Effect of the Composition of Lanthanide Complexes on Their Luminescence Enhancement by Ag@SiO2 Core-Shell Nanoparticles
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Characterizations
2.2. The Preparation of Core-Shell Ag@SiO2 Nanoparticles
2.3. Synthesis of Lanthanide Complexes
2.4. The Preparation of Complexes Doped Core-Shell Ag@SiO2 Nanocomposites
3. Results and Discussions
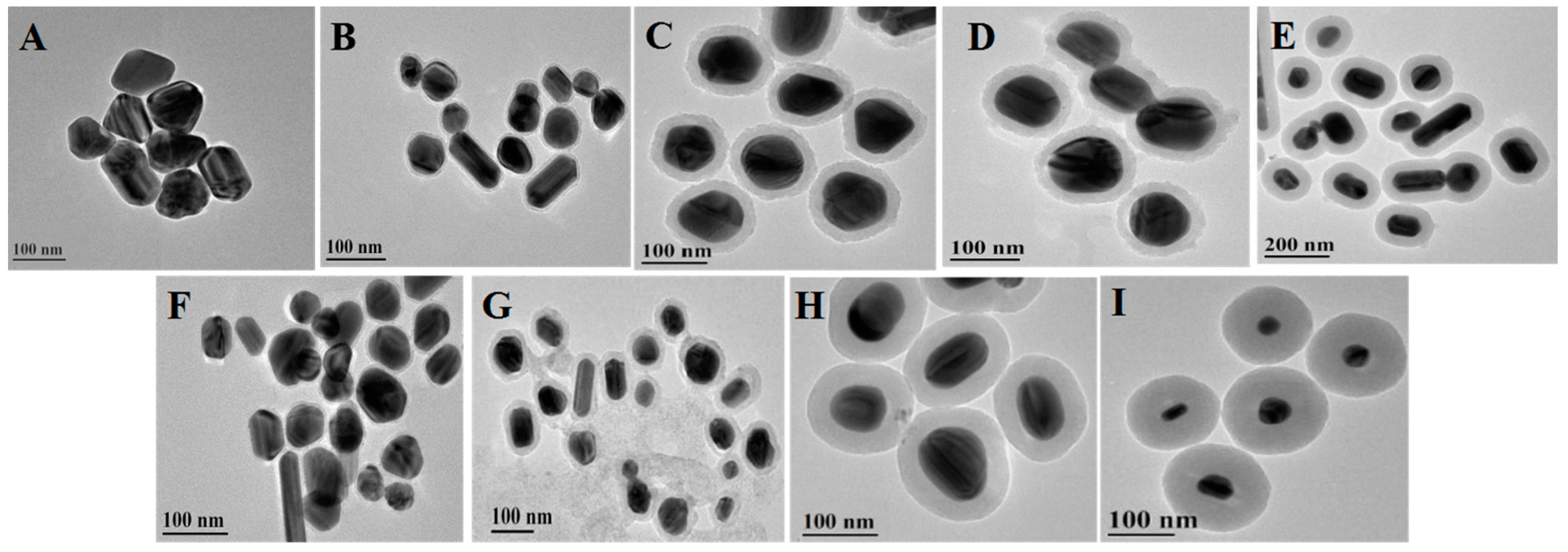
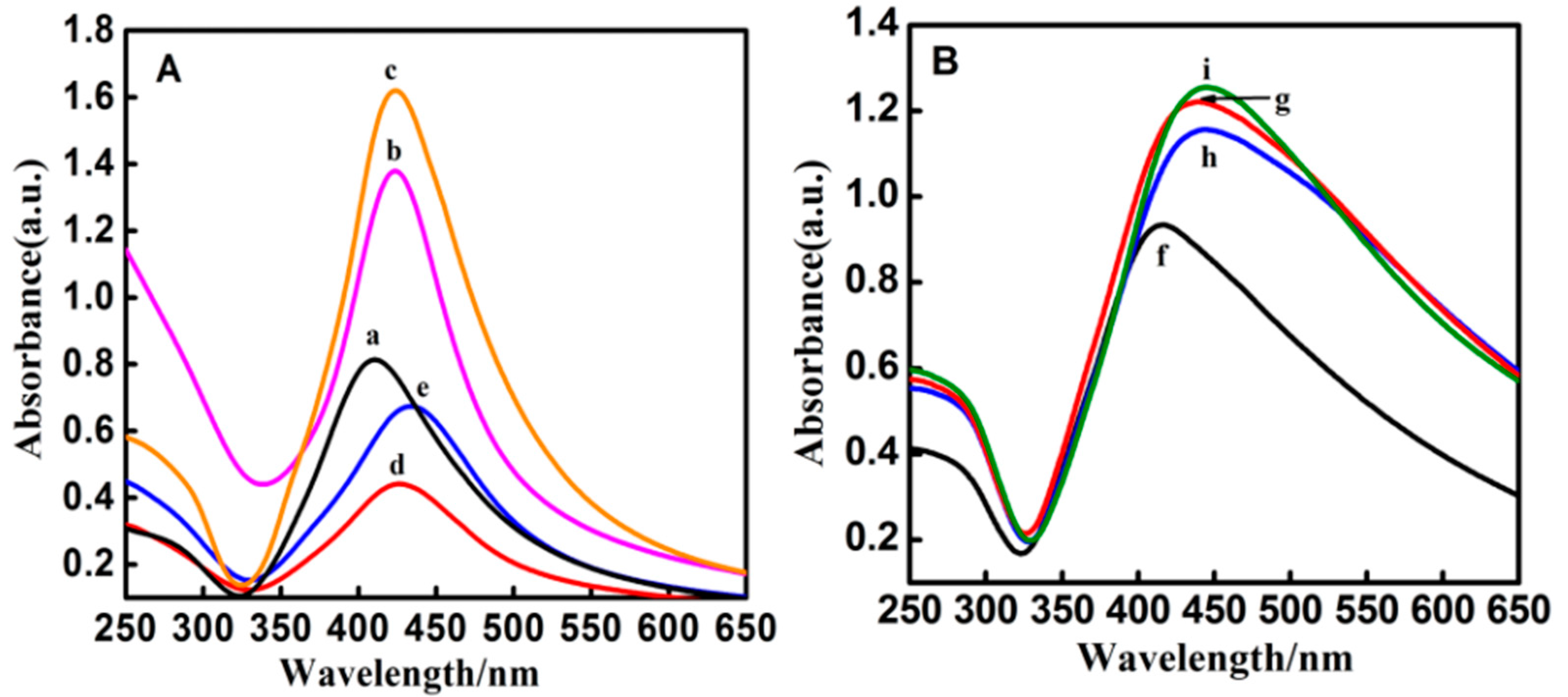
3.1. Characterizations of the Core-Shell Ag@SiO2 Nanoparticles
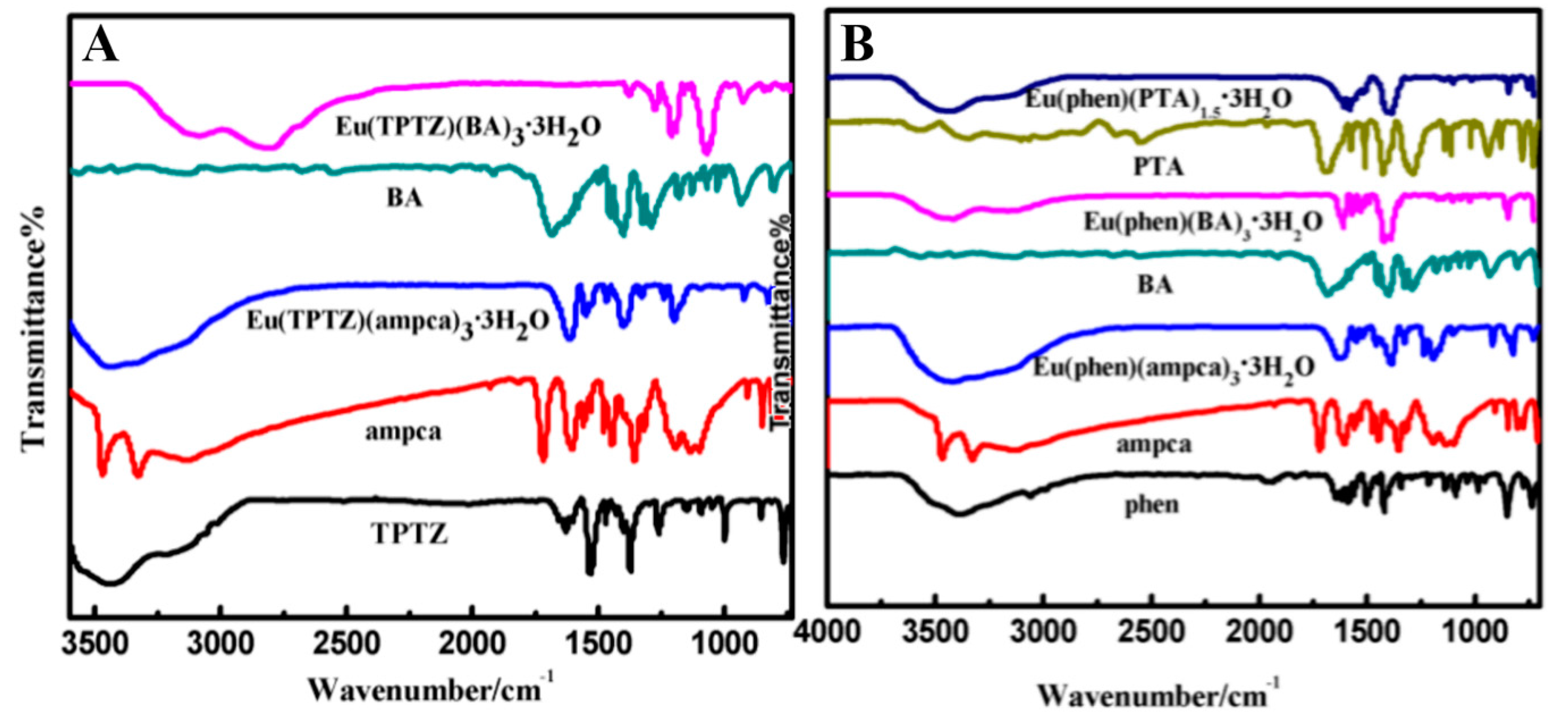
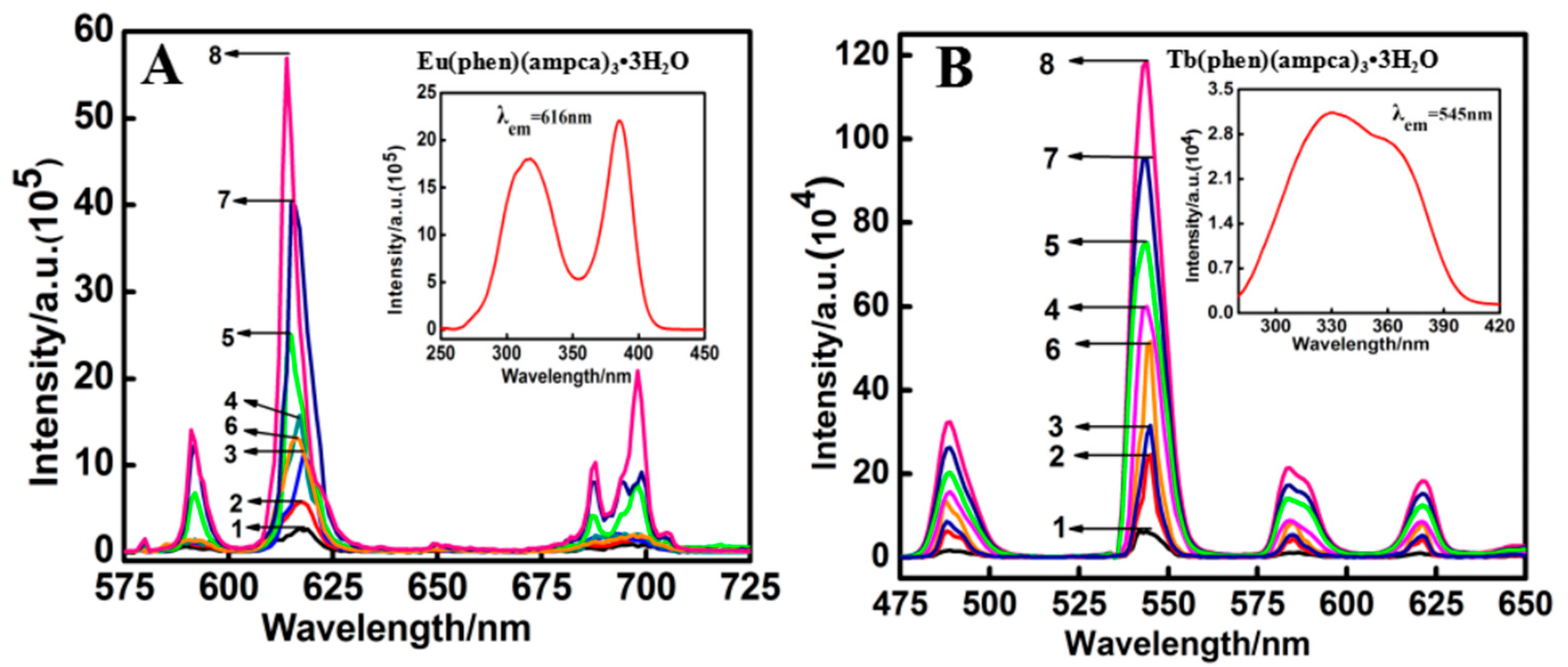
3.2. Characterizations of Lanthanide Complexes
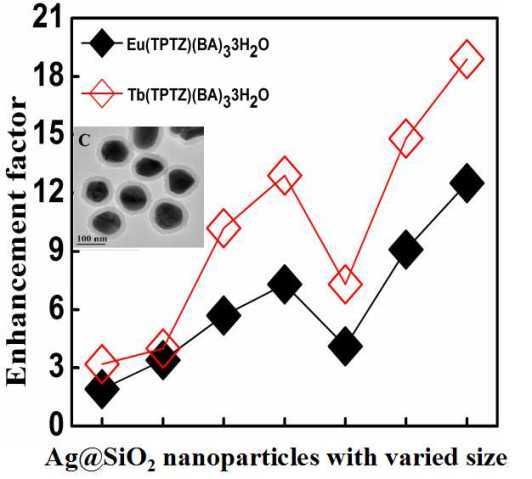
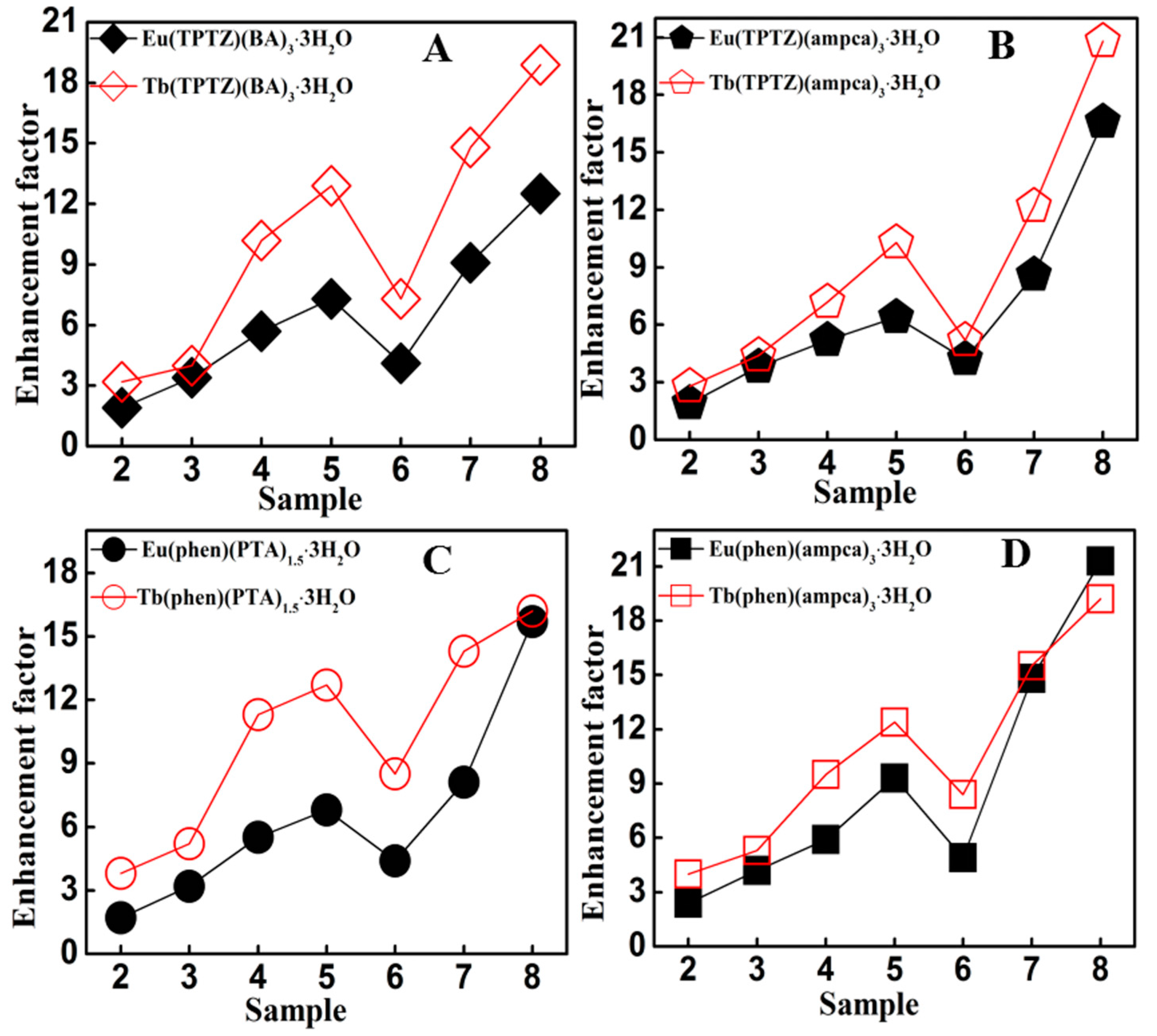
3.3. Luminescence Enhancement of the Lanthanide Complexes by Ag@SiO2 Nanoparticles
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Eliseeva, S.-V.; Bünzli, J.C.-G. Lanthanide luminescence for functional materials and bio-sciences. Chem. Soc. Rev. 2010, 39, 189–227. [Google Scholar] [CrossRef] [PubMed]
