Au Nanoparticles as Template for Defect Formation in Memristive SrTiO3 Thin Films
Abstract
:1. Introduction
2. Results
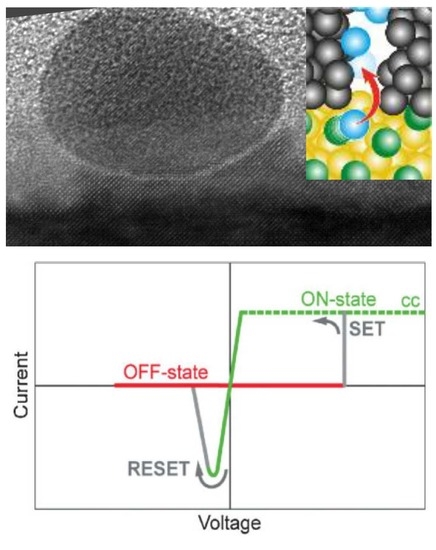
2.1. SrTiO Growth on Au Nanoparticles
2.2. Nanoscale Switching Properties
3. Discussion
4. Materials and Methods
4.1. Formation of AuNPs
4.2. SrTiO Thin Film Growth
4.3. Atomic Force Microscopy
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Waser, R.; Dittmann, R.; Staikov, G.; Szot, K. Redox-Based Resistive Switching Memories—Nanoionic Mechanisms, Prospects, and Challenges. Adv. Mater. 2009, 21, 2632–2663. [Google Scholar] [CrossRef]
- Ielmini, D.; Waser, R. Resistive Switching—From Fundamentals of Nanoionic Redox Processes to Memristive Device Applications; Wiley-VCH: Weinheim, Germany, 2016. [Google Scholar]
- Burr, G.W.; Shelby, R.M.; Sebastian, A.; Kim, S.; Kim, S.; Sidler, S.; Virwani, K.; Ishii, M.; Narayanan, P.; Fumarola, A.; et al. Neuromorphic computing using non-volatile memory. Adv. Phys. X 2016, 2, 89–124. [Google Scholar] [CrossRef] [Green Version]
- Baeumer, C.; Schmitz, C.; Marchewka, A.; Mueller, D.N.; Valenta, R.; Hackl, J.; Raab, N.; Rogers, S.P.; Khan, M.I.; Nemsak, S.; et al. Quantifying redox-induced Schottky barrier variations in memristive devices via in operando spectromicroscopy with graphene electrodes. Nat. Commun. 2016, 7, 12398. [Google Scholar] [CrossRef] [PubMed]
- Baeumer, C.; Dittmann, R. Redox-based memristive metal-oxide devices. In Metal Oxide-Based Thin Film Structures; Korotcenkov, N.P.V.E.G., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 489–522. [Google Scholar]
- Sharath, S.U.; Bertaud, T.; Kurian, J.; Hildebrandt, E.; Walczyk, C.; Calka, P.; Zaumseil, P.; Sowinska, M.; Walczyk, D.; Gloskovskii, A.; Schroeder, T.; Alff, L. Towards forming-free resistive switching in oxygen engineered HfO2-x. Appl. Phys. Lett. 2014, 104, 063502. [Google Scholar] [CrossRef]
- Sharath, S.U.; Kurian, J.; Komissinskiy, P.; Hildebrandt, E.; Bertaud, T.; Walczyk, C.; Calka, P.; Schroeder, T.; Alff, L. Thickness independent reduced forming voltage in oxygen engineered HfO2 based resistive switching memories. Appl. Phys. Lett. 2014, 105, 73505. [Google Scholar] [CrossRef]
- Sharath, S.U.; Joseph, M.J.; Vogel, S.; Hildebrandt, E.; Komissinskiy, P.; Kurian, J.; Schroeder, T.; Alff, L. Impact of oxygen stoichiometry on electroforming and multiple switching modes in TiN/TaOx/Pt based ReRAM. Appl. Phys. Lett. 2016, 109, 173503. [Google Scholar] [CrossRef]
- Skaja, K.; Andrae, M.; Rana, V.; Waser, R.; Dittmann, R.; Baeumer, C. Reduction of the forming voltage through tailored oxygen non-stoichiometry in tantalum oxide ReRAM devices. Sci. Rep. 2018, 8, 10861. [Google Scholar] [CrossRef] [PubMed]
- Aslam, N.; Longo, V.; Keuning, W.; Roozeboom, F.; Kessels, W.; Waser, R.; Hoffmann-Eifert, S. Influence of stoichiometry on the performance of MIM capacitors from plasma-assisted ALD SrxTiyOz films. Phys. Status Solidi 2014, 211, 389–396. [Google Scholar] [CrossRef]
- Raab, N.; Bäumer, C.; Dittmann, R. Impact of the cation-stoichiometry on the resistive switching and data retention of SrTiO3 thin films. AIP Adv. 2015, 5, 047150. [Google Scholar] [CrossRef]
- Szot, K.; Speier, W.; Bihlmayer, G.; Waser, R. Switching the electrical resistance of individual dislocations in single-crystalline SrTiO3. Nat. Mater. 2006, 5, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Lenser, C.; Connell, Z.; Kovács, A.; Dunin-Borkowski, R.; Köhl, A.; Waser, R.; Dittmann, R. Identification of screw dislocations as fast-forming sites in Fe-doped SrTiO3. Appl. Phys. Lett. 2013, 102, 183504. [Google Scholar] [CrossRef]
- Lanza, M.; Bersuker, G.; Porti, M.; Miranda, E.; Nafria, M.; Aymerich, X. Resistive switching in hafnium dioxide layers: Local phenomenon at grain boundaries. Appl. Phys. Lett. 2012, 101, 193502. [Google Scholar] [CrossRef] [Green Version]
