Characteristics of Graphene Oxide Films Reduced by Using an Atmospheric Plasma System
Abstract
:1. Introduction
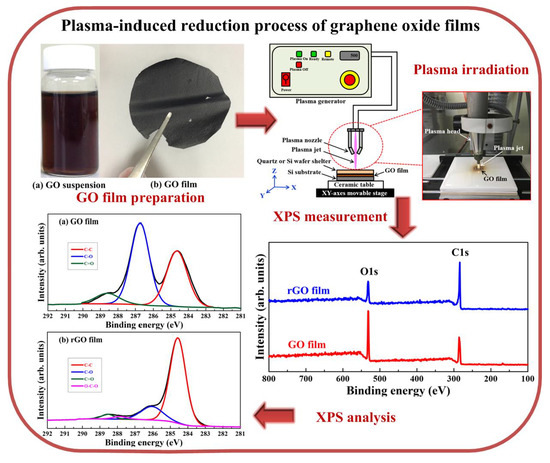
2. Experimental Details
3. Results and Discussion
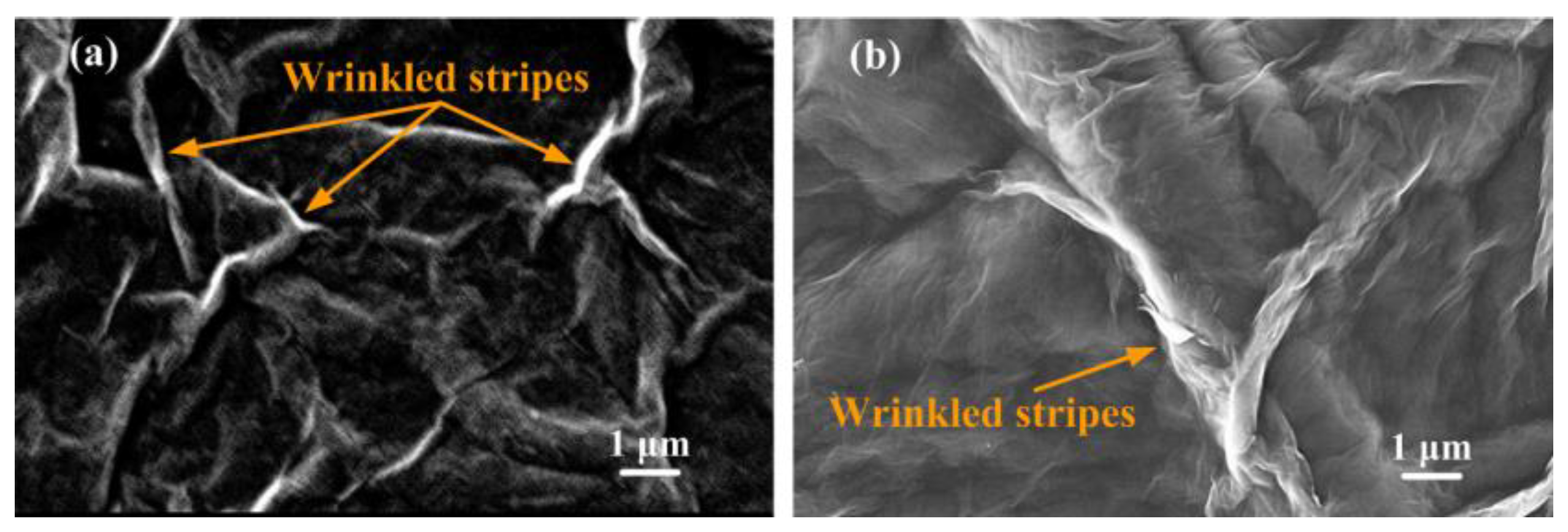
3.1. Surface Morphologies of GO Films before and after Plasma Reduction
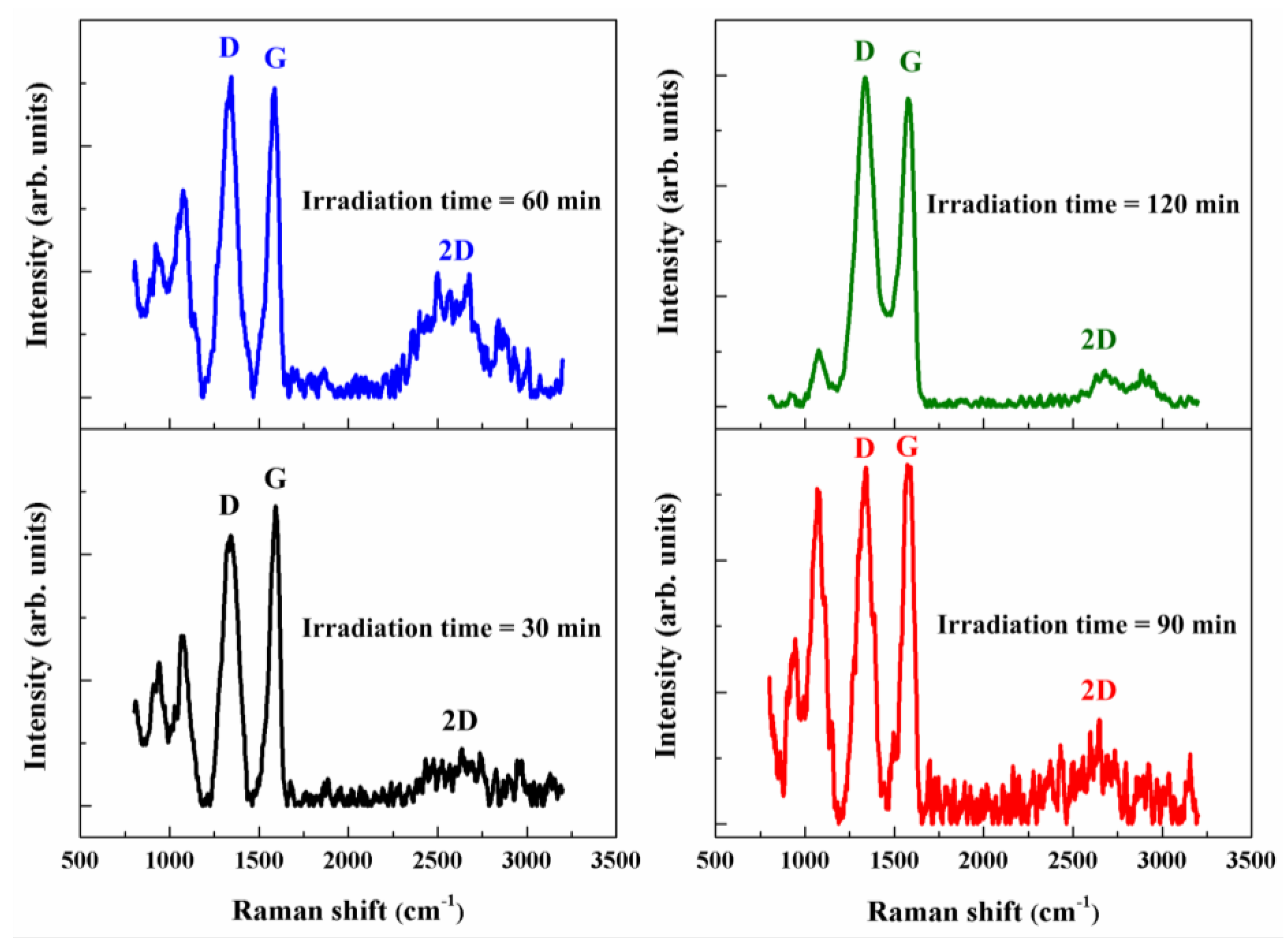
3.2. Raman Spectra Analysis
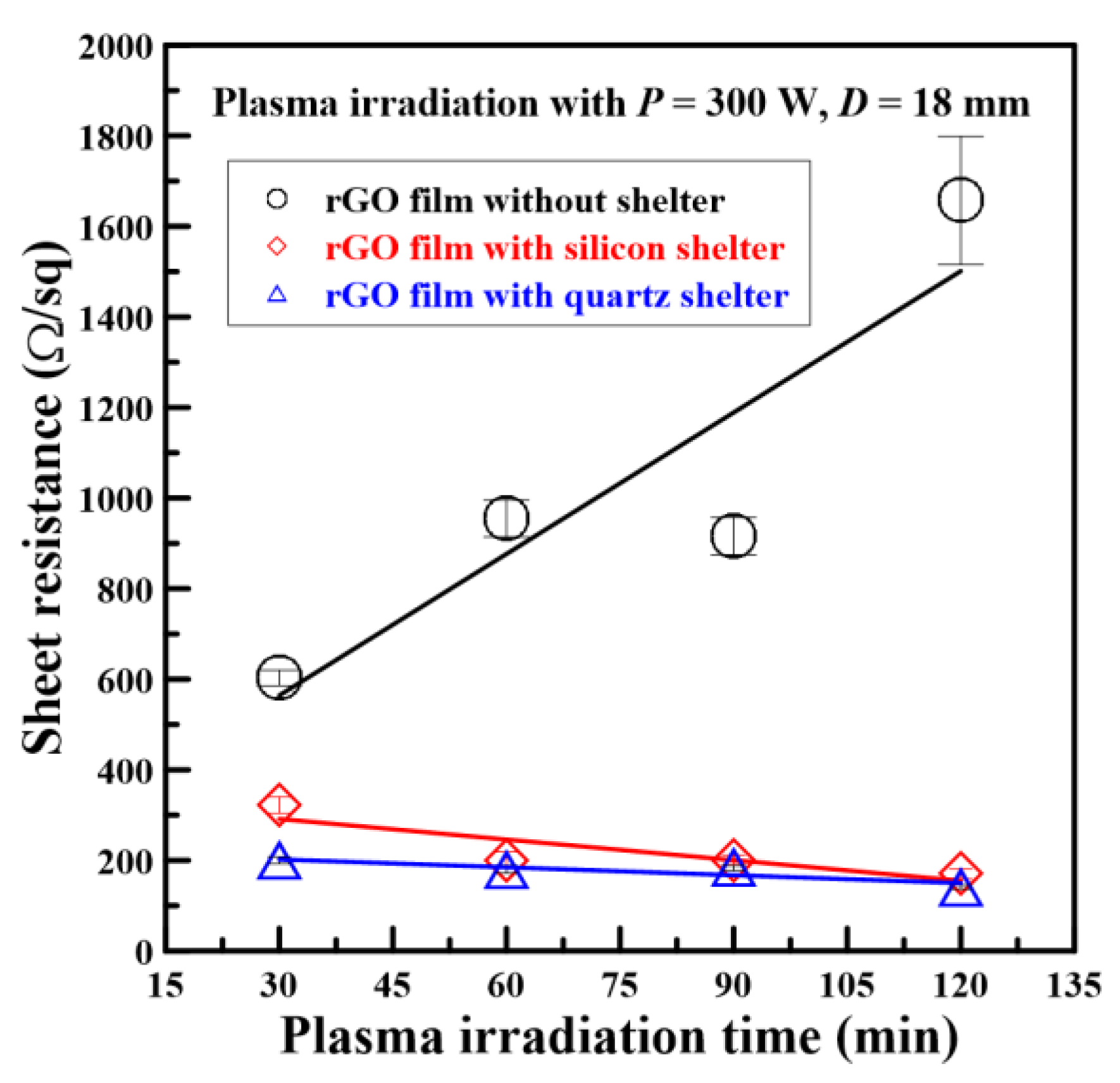
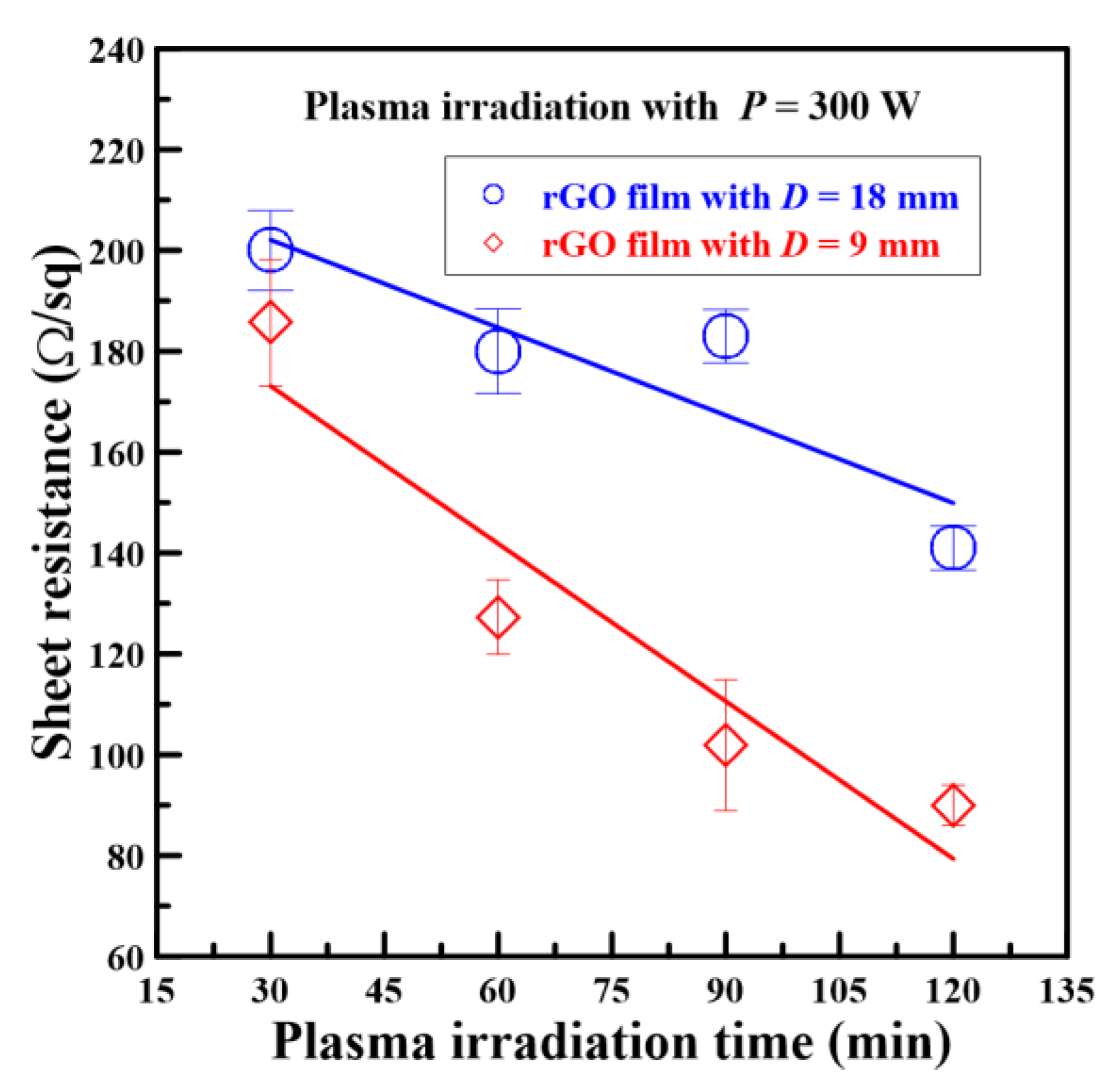
3.3. Electrical Resistance Analysis
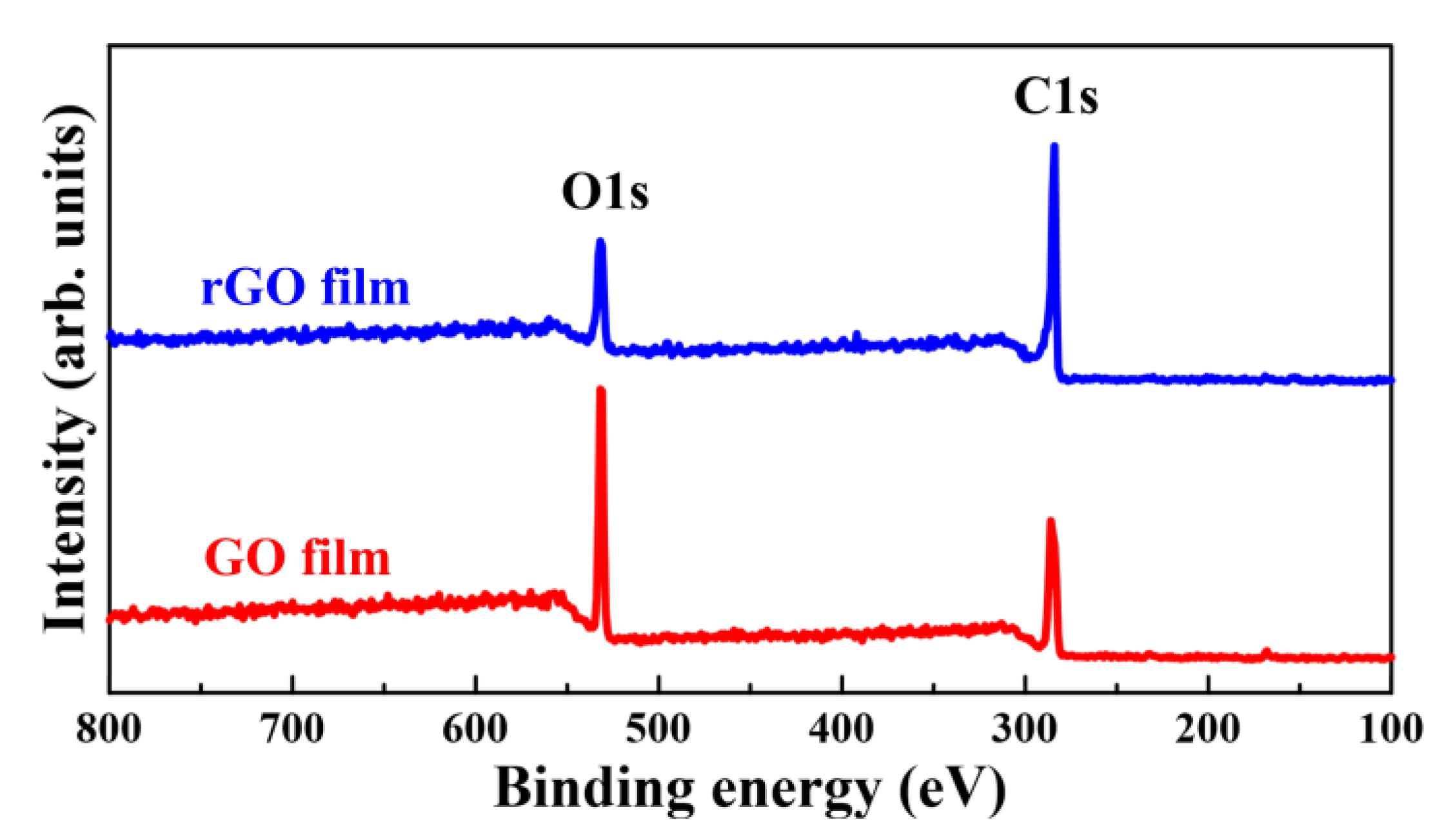
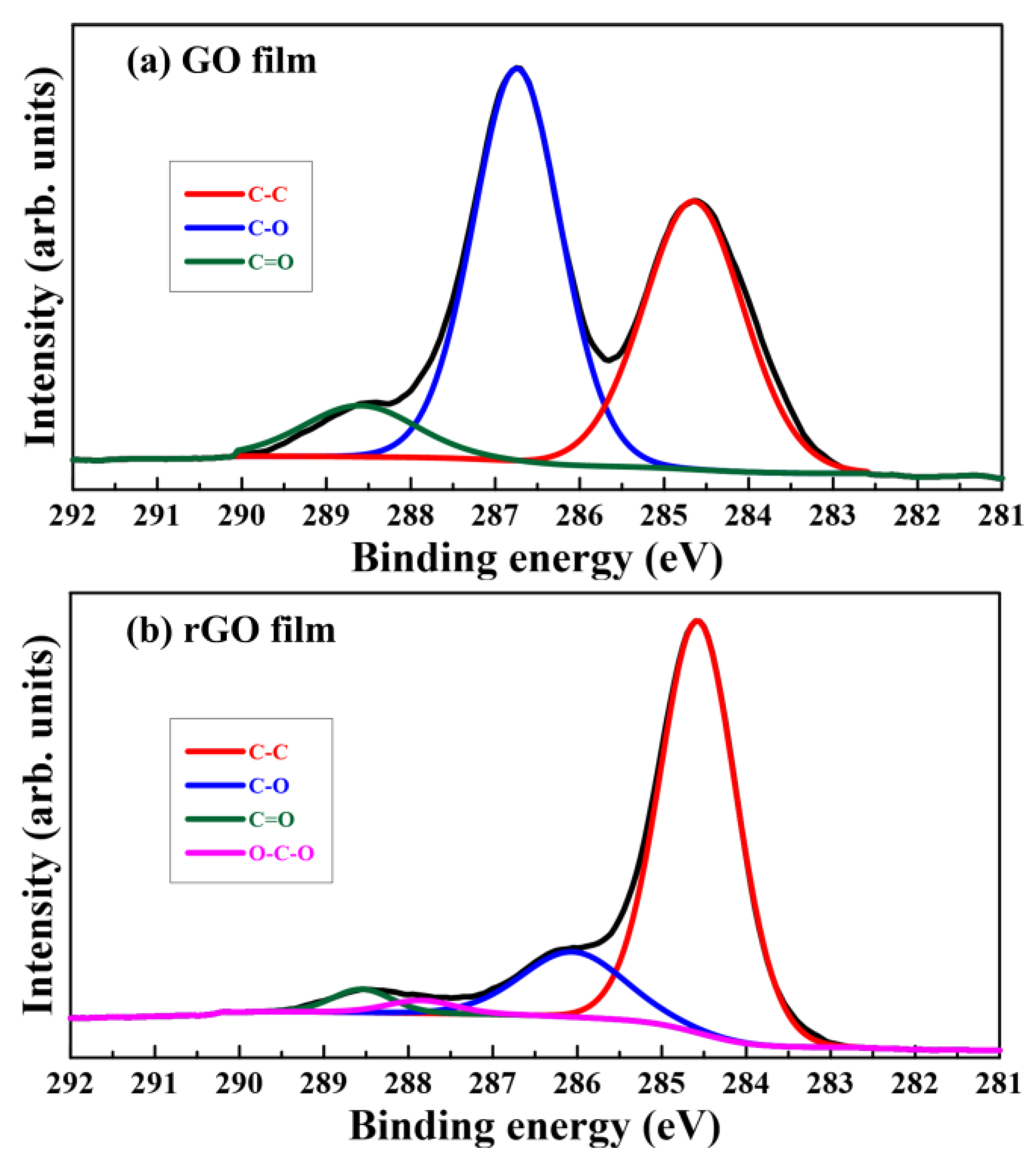
3.4. XPS Analysis
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K. Graphene: status and prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Kim, H.; Lee, Y.; Xu, X.; Park, J.S.; Zheng, Y.; Balakrishnan, J.; Lei, T.; Kim, H.R.; Song, Y.I.; et al. Roll-to-roll production of 30-inch graphene films for transparent electrodes. Nat. Nanotechnol. 