- Binnemans, K. Lanthanide-Based Luminescent Hybrid Materials. Chem. Rev. 2009, 109, 4283–4374. [Google Scholar] [CrossRef] [PubMed]
- Carvalhoa, C.-T.; Oliveir, G.-F.; Fernandes, J.; Siqueira, A.-B.; Ionashiroc, E.-Y.; Ionashiro, M. Rare-earth metal compounds with a novel ligand 2-methoxycinnamylidenepyruvate: A thermal and spectroscopic approach. Thermochim. Acta 2016, 637, 17–23. [Google Scholar] [CrossRef]
- Zondlo, S.-C.; Gao, F.; Zondlo, N.-J. Design of an encodable tyrosine kinase-inducible domain: Detection of tyrosine kinase activity by terbium luminescence. J. Am. Chem. Soc. 2010, 132, 5619–5621. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.-B.; Chen, B.; Sun, Y.-Y.; Lian, H.; Zhang, Q.-J. Effects of synergetic ligands on the thermal and radiative properties of Eu(TTA)3nL-doped poly(methyl methacrylate). J. Non-Cryst. Solids 2005, 351, 849–855. [Google Scholar] [CrossRef]
- Pazos, E.; Torrecill, D.; Lopez, M.-V.; Castedo, L.; Mascarena, J.-L.; Vidal, A.; Vázquez, M.-E. Cyclin A Probes by Means of Intermolecular Sensitization of Terbium-Chelating Peptides. J. Am. Chem. Soc. 2008, 130, 9652–9653. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Gunther, J.-R.; Katzenellenbogen, J.-A. Monitoring a Coordinated Exchange Process in a Four-Component Biological Interaction System: Development of a Time-Resolved Terbium-Based One-Donor/Three-Acceptor Multicolor FRET System. J. Am. Chem. Soc. 2010, 132, 4685–4692. [Google Scholar] [CrossRef] [PubMed]
- Luppa, P.-B.; Sokoll, L.-J.; Chan, D.-W. Immunosensors—Principles and applications to clinical chemistry. Clin. Chim. Acta 2001, 314, 1–26. [Google Scholar] [CrossRef]
- Zhong, W.-W. Nanomaterials in fluorescence-based biosensing. Anal. Bioanal. Chem. 2009, 394, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Davies, G.-L.; O’Brien, J.; Guńko, Y.-K. Rare earth doped silica nanoparticles via thermolysis of a single source metallasilsesquioxane precursor. Sci. Rep. 2017, 7, 45862. [Google Scholar] [CrossRef] [PubMed]
- Murray, K.; Cao, Y.-C.; Ali, S.; Hanley, Q. Lanthanide doped silica nanoparticles applied to multiplexed immunoassays. Analyst 2010, 135, 2132–2138. [Google Scholar] [CrossRef] [PubMed]
