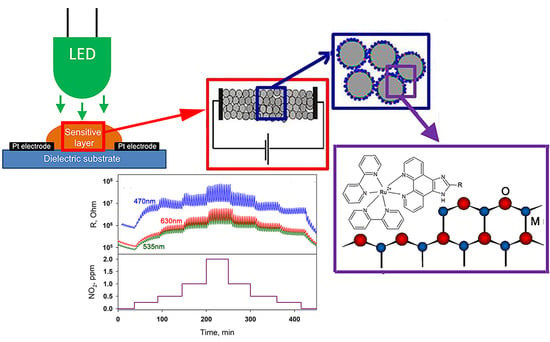
Photosensitive Organic-Inorganic Hybrid Materials for Room Temperature Gas Sensor Applications
Abstract
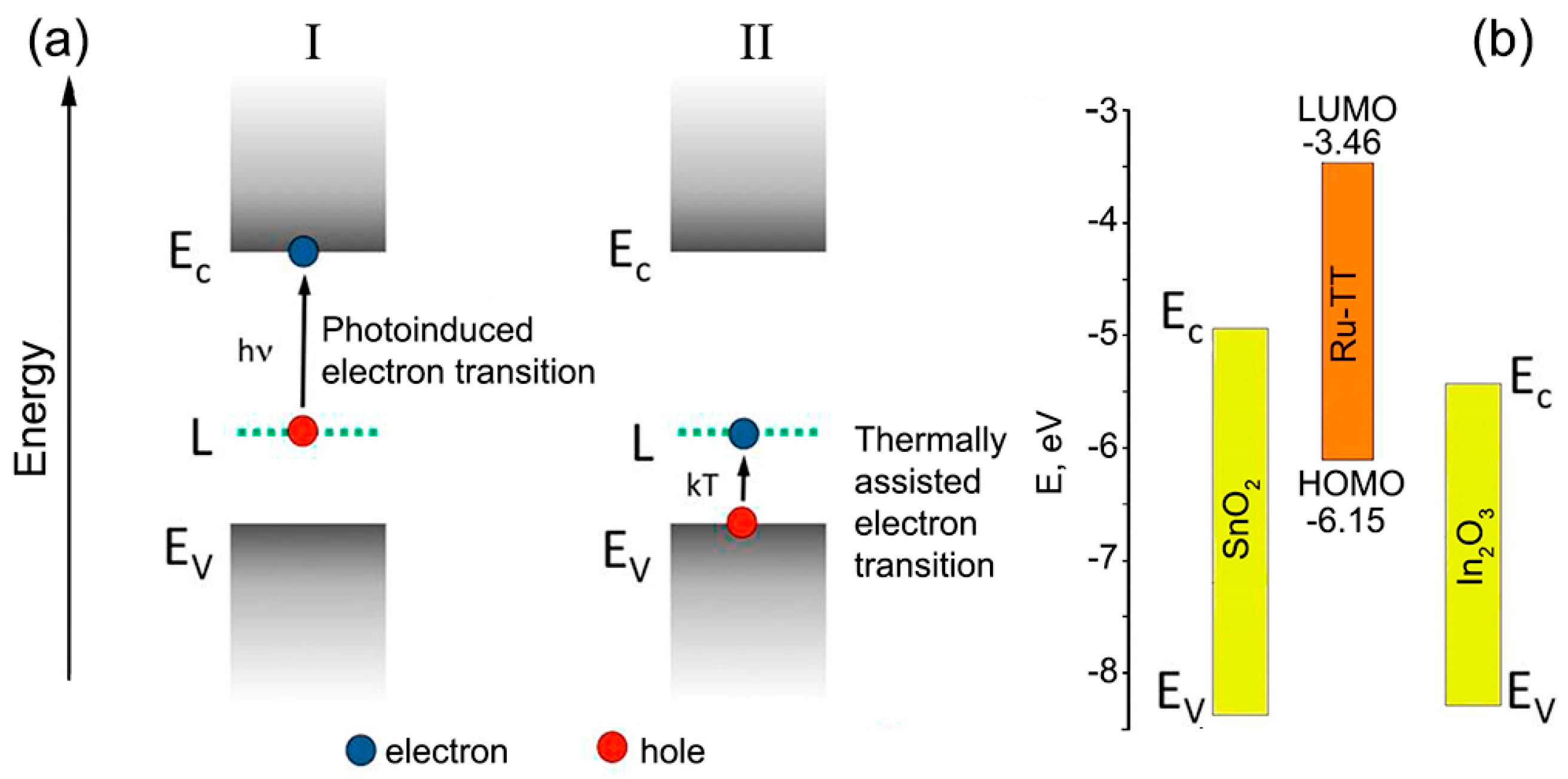
:1. Introduction
2. Results and Discussion
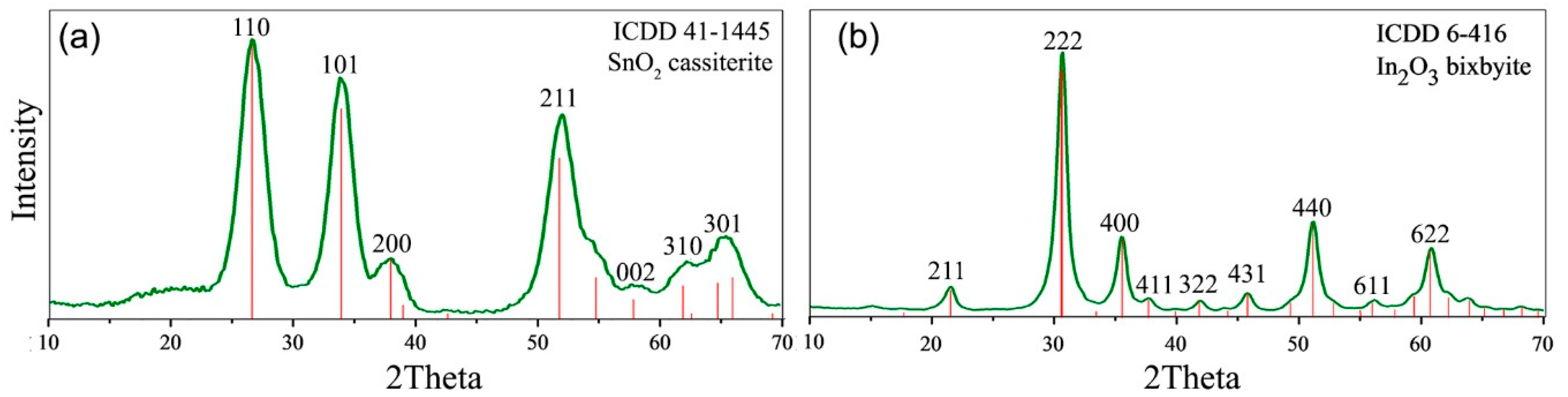
2.1. Characteristics of Nanocrystalline Semiconductor Oxides
2.2. Characteristics of Ru(II) Heterocyclic Complex
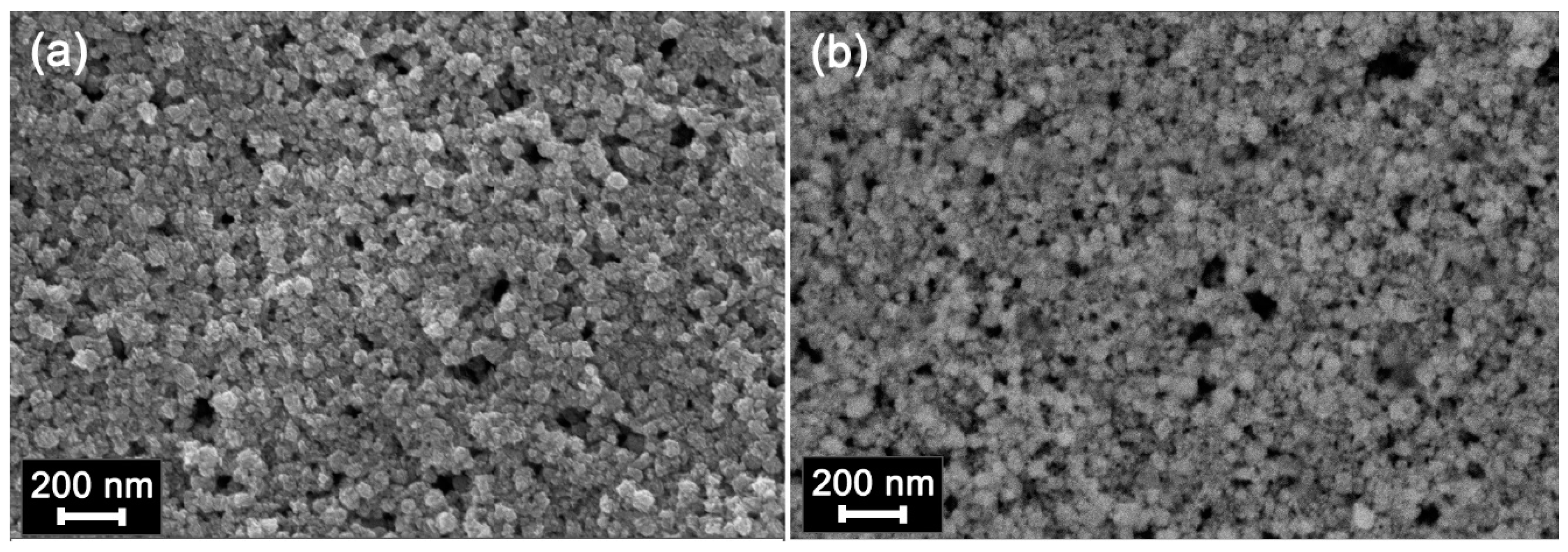
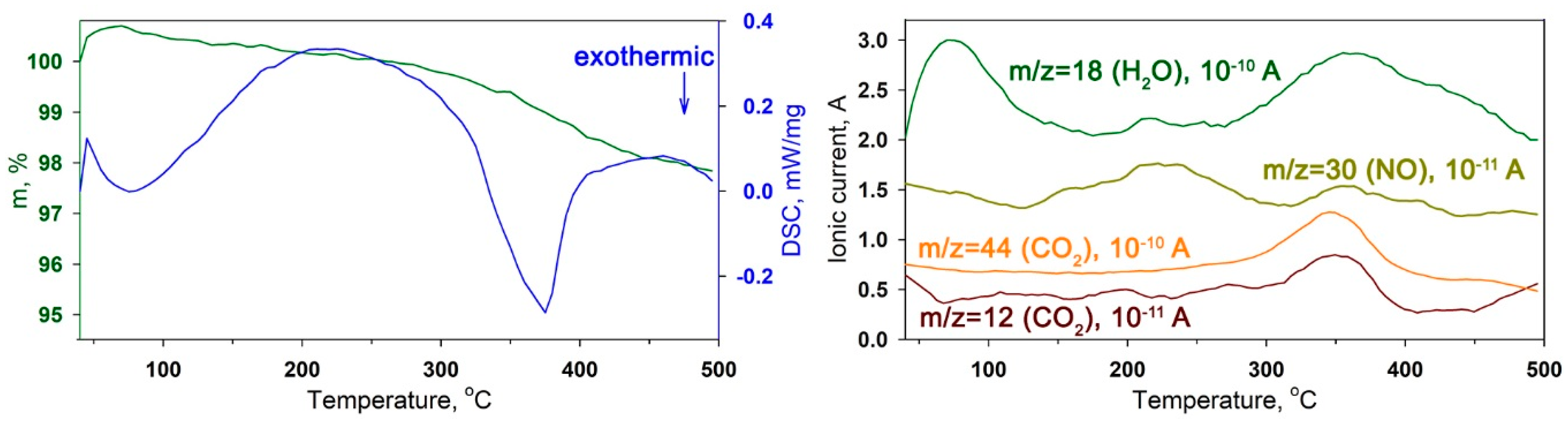
2.3. Characteristics of Hybrid Samples
2.4. Gas Sensor Properties
- (i)
- Pure tin dioxide did not exhibit photosensitivity (Figure 9c), and the effective photoresponse SPh = 1 for all NO2 concentrations under illumination with blue, green and red light (Figure 10a). Nevertheless, the observed change in the resistance as a function of the NO2 concentration makes it possible to calculate the magnitude of the sensor signal by Equation (7). The maximum sensor signal of blank SnO2 was measured under green light illumination (Figure 10b).This effect can be due to the participation of oxygen vacancies of tin dioxide in the adsorption of NO2. As shown by the authors of [65], the acceptor levels related to the oxygen vacancies in SnO2 lie at 1.4 eV (bridging vacancies) and 0.9 eV (in-plane vacancies) above the valence band, which correspond to the energy of an