- Shibuya, K.; Dittmann, R.; Mi, S.; Waser, R. Impact of Defect Distribution on Resistive Switching Characteristics of Sr2TiO4 Thin Films. Adv. Mater. 2010, 22, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Niu, G.; Calka, P.; der Maur, M.A.; Santoni, F.; Guha, S.; Fraschke, M.; Hamoumou, P.; Gautier, B.; Perez, E.; Walczyk, C.; et al. Geometric conductive filament confinement by nanotips for resistive switching of HfO2-RRAM devices with high performance. Sci. Rep. 2016, 6, 25757. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, Y.; Himeno, A.; Yasuhara, R.; Boullart, W.; Vecchio, E.; Vandeweyer, T.; Witters, T.; Crotti, D.; Jurczak, M.; Fujii, S.; et al. Highly reliable TaOx ReRAM with centralized filament for 28-nm embedded application. In Proceedings of the 2015 Symposium on VLSI Technology (VLSI Technology), Kyoto, Japan, 16–18 June 2015. [Google Scholar]
- Katzer, C.; Schmidt, M.; Michalowski, P.; Kuhwald, D.; Schmidl, F.; Grosse, V.; Treiber, S.; Stahl, C.; Albrecht, J.; Huebner, U.; et al. Increased flux pinning in YBa2Cu3O7-delta thin-film devices through embedding of Au nano crystals. Europhys. Lett. 2011, 95, 68005. [Google Scholar] [CrossRef]
- Katzer, C.; Stahl, C.; Michalowski, P.; Treiber, S.; Schmidl, F.; Seidel, P.; Albrecht, J.; Schuetz, G. Gold nanocrystals in high-temperature superconducting films: creation of pinning patterns of choice. New J. Phys. 2013, 15, 113029. [Google Scholar] [CrossRef] [Green Version]
- Christke, S.; Katzer, C.; Grosse, V.; Schmidl, F.; Schmidl, G.; Fritzsche, W.; Petschulat, J.; Pertsch, T.; Rettenmayr, M. Optical resonances of self-organized monocrystalline Au nanoparticles embedded in SrTiO3 matrix. Opt. Mater. Express 2011, 1, 890–897. [Google Scholar] [CrossRef]
- Bernhardt, H.; Diener, R.; Sungur, P.; Katzer, C.; Schmidl, G.; Huebner, U.; Uschmann, I.; Fritzsche, W.; Schmidl, F. Engineering crystalline Au nanoparticles of anisotropic shape in epitaxially grown high-index SrTiO3. J. Mater. Sci. 2015, 50, 5562–5570. [Google Scholar] [CrossRef]
- Brown, K.R.; Walter, D.G.; Natan, M.J. Seeding of Colloidal Au Nanoparticle Solutions. 2. Improved Control of Particle Size and Shape. Chem. Mater. 2000, 12, 306–313. [Google Scholar] [CrossRef]
- Ye, T.; Chen, X.; Fan, X.; Shen, Z. Ordered gold nanoparticle arrays obtained with supramolecular block copolymers. Soft Matter 2013, 9, 4715–4724. [Google Scholar] [CrossRef]
- Ghalgaoui, A.; Doudin, N.; Calaza, F.; Surnev, S.; Sterrer, M. Ordered Au Nanoparticle Array on Au(111) through Coverage Control of Precursor Metal–Organic Chains. J. Phys. Chem. C 2016, 120, 17418–17426. [Google Scholar] [CrossRef]
- Baeumer, C.; Xu, C.; Gunkel, F.; Raab, N.; Heinen, R.A.; Koehl, A.; Dittmann, R. Surface Termination Conversion during SrTiO3 Thin Film Growth Revealed by X-ray Photoelectron Spectroscopy. Sci. Rep. 2015, 5, 11829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohnishi, T.; Lippmaa, M.; Yamamoto, T.; Meguro, S.; Koinuma, H. Improved stoichiometry and misfit control in perovskite thin film formation at a critical fluence by pulsed laser deposition. Appl. Phys. Lett. 2005, 87, 241919. [Google Scholar] [CrossRef]
- Du, H.; Jia, C.; Mayer, J.; Barthel, J.; Lenser, C.; Dittmann, R. Atomic Structure of Antiphase Nanodomains in Fe-doped SrTiO3 films. Adv. Funct. Mater. 2015, 25, 6369–6373. [Google Scholar] [CrossRef]
- Muenstermann, R.; Menke, T.; Dittmann, R.; Waser, R. Coexistence of Filamentary and Homogeneous Resistive Switching in Fe-doped SrTiO3 Thin-Film Memristive Devices. Adv. Mater. 2010, 22, 4819–4822. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.; Baeumer, C.; Bernier, N.; Marchewka, A.; Torre, C.L.; Dunin-Borkowski, R.E.; Menzel, S.; Waser, R.; Dittmann, R. Anomalous Resistance Hysteresis in Oxide ReRAM: Oxygen Evolution and Reincorporation Revealed by in situ TEM. Adv. Mater. 2017, 29, 1700212. [Google Scholar] [CrossRef] [PubMed]