- Chiriua, D.; Stagia, L.; Carbonaroa, C.-M.; Corpinoa, R.; Casula, M.-F.; Ricci, P.-C. Towards the development of new phosphors with reduced content of rare earth elements: Structural and optical characterization of Ce:Tb: Al2SiO5. Mater. Res. Bull. 2016, 77, 15–22. [Google Scholar] [CrossRef]
- Bünzli, J.-C.G.; Eliseeva, S.-V. Intriguing aspects of lanthanide luminescence. Chem. Sci. 2013, 4, 1939–1949. [Google Scholar] [CrossRef]
- Atabaev, T.S.; Lee, J.H.; Shin, Y.C.; Han, D.-W.; Choo, K.S.; Jeon, U.B.; Hwang, J.Y.; Yeom, J.A.; Kim, H.-K.; Hwang, Y.-H. Eu, Gd-Codoped Yttria Nanoprobes for Optical and T1-Weighted Magnetic Resonance Imaging. Nanomaterials 2017, 7, 35. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ye, Z.-Q.; Wang, G.; Jin, D.-Y.; Yuan, J.-L.; Guan, Y.-F.; James, P. Visible-light-sensitized highly luminescent europium nanoparticles: Preparation and application for time-gated luminescence bioimaging. J. Mater. Chem. 2009, 19, 1258–1264. [Google Scholar] [CrossRef]
- Zhang, D.-J.; Wang, X.-M.; Qiao, Z.-A.; Tang, D.-H.; Liu, Y.-L.; Huo, Q.-S. Synthesis and characterization of novel lanthanide(III) complexes-functionalized mesoporous silica nanoparticles as fluorescent nanomaterials. J. Phys. Chem. C 2010, 114, 12505–12510. [Google Scholar] [CrossRef]
