Use of Electrosprayed Agave Fructans as Nanoencapsulating Hydrocolloids for Bioactives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Formulation
2.3. Characterization of Different Solutions and Emulsions
2.4. Preparation of Capsules by Electrospraying
2.5. Scanning Electron Microscopy (SEM)
2.6. Fourier Transform Infrared Spectroscopy
2.7. Thermogravimetric Analysis (TGA)
2.8. Ultraviolet (UV) Photostability
3. Results and Discussion
3.1. Solution Properties
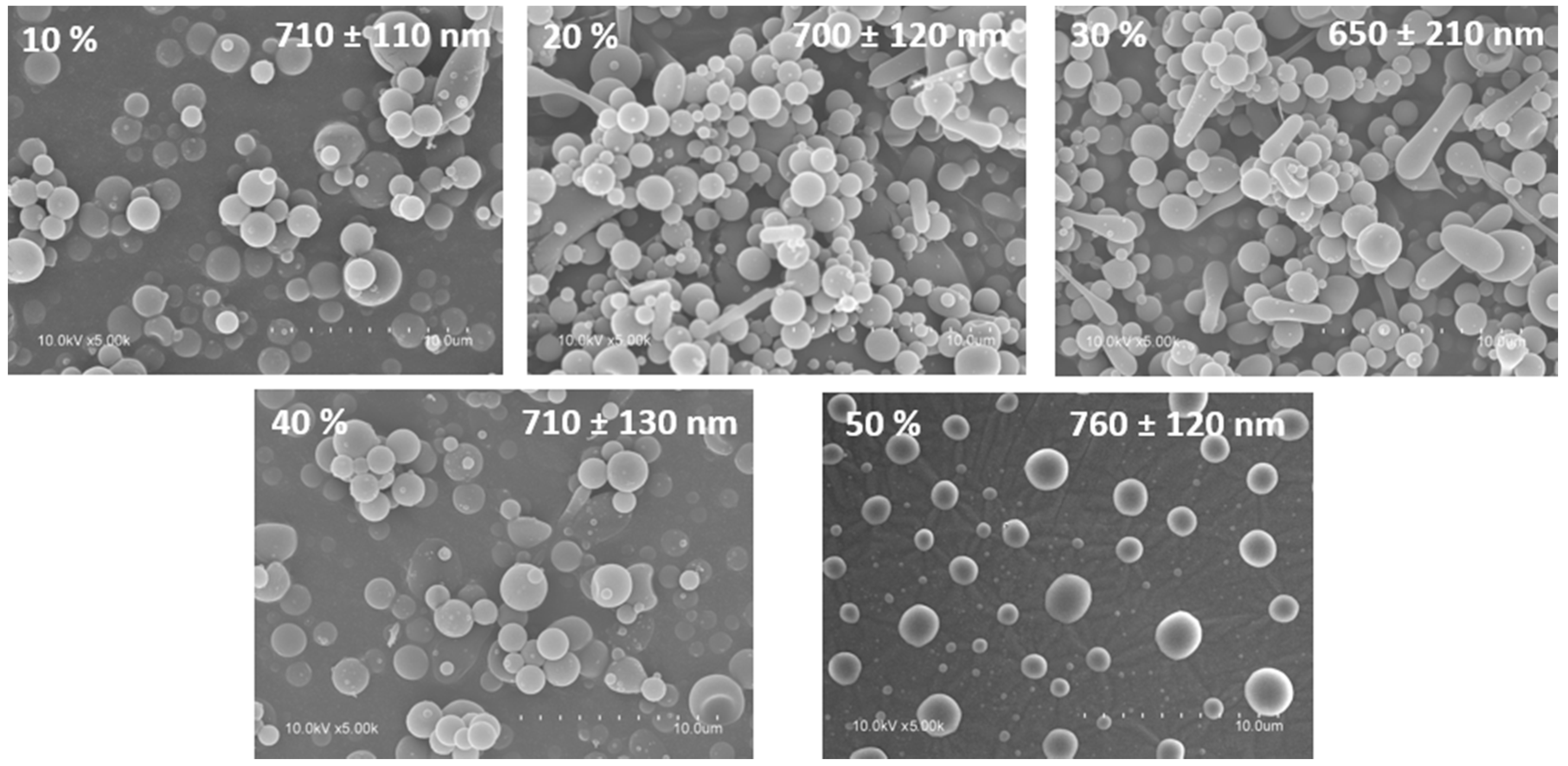
3.2. Capsule Morphology
3.3. β-Carotene Encapsulation
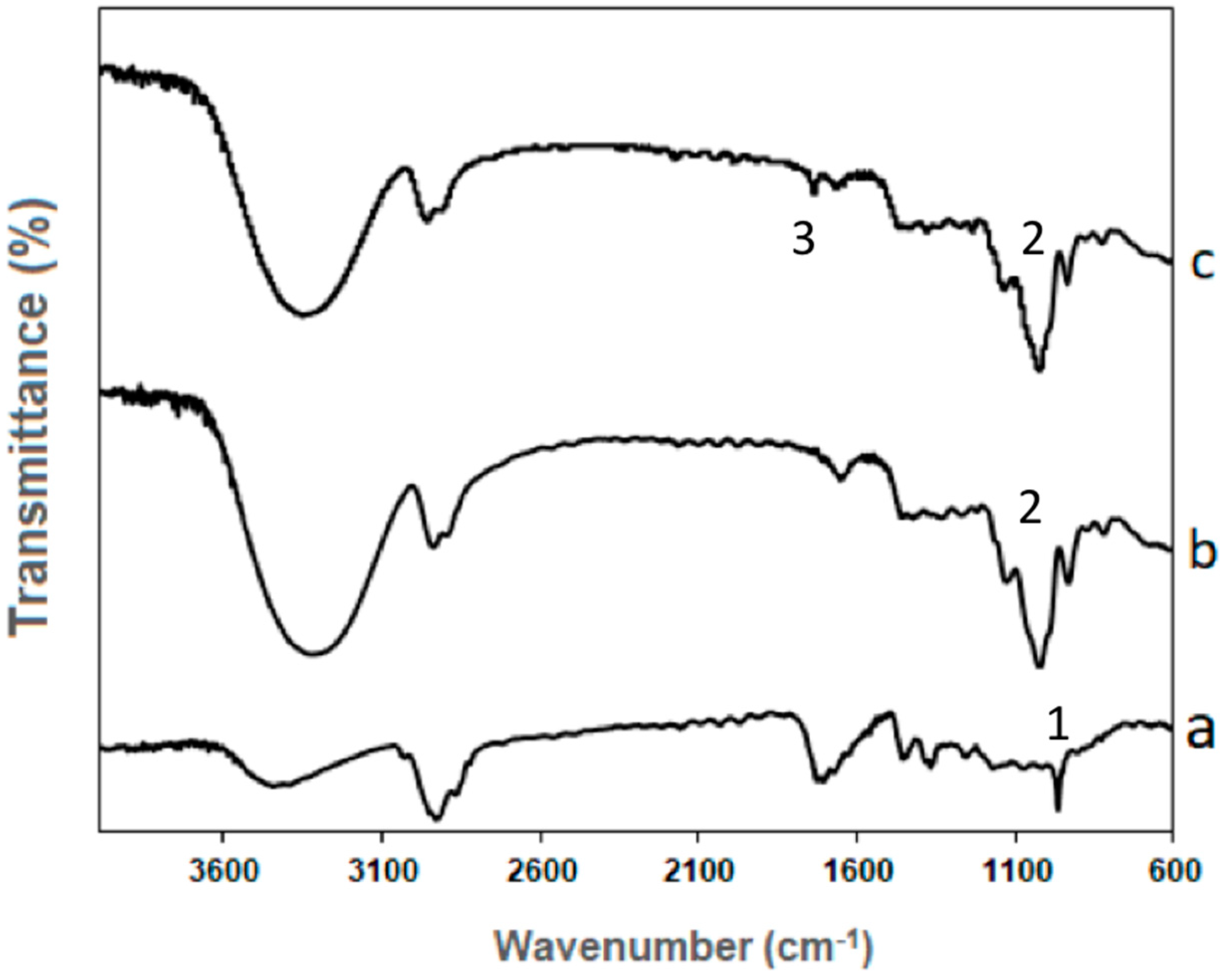
3.4. FTIR Analysis of the Encapsulation Structures
3.5. Thermal Stability of β-Carotene and HDPAF Nanocapsules
3.6. Ultraviolet (UV) Photostability of Encapsulated β-Carotene
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lopez, M.G.; Mancilla-Margalli, N.A.; Mendoza-Diaz, G. Molecular structures of fructans from agave tequilana weber var. azul. J. Agric. Food Chem. 2003, 51, 7835–7840. [Google Scholar] [CrossRef] [PubMed]
- Ritsema, T.; Smeekens, S.C. Engineering fructan metabolism in plants. J. Plant. Physiol. 2003, 160, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Palatnik, D.R.; Aldrete Herrera, P.; Rinaldoni, A.N.; Ortiz Basurto, R.I.; Campderrós, M.E. Development of reduced-fat cheeses with the addition of agave fructanos. Dairy Technol. 2016, 70, 212–219. [Google Scholar] [CrossRef]
- Guggisberg, D.; Cuthbert-Steven, J.; Piccinali, P.; Bütikofer, U.; Eberhard, P. Rheological, microstructural and sensory characterization of low-fat and whole milk set yoghurt as influenced by inulin addition. Int. Dairy J. 2009, 19, 107–115. [Google Scholar] [CrossRef]
- Furlán, L.T.R.; Herrera, P.A.; Padilla, A.P.; Basurto, R.I.O.; Campderrós, M.E. Assessment of agave fructans as lyoprotectants of bovine plasma proteins concentrated by ultrafiltration. Food Res. Int. 2014, 56, 146–158. [Google Scholar] [CrossRef]
- Ortiz-Basurto, R.I.; Rubio-Ibarra, M.E.; Ragazzo-Sanchez, J.A.; Beristain, C.I.; Jiménez-Fernández, M. Microencapsulation of Eugenia uniflora L. juice by spray drying using fructans with different degrees of polymerisation. Carbohydr. Polym. 2017, 175, 603–609. [Google Scholar] [CrossRef] [PubMed]