2010, 5, 574–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Sun, K.; Tao, F.; Stacchiola, D.J.; Hu, Y.H. 3D honeycomb-like structured graphene and its high efficiency as a counter-electrode catalyst for dye-sensitized solar cells. Angew. Chem. Int. Ed. 2013, 52, 9210–9214. [Google Scholar] [CrossRef] [PubMed]
- Hyun, W.J.; Park, O.O.; Chin, B.D. Foldable graphene electronic circuits based on paper substrates. Adv. Mater. 2013, 25, 4729–4734. [Google Scholar] [CrossRef] [PubMed]
- Prasai, D.; Tuberquia, J.C.; Harl, R.R.; Jennings, G.K.; Bolotin, K.I. Graphene: Corrosion-Inhibiting Coating. ACS Nano 2012, 6, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.C.; Huang, J.H.; Li, C.P.; Chang-Jian, C.W.; Lee, K.C.; Hsiao, Y.S.; Huang, J.H. Graphene-based thermoplastic composites and their application for LED thermal management. Carbon 2016, 102, 66–73. [Google Scholar] [CrossRef]
- Ke, Q.; Wang, J. Graphene-based materials for supercapacitor electrodes—A review. J. Materiomics 2016, 2, 37–54. [Google Scholar] [CrossRef]