electron transition from an acceptor level of Ea = 2.2 eV (563 nm) and Ea = 2.3 eV (538 nm), respectively. The absence of the photosensitivity (SPh = 1), together with the measurable sensor signal (S > 1), can be due to the fact that, for the finely dispersed tin dioxide, the main process of interaction with NO2 is the reaction (5). Since the electron affinity of NO2 is larger than the one for O2, the position of energy levels of electrons localized on NO2 molecules chemisorbed on a SnO2 surface is deeper than that for chemisorbed oxygen. Thus, electron transfer in accordance with the reaction (5) will lead to a decrease in the Fermi level of the semiconductor. Since the band structure of nanocrystalline SnO2 with a particle size of 3–4 nm corresponds to the situation of flat zones [66], the decrease in the Fermi level indicates the decrease in the electrical conductivity, providing the sensor signal in the presence of NO2.
- (ii)
- With a blue illumination, pure In2O3 exhibited a noticeable photosensitivity (Figure 9a). The value of the effective photoresponse at a fixed concentration of NO2 decreased with the increasing wavelength of the activating light (wavelengths of 470, 535 and 630 nm were used) (Figure 10a). A similar trend was observed for the concentration dependence of sensor signal. The maximum values of sensor signal in the whole range of NO2 concentrations were obtained under blue light (Figure 10b). This tendency is in accordance with the fact that for nanocrystalline In2O3, the photoconductivity is nonzero at photon energies more than 2.25 eV (λ < 550 nm), which can be explained by the generation of electrons from localized levels located in the bandgap [64].
- (iii)
- The sensitization of semiconducting oxides with the Ru-TT organic complex leads to the increase in both the effective photoresponse SPh of the materials and their sensor signal S towards NO2 (Figure 9b,d). For the SnO2 R-TT hybrid material, the effective photoresponse and sensor signal values decreased upon transition from blue to green and then to red light illumination (Figure 10). The observed tendency agrees with the absorption spectrum and the spectral dependence of the photoconductivity of this hybrid material. In the case of the In2O3 Ru-TT hybrid, the maximum values of the photoresponse and the sensor signal at high concentrations of NO2 were obtained under red light. It appears to be an artifact. The values of the dark resistance Rdark observed under these measurement conditions exceeded 109 Ohm, which is the upper limit of the measuring system. The measurement of such resistances occurred with a large error and a high noise level.
