov AV, Yakushev IA, Vargaftik MN, Stakheev AY. Highly-Ordered PdIn Intermetallic Nanostructures Obtained from Heterobimetallic Acetate Complex: Formation and Catalytic Properties in Diphenylacetylene Hydrogenation. Nanomaterials. 2018; 8(10):769. https://doi.org/10.3390/nano8100769
Chicago/Turabian StyleMashkovsky, Igor S., Pavel V. Markov, Galina O. Bragina, Galina N. Baeva, Alexander V. Rassolov, Ilya A. Yakushev, Michael N. Vargaftik, and Alexander Yu. Stakheev. 2018. "Highly-Ordered PdIn Intermetallic Nanostructures Obtained from Heterobimetallic Acetate Complex: Formation and Catalytic Properties in Diphenylacetylene Hydrogenation" Nanomaterials 8, no. 10: 769. https://doi.org/10.3390/nano8100769