- Dunst, K.; Author Vitae Jurków, D.; Author Vitae Jasiński, P. Laser patterned platform with PEDOT-graphene composite film for NO2 sensing. Sens. Actuators B Chem. 2016, 229, 155–165. [Google Scholar] [CrossRef]
- Tseng, S.F.; Hsiao, W.T.; Cheng, P.Y.; Chung, C.K.; Lin, Y.S.; Chien, S.C.; Huang, W.Y. Graphene-based chips fabricated by ultraviolet laser patterning for an electrochemical impedance spectroscopy. Actuators B Chem. 2016, 226, 342–348. [Google Scholar] [CrossRef]
- Tseng, S.F. Picosecond laser micropatterning of graphene films for rapid heating chips. Appl. Surf. Sci. 2018, 450, 380–386. [Google Scholar] [CrossRef]
- Singh, V.; Joung, D.; Zhai, L.; Das, S.; Khondaker, S.I.; Seal, S. Graphene based materials: Past, present and future. Prog. Mater. Sci. 2011, 56, 1178–1271. [Google Scholar] [CrossRef]
- Ouerghi, A.; Ridene, M.; Mathieu, C.; Gogneau, N.; Belkhou, R. From nanographene to monolayer graphene on 6H-SiC (0001) substrate. Appl. Phys. Lett. 2013, 102, 253108. [Google Scholar] [CrossRef]