3. Materials and Methods
3.1. Materials Synthesis
3.1.1. Synthesis of Ru(II) Complex
3.1.2. Synthesis of Nanocrystalline SnO2 and In2O3 and Hybrid Materials
3.2. Materials Characterization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Guidelines for Indoor Air Quality: Selected Pollutants, Geneva. 2010. Available online: http://www.euro.who.int/__data/assets/pdf_file/0009/128169/e94535.pdf (accessed on 12 August 2018).
- Lian, K.-Y.; Hsiao, S.-J.; Sung, W.-T. Mobile Monitoring and Embedded Control System for Factory Environment. Sensors 2013, 13, 17379–17413. [Google Scholar] [CrossRef] [PubMed]
- Krivetskiy, V.; Ponzoni, A.; Comini, E.; Badalyan, S.; Rumyantseva, M.; Gaskov, A. Selectivity Modification of SnO2-Based Materials for Gas Sensor Arrays. Electroanalysis 2010, 22, 2809–2816. [Google Scholar] [CrossRef]
- Marikutsa, A.; Rumyantseva, M.; Baranchikov, A.; Gaskov, A. Nanocrystalline BaSnO3 as an alternative gas sensor material: Surface reactivity and high sensitivity to SO2. Materials 2015, 8, 6437–6454. [Google Scholar] [CrossRef] [PubMed]
- Korotcenkov, G.; Brinzari, V.; Cho, B.K. In2O3- and SnO2-Based Thin Film Ozone Sensors: Fundamentals. J. Sens. 2016, 2016, 3816094. [Google Scholar] [CrossRef]
- Saura, J. Gas-sensing properties of SnO2 pyrolytic films subjected to ultraviolet radiation. Sens. Actuators B 1994, 17, 211–214. [Google Scholar] [CrossRef]
- Mishra, S.; Ghanshyam, C.; Ram, N.; Bajpai, R.P.; Bedi, R.K. Detection mechanism of metal oxide gas sensor under UV radiation. Sens. Actuators B 2004, 97, 387–390. [Google Scholar] [CrossRef]
- Prades, J.D.; Diaz, R.J.; Hernandez-Ramirez, F.; Barth, S.; Cirera, A.; Romano-Rodriguez, A.; Mathur, S.; Morante, J.R. Equivalence between thermal and room temperature UV light modulated responses of gas sensors based on individual SnO2 nanowires. Sens. Actuators B 2009, 140, 337–341. [Google Scholar] [CrossRef]
- Espid, E.; Taghipour, F. UV-LED Photo-activated Chemical Gas Sensors: A Review. Crit. Rev. Solid State Mater. Sci. 2017, 42, 416–432. [Google Scholar] [CrossRef]
- Espid, E.; Taghipour, F. Development of highly sensitive ZnO/In2O3 composite gas sensor activated by UV-LED. Sens. Actuators B 2017, 241, 828–839. [Google Scholar] [CrossRef]
- Hsu, C.-L.; Chang, L.-F.; Hsueh, T.-J. Light-activated humidity and gas sensing by ZnO nanowires grown on LED at room temperature. Sens. Actuators B 2017, 249, 265–277. [Google Scholar] [CrossRef]
- Ilin, A.; Martyshov, M.; Forsh, E.; Forsh, P.; Rumyantseva, M.; Abakumov, A.; Gaskov, A.; Kashkarov, P. UV effect on NO2 sensing properties of nanocrystalline In2O3. Sens. Actuators B 2016, 231, 491–496. [Google Scholar] [CrossRef]
- Trawka, M.; Smulko, J.; Hasse, L.; Granqvist, C.-G.; Annanouch, F.E.; Ionescu, R. Fluctuation enhanced gas sensing with WO3-based nanoparticle gas sensors modulated by UV light at selected wavelengths. Sens. Actuators B 2016, 234, 453–461. [Google Scholar] [CrossRef]
- Wongrat, E.; Chanlek, N.; Chueaiarrom, C.; Samransuksamer, B.; Hongsith, N.; Choopun, S. Low temperature ethanol response enhancement of ZnO nanostructures sensor decorated with gold nanoparticles exposed to UV illumination. Sens. Actuators A 2016, 251, 188–197. [Google Scholar] [CrossRef]
- Saboor, F.H.; Ueda, T.; Kamada, K.; Hyodo, T.; Mortazavi, Y.; Khodadadi, A.A.; Shimizu, Y. Enhanced NO2 gas sensing performance of bare and Pd-loaded SnO2 thick film sensors under UV-light irradiation at room temperature. Sens. Actuators B 2016, 223, 429–439. [Google Scholar] [CrossRef]