- FDA. GRAS Notification—Premium Agave Inulin-Resubmission. GRAS Notice (GRN) No. 687. Available online: http://www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/ NoticeInventory/default.htm (accessed on 16 December 2016).
- Li, X.Y.; Chen, X.G.; Sun, Z.W.; Park, H.J.; Cha, D.S. Preparation of alginate/chitosan/carboxymethyl chitosan complex microcapsules and application in Lactobacillus casei ATCC 393. Carbohydr. Polym. 2011, 83, 1479–1485. [Google Scholar] [CrossRef]
- Zuidam, N.J.; Shimoni, E. Overview of microencapsulates for use in food products or processes and methods to make them. In Encapsulation Technologies for Active Food Ingredients and Food Processing; Zuidam, N.J., Nedovic, V., Eds.; Springer: New York, NY, USA, 2010; Volume 1, pp. 3–29. ISBN 978-1-4419-1007-3. [Google Scholar]
- Nedovic, V.; Kalusevic, A.; Manojlovic, V.; Levic, S.; Bugarski, B. An overview of encapsulation technologies for food applications. Procedia Food Sci. 2011, 1, 1806–1815. [Google Scholar] [CrossRef]
- Murugesan, R.; Orsat, V. Spray drying for the production of nutraceutical ingredients–A review. Food Bioprocess Technol. 2011, 5, 3–14. [Google Scholar] [CrossRef]
- Gharsallaoui, A.; Roudaut, G.; Chambin, O.; Voilley, A.; Saurel, R. Applications of spray-drying in microencapsulation of food ingredients: An overview. Food Res. Int. 2007, 40, 1107–1121. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Martinez-Abad, A.; Ocio, M.J.; Lagaron, J.M. Stabilization of a nutraceutical ω-3 fatty acid by encapsulation in ultrathin electrosprayed zein prolamine. J. Food Sci. 2010, 75, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Rubio, A.; Lagaron, J.M. Whey protein capsules obtained through electrospraying for the encapsulation of bioactives. Innov. Food Sci. Emerg. Technol. 2011, 13, 200–206. [Google Scholar] [CrossRef]
- Drosou, C.G.; Krokida, M.K.; Biliaderis, C.G. Encapsulation of bioactive compounds through electrospinning/electrospraying and spray drying: A comparative assessment of food-related applications. Drying Technol. 2016, 35, 139–162. [Google Scholar] [CrossRef]