- Su, C.Y.; Lu, A.Y.; Xu, Y.; Chen, F.R.; Khlobystov, A.N.; Li, L.J. High-quality thin graphene films from fast electrochemical exfoliation. ACS Nano 2011, 5, 2332–2339. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Parvez, M.K.; Chhowalla, M. UV-reduction of graphene oxide and its application as an interfacial layer to reduce the back-transport reactions in dye-sensitized solar cells. Chem. Phys. Lett. 2009, 483, 124–127. [Google Scholar] [CrossRef]
- Ciesielski, A.; Samori, P. Graphene via sonication assisted liquid-phase exfoliation. Chem. Soc. Rev. 2014, 43, 381–398. [Google Scholar] [CrossRef] [PubMed]
- Buglione, L.; Chng, E.L.K.; Ambrosi, A.; Sofer, Z.; Pumera, M. Graphene materials preparation methods have dramatic influence upon their capacitance. Electrochem. Commun. 2012, 14, 5–8. [Google Scholar] [CrossRef]
- Schniepp, H.C.; Li, J.L.; McAllister, M.J.; Sai, H.; Herrera-Alonso, M.; Adamson, D.H.; Prud’homme, R.K.; Car, R.; Saville, D.A.; Aksay, I.A. Functionalized single graphene sheets derived from splitting graphite oxide. J. Phys. Chem. B 2006, 110, 8535–8539. [Google Scholar] [CrossRef] [PubMed]