- Nakate, U.T.; Patil, P.; Bulakhe, R.N.; Lokhande, C.D.; Kale, S.N.; Naushad, M.; Mane, R.S. Sprayed zinc oxide films: Ultra-violet light-induced reversible surface wettability and platinum-sensitization-assisted improved liquefied petroleum gas response. J. Colloid Interface Sci. 2016, 480, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Klaus, D.; Klawinski, D.; Amrehn, S.; Tiemann, M.; Wagner, T. Light-activated resistive ozone sensing at room temperature utilizing nanoporous In2O3 particles: Influence of particle size. Sens. Actuators B 2015, 217, 181–185. [Google Scholar] [CrossRef]
- Wagner, T.; Kohl, C.-D.; Malagù, C.; Donato, N.; Latino, M.; Neri, G.; Tiemann, M. UV light-enhanced NO2 sensing by mesoporous In2O3: Interpretation of results by a new sensing model. Sens. Actuators B 2013, 187, 488–494. [Google Scholar] [CrossRef]
- Cui, J.; Wang, D.; Xie, T.; Lin, Y. Study on photoelectric gas-sensing property and photogenerated carrier behavior of Ag-ZnO at the room temperature. Sens. Actuators B 2013, 186, 165–171. [Google Scholar] [CrossRef]
- Wang, C.Y.; Becker, R.W.; Passow, T.; Pletsche, W.; Kohler, K.; Cimalla, V.; Ambacher, O. Photon-stimulated sensor based on indium oxide nanoparticles I: Wide-concedntration-range ozone monitoring in air. Sens. Actuators B 2011, 152, 235–240. [Google Scholar] [CrossRef]
- Carotta, M.C.; Cervi, A.; Fioravanti, A.; Gherardi, S.; Giberti, A.; Vendemiati, B.; Vincenzi, D.; Sacerdoti, M. A novel ozone detection at room temperature through UV-LED-assisted ZnO thick film sensors. Thin Solid Films 2011, 520, 939–946. [Google Scholar] [CrossRef]
- Peng, L.; Zhao, Q.; Wang, D.; Zhai, J.; Wang, P.; Pang, S.; Xie, T. Ultraviolet-assisted gas sensing: A potential formaldehyde detection approach at room temperature based on zinc oxide nanorods. Sens. Actuators B 2009, 136, 80–85. [Google Scholar] [CrossRef]
- Prades, J.D.; Jimenez-Diaz, P.; Manzanares, M.; Hernandez-Ramirez, F.; Cirera, A.; Romano-Rodriguez, V.; Mathur, S.; Morante, J.R. A model for the response towards oxidizing gases of photoactivated sensors based on individual SnO2 nanowires. Phys. Chem. Chem. Phys. 2009, 11, 10881–10889. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Xie, T.; Yang, M.; Wang, P.; Xu, D.; Pang, S.; Wang, D. Light induced enhancing gas sensitivity of copper-doped zinc oxide at room temperature. Sens. Actuators B 2008, 131, 660–664. [Google Scholar] [CrossRef]
- De Lacy Costello, B.P.J.; Ewen, R.J.; Ratcliffe, N.M.; Richards, M. Highly sensitive room temperature sensors based on the UV-LED activation of zinc oxide nanoparticles. Sens. Actuators B 2008, 134, 945–952. [Google Scholar] [CrossRef]
- Malagu, C.; Carotta, M.C.; Comini, E.; Faglia, G.; Giberti, A.; Guidi, V.; Maffeis, T.G.G.; Martinelli, G.; Sberveglieri, G.; Wilks, S.P. Photo-Induced Unpinning of Fermi Level in WO3. Sensors 2005, 5, 594–603. [Google Scholar] [CrossRef]
- Zhang, C.; Geng, X.; Li, J.; Luo, Y.; Lu, P. Role of oxygen vacancy in tuning of optical, electrical and NO2 sensing properties of ZnO1−x coatings at room temperature. Sens. Actuators B 2017, 248, 886–893. [Google Scholar] [CrossRef]
- Chizhov, A.; Rumyantseva, M.; Vasiliev, R.; Filatova, D.; Drozdov, K.; Krylov, I.; Marchevsky, A.; Karakulina, O.; Abakumov, A.; Gaskov, A. Visible light activation of room temperature NO2 gas sensors based on ZnO, SnO2 and In2O3 sensitized with CdSe quantum dots. Thin Solid Films 2016, 618, 253–262. [Google Scholar] [CrossRef]
- Han, L.; Wang, D.; Lu, Y.; Jiang, T.; Chen, L.; Xie, T.; Lin, Y. Influence of annealing temperature on the photoelectric gas sensing of Fe-doped ZnO under visible light irradiation. Sens. Actuators B 2013, 177, 34–40. [Google Scholar] [CrossRef]
- Geng, Q.; He, Z.; Chen, X.; Dai, W.; Wang, X. Gas sensing property of ZnO under visible light irradiation at room temperature. Sens. Actuators B 2013, 188, 293–297. [Google Scholar] [CrossRef]