- Paximada, P.; Echegoyen, Y.; Koutinas, A.A.; Mandala, I.G.; Lagaron, J.M. Encapsulation of hydrophilic and lipophilized catechin into nanoparticles through emulsion electrospraying. Food Hydrocoll. 2016, 64, 123–132. [Google Scholar] [CrossRef]
- Lagaron, J.M.; Pérez-Masia, R.; López-Rubio, A. Procedimiento de Protección de Material Biológico y Compuestos Termolábiles Para Posibles Aplicaciones Industriales. Patent Application Number ES P201430034, 23 July 2015. [Google Scholar]
- Librán, C.M.; Castro, S.; Lagaron, J.M. Encapsulation by electrospray coating atomization of probiotic strains. Innov. Food Sci. Emerg. Technol. 2016, 39, 216–222. [Google Scholar] [CrossRef]
- Fernández, A.; Torres-Giner, S.; Lagaron, J.M. Novel route to stabilization of bioactive antioxidants by encapsulation in electrospun fibers of zein prolamine. Food Hydrocoll. 2009, 23, 1427–1432. [Google Scholar] [CrossRef]
- Ramakrishna, S.; Fujihara, K.; Teo, W.E.; Yong, T.; Ma, Z.; Ramaseshan, R. Electrospun nanofibers: Solving global issues. Mater. Today 2006, 9, 40–50. [Google Scholar] [CrossRef]
- De Freitas Zômpero, R.H.; López-Rubio, A.; de Pinho, S.C.; Lagaron, J.M.; de la Torre, L.G. Hybrid encapsulation structures based on β-carotene-loaded nanoliposomes within electrospun fibers. Coll. Surf. B 2015, 134, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Jaworek, A. Micro- and nanoparticle production by electrospraying. Powder Technol. 2007, 176, 18–35. [Google Scholar] [CrossRef]
- Gómez-Mascaraque, L.G.; Perez-Masiá, R.; González-Barrio, R.; Periago, M.J.; López-Rubio, A. Potential of microencapsulation through emulsion-electrospraying to improve the bioaccesibility of β-carotene. Food Hydrocoll. 2017, 73, 1–12. [Google Scholar] [CrossRef]
- Fernandes, R.V.; Borges, S.V.; Botrel, D.A. Gum arabic/starch/maltodextrin/inulin as wall materials on the microencapsulation of rosemary essential oil. Carbohydr. Polym. 2013, 101, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.P.; Nakajima, M. Beta-carotene nanodispersions: Preparation, characterization and stability evaluation. Food Chem. 2005, 92, 661–671. [Google Scholar] [CrossRef]