- Ramesha, G.K.; Sampath, S. Electrochemical reduction of oriented graphene oxide films: an in situ raman spectroelectrochemical study. J. Phys. Chem. C 2009, 113, 7985–7989. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.B.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Williams, G.; Seger, B.; Kamat, P.V. TiO2-graphene nanocomposites. UV-assisted photocatalytic reduction of graphene oxide. ACS Nano 2008, 2, 1487–1491. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Murali, S.; Stoller, M.D.; Velamakanni, A.; Piner, R.D.; Ruoff, R.S. Microwave assisted exfoliation and reduction of graphite oxide for ultracapacitors. Carbon 2010, 48, 2118–2122. [Google Scholar] [CrossRef]
- Kasischke, M.; Maragkaki, S.; Volz, S.; Ostendorf, A.; Gurevich, E.L. Simultaneous nanopatterning and reduction of graphene oxide by femtosecond laser pulses. Appl. Surf. Sci. 2018, 445, 197–203. [Google Scholar] [CrossRef] [Green Version]
- Baraket, M.; Walton, S.G.; Wei, Z.; Lock, E.H.; Robinson, J.T.; Sheehan, P. Reduction of graphene oxide by electron beam generated plasmas produced in methane/argon mixtures. Carbon 2010, 48, 3382–3390. [Google Scholar] [CrossRef]
- Dey, A.; Krishnamurthy, S.; Bowen, J.; Nordlund, D.; Meyyappan, M.; Gandhiraman, R.P. Plasma jet printing and in situ reduction of highly acidic graphene oxide. ACS Nano 2018, 12, 5473–5481. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.R.; Tseng, S.F.; Chen, Y.T. Laser-induced reduction of graphene oxide powders by high pulsed ultraviolet laser irradiations. Appl. Surf. Sci. 2018, 444, 578–583. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Le, H.D.; Nguyen, V.C.; Ngo, T.T.T.; Le, D.Q.; Nguyen, X.N.; Phan, N.M. Synthesis of multi-layer graphene films on copper tape by atmospheric pressure chemical vapor deposition method. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4, 035012. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Jang, J.; Nagase, S. Hydrazine and thermal reduction of graphene oxide: reaction mechanisms, product structures, and reaction design. J. Phys. Chem. C 2010, 114, 832–842. [Google Scholar] [CrossRef]

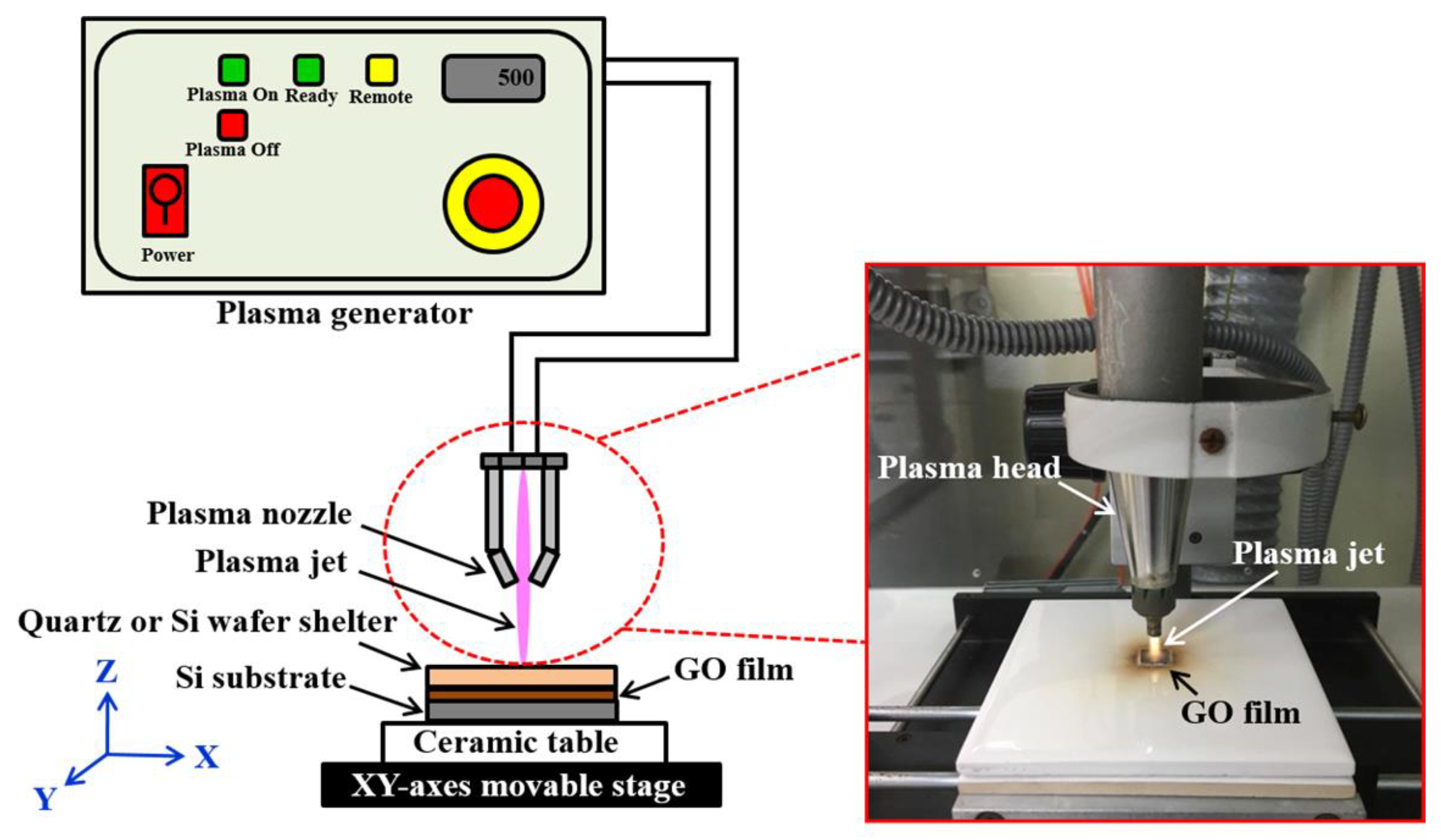


| Plasma Irradiation Parameters | Values or Methods | |||
|---|---|---|---|---|
| Plasma output power (W) | 300 | |||
| Plasma irradiation time (min) | 30 | 60 | 90 | 120 |
| Working distance (mm) | 9 | 18 | ||
| Shelter meterial | Quartz | Si wafer | ||
| Processing gas | Dry pressured air | |||
| Air pressure (kg/cm2) | 5 | |||
| Film Types | GO | rGO | |||
|---|---|---|---|---|---|
| Plasma irradiation time (min) | - | 30 | 60 | 90 | 120 |
| ID/IG | 0.77 | 0.89 | 1.08 | 0.97 | 1.09 |
| I2D/IG | - | 0.08 | 0.23 | 0.19 | 0.05 |
| Shelter Types | Silicon | Quartz | ||||||
|---|---|---|---|---|---|---|---|---|
| Plasma irradiation time (min) | 30 | 60 | 90 | 120 | 30 | 60 | 90 | 120 |
| ID/IG | 0.93 | 0.97 | 0.99 | 0.98 | 1 | 0.93 | 0.95 | 0.93 |