- Varechkina, E.N.; Rumyantseva, M.N.; Vasiliev, R.B.; Konstantinova, E.A.; Gaskov, A.M. UV-VIS photoconductivity of nanocrystalline tin oxide. J. Nanoelectron. Optoelectron. 2012, 7, 623–628. [Google Scholar] [CrossRef]
- Han, L.; Wang, D.; Cui, J.; Chen, L.; Jiang, T.; Lin, Y. Study on formaldehyde gas-sensing of In2O3-sensitized ZnO nanoflowers under visible light irradiation at room temperature. J. Mater. Chem. 2012, 22, 12915–12920. [Google Scholar] [CrossRef]
- Geng, X.; Zhang, C.; Debliquy, M. Cadmium sulfide activated zinc oxide coatings deposited by liquid plasma spray for room temperature nitrogen dioxide detection under visible light illumination. Ceram. Int. 2016, 42, 4845–4852. [Google Scholar] [CrossRef]
- Geng, X.; You, J.; Zhang, C. Microstructure and sensing properties of CdS-ZnO1−x coatings deposited by liquid plasma spray and treated with hydrogen peroxide solution for nitrogen dioxide detection at room temperature. J. Alloy. Compd. 2016, 68, 286–293. [Google Scholar] [CrossRef]
- Chizhov, A.S.; Rumyantseva, M.N.; Vasiliev, R.B.; Filatova, D.G.; Drozdov, K.A.; Krylov, I.V.; Abakumov, A.M.; Gaskov, A.M. Visible light activated room temperature gas sensors based on nanocrystalline ZnO sensitized with CdSe quantum dots. Sens. Actuators B 2014, 205, 305–312. [Google Scholar] [CrossRef]
- Yang, Z.; Guo, L.; Zu, B.; Guo, Y.; Xu, T.; Dou, X. CdS/ZnO Core/Shell Nanowire-Built Films for Enhanced Photodetecting and Optoelectronic Gas-Sensing Applications. Adv. Opt. Mater. 2014, 2, 738–745. [Google Scholar] [CrossRef]
- Vasiliev, R.; Babynina, A.; Maslova, O.; Rumyantseva, M.; Ryabova, L.; Dobrovolsky, A.; Drozdov, K.; Khokhlov, D.; Abakumov, A.; Gaskov, A. Photoconductivity of nanocrystalline SnO2 sensitized with colloidal CdSe quantum dots. J. Mater. Chem. 2013, 1, 1005–1010. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, J.; Olivier, M.-G.; Debliquy, M. Room temperature nitrogen dioxide sensors based on N719-dye sensitized amorphous zinc oxide sensors performed under visible-light illumination. Sens. Actuators B 2015, 209, 69–77. [Google Scholar] [CrossRef]
- Peng, L.; Qin, P.; Zeng, Q.; Song, H.; Lei, M.; Mwangi, J.J.N.; Wang, D.; Xie, T. Improvement of formaldehyde sensitivity of ZnO nanorods by modifying with Ru(dcbpy)2(NCS)2. Sens. Actuators B 2011, 160, 39–45. [Google Scholar] [CrossRef]
- Paolesse, R.; Nardis, S.; Monti, D.; Stefanelli, M.; Di Natale, C. Porphyrinoids for Chemical Sensor Applications. Chem. Rev. 2017, 117, 2517–2583. [Google Scholar] [CrossRef] [PubMed]
- Kalyanasundaram, K.; Grätzel, M. Applications of functionalized transition metal complexes in photonic and optoelectronic devices. Coord. Chem. Rev. 1998, 177, 347–414. [Google Scholar] [CrossRef]
- Roundhill, D.M.; Fackler, J.P., Jr. Photochemistry and Photophysics of Metal Complexes in Modern Inorganic Chemistry; Plenum Press: New York, NY, USA, 1994. [Google Scholar]
- Kalyanasundaram, K. Photophysics, photochemistry and solar energy conversion with tris(bipyridyl)ruthenium(II) and its analogues. Coord. Chem. Rev. 1982, 46, 159–244. [Google Scholar] [CrossRef]
- Bard, A.J.; Fox, M.A. Artificial Photosynthesis: Solar Splitting of Water to Hydrogen and Oxygen. Acc. Chem. Res. 1995, 28, 141–145. [Google Scholar] [CrossRef]
- Sutin, N.; Creutz, C. Light induced electron transfer reactions of metal complexes. Pure Appl. Chem. 1980, 52, 2717–2738. [Google Scholar] [CrossRef] [Green Version]
- Cook, M.J.; Lewis, A.P.; McAuliffe, G.S.G.; Skarda, V.; Thomson, A.J.; Glasper, J.L.; Robbins, D.J. Luminescent metal complexes. Part 2. A model for the luminescence properties of the tris-chelates of substituted 2,2′-bipyridyls with ruthenium (II). J. Chem. Soc. Perkin Trans. 2 1984, 8, 1303–1307. [Google Scholar] [CrossRef]