- Ribeiro, H.S.; Chu, B.-S.; Ichikawa, S.; Nakajima, M. Preparation of nanodispersions containing β-carotene by solvent displacement method. Food Hydrocoll. 2008, 22, 12–17. [Google Scholar] [CrossRef]
- Astete, C.E.; Sabliov, C.M.; Watanabe, F.; Biris, A. Ca2+ cross-linked alginic acid nanoparticles for solubilization of lipophilic natural colorants. J. Agric. Food Chem. 2009, 57, 7505–7512. [Google Scholar] [CrossRef] [PubMed]
- Camelo-Méndez, G.A.; Ragazzo-Sánchez, J.A.; Jiménez-Aparicio, A.R.; Vanegas-Espinoza, P.E.; Paredes-López, O.; Del Villar-Martínez, A.A. Comparative study of anthocyanin and aromatic compounds content of four varieties of Mexican roselle (Hibiscus sabdariffa L.) by multivariable analysis. Plant. Foods Hum. Nutr. 2013, 68, 229–234. [Google Scholar]
- Peinado, I.; Mason, M.; Romano, A.; Biasioli, F.; Scampicchio, M. Stability of β-carotene in polyethylene oxide electrospun nanofibers. Appl. Surf. Sci. 2016, 370, 111–116. [Google Scholar] [CrossRef]
- Ammawath, W.; Yaakob, C.M. A rapid method for determination of commercial β-carotene in RBD palm olein by Fourier transform infrared spectroscopy. Asian. J. Food Agro-Ind. 2010, 3, 443–452. [Google Scholar]
- Reksamunandar, R.P.; Edikresnha, D.; Munir, M.M.; Damayanti, S. Encapsulation of β-carotene in poly(vinylpyrrolidone) (PVP) by electrospinning Technique. Procedia Eng. 2017, 170, 19–23. [Google Scholar] [CrossRef]
- Grube, M.; Bekers, M.; Upite, D.; Kaminska, E. Infrared spectra of some fructans. Spectroscopy 2002, 16, 289–296. [Google Scholar] [CrossRef]
- Apolinário, A.C.; de Carvalho, E.M.; de Lima Damasceno, B.P.G.; da Silva, P.C.D.; Converti, A.; Pessoa, A., Jr.; da Silva, J.A. Extraction, isolation and characterization of inulin from Agave sisalana boles. Ind. Crops Prod. 2017, 108, 355–362. [Google Scholar] [CrossRef]
- Busolo, M.A.; Lagaron, J.M. Antioxidant polyethylene films based on a resveratrol containing clay of interest in food packaging applications. Food Packag. Shelf Life 2015, 6, 30–41. [Google Scholar] [CrossRef]