| I2D/IG | × | 0.4 | 0.3 | 0.34 | 0.1 | 0.2 | 0.21 | 0.3 |
| Film Types | GO | rGO | ||
|---|---|---|---|---|
| Shelter types | - | Silicon | Quartz | |
| Working distance (mm) | - | 18 | 18 | 9 |
| Oxygen content (%) | 75.27 | 42.91 | 43.53 | 39.65 |
| Carbon content (%) | 24.73 | 57.09 | 56.47 | 60.35 |
| C/O ratio | 0.329 | 1.33 | 1.297 | 1.522 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.-R.; Tseng, S.-F.; Chen, Y.-T. Characteristics of Graphene Oxide Films Reduced by Using an Atmospheric Plasma System. Nanomaterials 2018, 8, 802. https://doi.org/10.3390/nano8100802
Yang C-R, Tseng S-F, Chen Y-T. Characteristics of Graphene Oxide Films Reduced by Using an Atmospheric Plasma System. Nanomaterials. 2018; 8(10):802. https://doi.org/10.3390/nano8100802
Chicago/Turabian StyleYang, Chii-Rong, Shih-Feng Tseng, and Yu-Ting Chen. 2018. "Characteristics of Graphene Oxide Films Reduced by Using an Atmospheric Plasma System" Nanomaterials 8, no. 10: 802. https://doi.org/10.3390/nano8100802