- De Carvalho, I.M.M.; de Sousa Moreira, I.; Gehlen, M.H. Synthesis, Characterization, and Photophysical Studies of New Bichromophoric Ruthenium(II) Complexes. Inorg. Chem. 2003, 42, 1525–1531. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, N.; Kawanishi, Y.; Tazuke, S. Spectroscopic and electrochemical studies on ruthenium(II) complexes containing diazadiimine ligands. Chem. Phys. Lett. 1983, 97, 103–105. [Google Scholar] [CrossRef]
- Rillema, D.P.; Allen, G.; Meyer, T.J.; Conrad, D. Redox properties of ruthenium(II) tris chelate complexes containing the ligands 2,2′-bipyrazine, 2,2′-bipyridine, and 2,2′-bipyrimidine. Inorg. Chem. 1983, 22, 1617–1622. [Google Scholar] [CrossRef]
- Ross, H.B.; Boldaji, M.; Rillema, D.P.; Blanton, C.B.; White, R.P. Photosubstitution in tris chelate complexes of ruthenium(II) containing the ligands 2,2′-bipyrazine, 2,2′-bipyrimidine, 2,2′-bipyridine, and 4,4′-dimethyl-2,2′-bipyridine: Energy gap control. Inorg. Chem. 1989, 28, 1013–1021. [Google Scholar] [CrossRef]
- Man’uel, D.J.; Strommen, D.P.; Bhuiyan, A.; Sykora, M.; Kincaid, J.R. Resonance Raman and Time-Resolved Resonance Raman Studies of Complexes of Divalent Ruthenium with Bipyridine and 4,4′-Bipyrimidine Ligands. J. Raman Spectrosc. 1998, 28, 933–938. [Google Scholar] [CrossRef]
- Ernst, S.; Kaim, W. d6-Metal Complexes of 4,4′-Bipyrimidine, an Ambident Ligand with High π-Acceptor Ability. Angew. Chem. Int. Ed. Engl. 1985, 24, 430–431. [Google Scholar] [CrossRef]
- Ernst, S.; Kaim, W.J. Energy level tailoring in ruthenium(II) polyazine complexes based on calculated and experimental ligand properties. Inorg. Chem. 1989, 28, 1520–1528. [Google Scholar] [CrossRef]
- Jing, B.W.; Wu, T.; Tian, C.H.; Zhang, M.H.; Shen, T. pH-Dependent Luminescence of Ruthenium(II) Polypyridine Complexes. Bull. Chem. Soc. Jpn. 2000, 73, 1749–1755. [Google Scholar] [CrossRef]
- Tan, L.F.; Wang, F.; Chao, H. Synthesis, DNA Binding, and DNA Photocleavage of the Ruthenium(II) Complexes [Ru(bpy)(btip)]2+ and [Ru(dmb)(btip)]2+ (bpy = 2,2′-Bipyridine; btip = 2-Benzo[b]thien-2-yl-1H-imidazo[4,5-f] [1,10]phenanthroline; dmb = 4,4′-Dimethyl-2,2′-bipyridine). Helv. Chim. Acta 2007, 90, 205–215. [Google Scholar] [CrossRef]
- Li, M.; Liu, J.; Sun, L.; Pan, J.; Zhao, C. Oligothiophene-2-yl-vinyl bridged mono- and binuclear ruthenium(II) tris-bipyridine complexes: Synthesis, photophysics, electrochemistry and electrogenerated chemiluminescence. J. Organomet. Chem. 2008, 693, 46–56. [Google Scholar] [CrossRef]
- Sivalingam, Y.; Martinelli, E.; Catini, A.; Magna, G.; Pomarico, G.; Basoli, F.; Paolesse, R.; Di Natale, C. Gas-Sensitive Photoconductivity of Porphyrin-Functionalized ZnO Nanorods. J. Phys. Chem. 2012, 116, 9151–9157. [Google Scholar] [CrossRef]
- Chizhov, A.S.; Mordvinova, N.E.; Rumyantseva, M.N.; Krylov, I.V.; Drozdov, K.A.; Li, X.; Gaskov, A.M. The effect of CdSe and InP quantum dots on the interaction of ZnO with NO2 under visible light irradiation. Russ. J. Inorg. Chem. 2018, 63, 512–518. [Google Scholar] [CrossRef]
- Messias, F.; Vega, B.; Scalvi, L.; Li, M.; Santilli, C.; Pulcinelli, S. Electron scattering and effects of sources of light on photoconductivity of SnO2 coatings prepared by sol–gel. J. Non-Cryst. Solids 1999, 247, 171–175. [Google Scholar] [CrossRef]
- Kornblit, L.; Ignatiev, A. Photodesorption threshold energies in semiconductors. Surf. Sci. Lett. 1984, 136, L57–L66. [Google Scholar] [CrossRef]
- Ganose, A.M.; Scanlon, D.O. Band gap and work function tailoring of SnO2 for improved transparent conducting ability in photovoltaic. J. Mater. Chem. C 2016, 4, 1467–1475. [Google Scholar] [CrossRef]