- López-Rubio, A.; Lagaron, J.M. Improved incorporation and stabilisation of β-carotene in hydrocolloids using glycerol. Food Chem. 2010, 125, 997–1004. [Google Scholar] [CrossRef]


| Concentration (% w/w) | Viscosity (cP) | Surface Tension (mN/m) | Conductivity (µS/cm) |
|---|---|---|---|
| Solutions | |||
| 5 | 1.61 ± 0.05 a | 24.35 ± 0.05 a | 69.39 ± 0.03 a |
| 10 | 2.37 ± 0.07 b | 24.37 ± 0.02 a | 82.14 ± 0.03 b |
| 20 | 3.42 ± 0.01 c | 24.85 ± 0.05 b | 101.20 ± 0.05 c |
| 30 | 6.82 ± 0.02 d | 23.65 ± 0.05 c | 93.30 ± 0.06 d |
| 40 | 46.05 ± 0.05 e | 23.51 ± 0.05 d | 76.73 ± 0.05 e |
| 50 | 162.22 ± 0.60 f | 23.46 ± 0.05 d | 52.86 ± 0.01 f |
| Emulsions | |||
| 5 | 2.65 ± 0.03 a | 22.42 ± 0.04 a | 41.81 ± 0.04 a |
| 10 | 3.40 ± 0.03 b | 22.91 ± 0.02 b | 45.82 ± 0.04 b |
| 20 | 8.83 ± 0.08 c | 24.05 ± 0.03 c | 52.70 ± 0.02 c |
| 30 | 12.26 ± 0.09 d | 23.20 ± 0.02 d | 50.89 ± 0.02 d |
| 40 | 45.70 ± 0.12 e | 22.73 ± 0.03 e | 39.11 ± 0.04 e |
| 50 | 93.54 ± 0.24 f | 21.13 ± 0.03 f | 30.26 ± 0.01 f |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos-Hernández, J.A.; Ragazzo-Sánchez, J.A.; Calderón-Santoyo, M.; Ortiz-Basurto, R.I.; Prieto, C.; Lagaron, J.M. Use of Electrosprayed Agave Fructans as Nanoencapsulating Hydrocolloids for Bioactives. Nanomaterials 2018, 8, 868. https://doi.org/10.3390/nano8110868
Ramos-Hernández JA, Ragazzo-Sánchez JA, Calderón-Santoyo M, Ortiz-Basurto RI, Prieto C, Lagaron JM. Use of Electrosprayed Agave Fructans as Nanoencapsulating Hydrocolloids for Bioactives. Nanomaterials. 2018; 8(11):868. https://doi.org/10.3390/nano8110868
Chicago/Turabian StyleRamos-Hernández, Jorge A., Juan A. Ragazzo-Sánchez, Montserrat Calderón-Santoyo, Rosa I. Ortiz-Basurto, Cristina Prieto, and Jose M. Lagaron. 2018. "Use of Electrosprayed Agave Fructans as Nanoencapsulating Hydrocolloids for Bioactives" Nanomaterials 8, no. 11: 868. https://doi.org/10.3390/nano8110868
APA StyleRamos-Hernández, J. A., Ragazzo-Sánchez, J. A., Calderón-Santoyo, M., Ortiz-Basurto, R. I., Prieto, C., & Lagaron, J. M. (2018). Use of Electrosprayed Agave Fructans as Nanoencapsulating Hydrocolloids for Bioactives. Nanomaterials, 8(11), 868. https://doi.org/10.3390/nano8110868