- Heisig, T.; Baeumer, C.; Gries, U.N.; Mueller, M.P.; Torre, C.L.; Luebben, M.; Raab, N.; Du, H.; Menzel, S.; Mueller, D.N.; et al. Oxygen exchange processes between oxide memristive devices and water molecules. Adv. Mater. 2018, 30, 1800957–1800964. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Kanki, T.; Hirooka, M.; Takagi, A.; Matsumoto, T.; Tanaka, H.; Kawai, T. Relaxation of nanopatterns on Nb-doped SrTiO3 surface. Appl. Phys. Lett. 2004, 84, 2670–2672. [Google Scholar] [CrossRef]
- Pellegrino, L.; Bellingeri, E.; Pallecchi, I.; Siri, A.S.; Marre, D. Submicrometric SrTiO3-x based devices realized by an atomic force microscope. Solid-State Electron. 2003, 47, 2193–2198. [Google Scholar] [CrossRef]
- Pellegrino, L.; Bellingeri, E.; Siri, A.S.; Marre, D. Current-controlled lithography on conducting SrTiO3-d thin films by atomic force microscopy. Appl. Phys. Lett. 2005, 87, 64102. [Google Scholar] [CrossRef]
- Chiabrera, F.; Morata, A.; Pacios, M.; Tarancon, A. Insights into the enhancement of oxygen mass transport properties of strontium-doped lanthanum manganite interface-dominated thin films. Solid State Ion. 2017, 299, 70–77. [Google Scholar] [CrossRef]
- Lenser, C.; Patt, M.; Menzel, S.; Köhl, A.; Wiemann, C.; Schneider, C.M.; Waser, R.; Dittmann, R. Insights into Nanoscale Electrochemical Reduction in a Memristive Oxide: The Role of Three-Phase Boundaries. Adv. Funct. Mater. 2014, 24, 4466–4472. [Google Scholar] [CrossRef]
- Muenstermann, R.; Menke, T.; Dittmann, R.; Mi, S.; Jia, C.L.; Park, D.; Mayer, J. Correlation between growth kinetics and nanoscale resistive switching properties of SrTiO3 thin films. J. Appl. Phys. 2010, 108, 124504. [Google Scholar] [CrossRef]
- Schaal, P.A.; Simon, U. Guided immobilisation of single gold nanoparticles by chemical electron beam lithography. Beilstein J. Nanotechnol. 2013, 4, 336–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processesin the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 180, 55–75. [Google Scholar] [CrossRef]
- Gilles, S. Nanoimprint Lithographie als Methode zur Chemischen Oberflaechenstrukturierung für Anwendungen in der Bioelektronik. Ph.D. Thesis, RWTH Aachen, Aachen, Germany, 2010. [Google Scholar]
- Irissou, E.; Denis, M.C.; Chaker, M.; Guay, D. Gold oxide thin film grown by pulsed laser deposition in an O2 atmosphere. Thin Solid Films 2005, 472, 49–57. [Google Scholar] [CrossRef]
- Dick, K.; Dhanasekaran, T.; Zhang, Z.; Meisel, D. Size-Dependent Melting of Silica-Encapsulated Gold Nanoparticles. J. Am. Chem. Soc. 2002, 124, 2312–2317. [Google Scholar] [CrossRef] [PubMed]
- Buffat, P.; Borel, J.P. Size effect on the melting temperature of gold particles. Phys. Rev. A 1976, 13, 2287–2298. [Google Scholar] [CrossRef]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raab, N.; Schmidt, D.O.; Du, H.; Kruth, M.; Simon, U.; Dittmann, R. Au Nanoparticles as Template for Defect Formation in Memristive SrTiO3 Thin Films. Nanomaterials 2018, 8, 869. https://doi.org/10.3390/nano8110869
Raab N, Schmidt DO, Du H, Kruth M, Simon U, Dittmann R. Au Nanoparticles as Template for Defect Formation in Memristive SrTiO3 Thin Films. Nanomaterials. 2018; 8(11):869. https://doi.org/10.3390/nano8110869
Chicago/Turabian StyleRaab, Nicolas, Dirk Oliver Schmidt, Hongchu Du, Maximilian Kruth, Ulrich Simon, and Regina Dittmann. 2018. "Au Nanoparticles as Template for Defect Formation in Memristive SrTiO3 Thin Films" Nanomaterials 8, no. 11: 869. https://doi.org/10.3390/nano8110869