- Zhou, Z.; Gao, H.; Liu, R.; Du, B. Study of structure and property for the NO2 + NO2− electron transfer system. J. Mol. Struct. THEOCHEM 2001, 545, 179–186. [Google Scholar] [CrossRef]
- Šulka, M.; Pitoňák, M.; Neogrády, P.; Urban, M. Electron affinity of the O2 molecule: CCSD(T) calculations using the optimized virtual orbitals space approach. Int. J. Quantum Chem. 2008, 108, 2159–2171. [Google Scholar] [CrossRef]
- Forsh, E.A.; Abakumov, A.M.; Zaytsev, V.B.; Konstantinova, E.A.; Forsh, P.A.; Rumyantseva, M.N.; Gaskov, A.M.; Kashkarov, P.K. Optical and photoelectrical properties of nanocrystalline indium oxide with small grains. Thin Solid Films 2015, 595, 25–31. [Google Scholar] [CrossRef]
- Prades, D.; Arbiol, J.; Cirera, A.; Morante, J.R.; Avella, M.; Zanotti, L.; Comini, E.; Faglia, G.; Sberveglieri, G. Defect study of SnO2 nanostructures by cathodoluminescence analysis: Application to nanowires. Sens. Actuators B 2007, 126, 6–12. [Google Scholar] [CrossRef] [Green Version]
- Bârsan, N.; Weimar, U. Conduction Model of Metal Oxide Gas Sensors. J. Electroceram. 2001, 7, 143–167. [Google Scholar] [CrossRef]
- Shuzhang, X.; Tao, Y.; Yifeng, Z.; Zhao, Q.; Fuyou, L.; Chunhui, H. Multi-state molecular switches based on dithienylperfluorocyclopentene and imidazo[4,5-f] [1,10]phenanthroline. Tetrahedron 2006, 62, 10072–10078. [Google Scholar] [CrossRef]
- Batista, R.M.F.; Costa, S.P.G.; Belsley, M.; Lodeiro, C.; Raposo, M.M.M. Synthesis and characterization of novel (oligo)thienyl-imidazo-phenanthrolines as versatile p-conjugated systems for several optical applications. Tetrahedron 2008, 64, 9230–9238. [Google Scholar] [CrossRef]
- Cai, Z.; Zhou, M.; Xu, J. Degenerate four-wave mixing determination of third-order optical nonlinear of three mixed ligand nickel (II) complexes. J. Mol. Struct. 2011, 1006, 282–287. [Google Scholar] [CrossRef]
- Li, Z.; Yang, H.; Zhang, A.; Luo, H.; Wang, K. pH effects on optical and DNA binding properties of a thiophene-containing ruthenium(II) complex. Inorg. Chim. Acta 2011, 370, 132–140. [Google Scholar] [CrossRef]
- Rumyantseva, M.; Makeeva, E.; Gaskov, A.; Shepel, N.; Peregudova, S.; Khoroshutin, A.; Tokarev, S.; Fedorova, O. H2S sensing by hybrids based on nanocrystalline SnO2 functionalized with Cu(II) organometallic complexes: The role of the ligand platform. Nanomaterials 2017, 7, 384. [Google Scholar] [CrossRef] [PubMed]








| E1/2(red), V | E1/2(ox), V | EHOMO, eV | ELUMO, eV |
|---|---|---|---|
| −1.27/−1.21 | 1.23 | −6.15 | −3.46 |
| −1.48/−1.41 | 1.42 | ||
| −1.96/−1.84 | 1.52 |
| Sample | dXRD a, nm | dTEM b, nm | Ssurf c, m2/g | SPh e in Pure Air (λ = 470 nm) | |
|---|---|---|---|---|---|
| SnO2 | 4 ± 1 | 4 ± 1 | 110 ± 5 | - | 1.00 |
| SnO2 Ru-TT | 1.4 ± 0.1 | 2.72 | |||
| In2O3 | 7 ± 1 | 7 ± 2 | 60 ± 5 | - | 1.30 |
| In2O3 Ru-TT | 2.1 ± 0.2 | 3.15 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rumyantseva, M.; Nasriddinov, A.; Vladimirova, S.; Tokarev, S.; Fedorova, O.; Krylov, I.; Drozdov, K.; Baranchikov, A.; Gaskov, A. Photosensitive Organic-Inorganic Hybrid Materials for Room Temperature Gas Sensor Applications. Nanomaterials 2018, 8, 671. https://doi.org/10.3390/nano8090671
Rumyantseva M, Nasriddinov A, Vladimirova S, Tokarev S, Fedorova O, Krylov I, Drozdov K, Baranchikov A, Gaskov A. Photosensitive Organic-Inorganic Hybrid Materials for Room Temperature Gas Sensor Applications. Nanomaterials. 2018; 8(9):671. https://doi.org/10.3390/nano8090671
Chicago/Turabian StyleRumyantseva, Marina, Abulkosim Nasriddinov, Svetlana Vladimirova, Sergey Tokarev, Olga Fedorova, Ivan Krylov, Konstantin Drozdov, Alexander Baranchikov, and Alexander Gaskov. 2018. "Photosensitive Organic-Inorganic Hybrid Materials for Room Temperature Gas Sensor Applications" Nanomaterials 8, no. 9: 671. https://doi.org/10.3390/nano8090671