- Zhao, Y.F.; Chu, H.B.; Bai, F.; Gao, D.Q.; Zhang, H.X.; Zhou, Y.S.; Wei, X.Y.; Shan, M.N.; Li, H.Y.; Zhao, Y.L. Synthesis, crystal structure, luminescent property and antibacterial activity of lanthanide ternary complexes with 2,4,6-tri(2-pyridyl)-s-triazine. J. Organomet. Chem. 2012, 716, 167–174. [Google Scholar] [CrossRef]
- Kong, L.J.; Kong, K.; Zhao, Y.L.; Chu, H.B. Tuning the luminescence properties of lanthanide coordination polymers with Ag@SiO2 nanoparticles. Dalton Trans. 2017, 46, 6447–6455. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.L.; Zhou, D.; Chen, Y.N.; Li, J.J.; Zhang, H.X.; Zhao, Y.L.; Chu, H.B. Crystal structure and photoluminescence of europium, terbium and samarium compounds with halogen-benzoate and 2,4,6-tri(2-pyridyl)-s-triazine. J. Lumin. 2016, 177, 22–30. [Google Scholar] [CrossRef]
- Aslan, K.; Wu, M.; Lakowicz, J.-R.; Geddes, C.-D. Fluorescent Core−Shell Ag@SiO2 Nanocomposites for Metal-Enhanced Fluorescence and Single Nanoparticle Sensing Platforms. J. Am.Chem. Soc. 2007, 129, 1524–1525. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.-J.; Lu, N.; Qi, D.-P.; Ma, R.-P.; Wu, Q. Tuning the Intensity of Metal-Enhanced Fluorescence by Engineering Silver Nanoparticle Arrays. Small 2010, 6, 1038–1043. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Han, H.; Bae, J.-G.; Lee, E.-Y.; Im, S.-H.; Kim, D.-H.; Seo, T.-S. Growth of silver nanowires from controlled silver chloride seeds and their application for fluorescence enhancement based on localized surface plasmon resonance. Small 2017, 13, 1603392. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Jin, D.; Drozdowicz-Tomsia, K.; Yuan, J.; Wu, J.; Goldys, E.-M. Ultrabright Eu–Doped Plasmonic Ag@SiO2 Nanostructures: Time-gated Bioprobes with Single Particle Sensitivity and Negligible Background. Adv. Mater. 2011, 23, 4649–4654. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-J.; Lee, S.-S.; Jung, J.-H. Recyclable fluorimetric and colorimetric mercury-specific sensor using porphyrin-functionalized Au@SiO2 core/shell nanoparticles. Analyst 2010, 135, 1551–1555. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Braun, G.-B.; Shi, Y.-F.; Zhang, Y.-C.; Sun, X.-H.; Reich, N.-O.; Zhao, D.-Y.; Stucky, G. Fabrication of Ag@SiO2@Y2O3:Er Nanostructures for Bioimaging: Tuning of the Upconversion Fluorescence with Silver Nanoparticles. J. Am. Chem. Soc. 2010, 132, 2850–2851. [Google Scholar] [CrossRef] [PubMed]
- Kudelski, A.; Wojtysiak, S. Silica-Covered Silver and Gold Nanoresonators for Raman Analysis of Surfaces of Various Materials. J. Phys. Chem. C. 2012, 116, 16167–16174. [Google Scholar] [CrossRef]
- Pham, X.-H.; Hahm, E.; Kim, H.M.; Shim, S.; Kim, T.H.; Jeong, D.H.; Lee, Y.-S.; Jun, B.-H. Silver Nanoparticle-Embedded Thin Silica-Coated Graphene Oxide as an SERS Substrate. Nanomaterials 2016, 6, 176. [Google Scholar] [CrossRef] [PubMed]
- Geddes, C.D. Metal-enhanced fluorescence. Phys. Chem. Chem. Phys. 2013, 15, 19537. [Google Scholar] [CrossRef] [PubMed]
- Geddes, C.D.; Lakowicz, J.R. Editorial: Metal-Enhanced Fluorescence. J. Fluoresc. 2002, 12, 121–129. [Google Scholar] [CrossRef]
- Geddes, C.D. Metal-Enhanced Fluorescence; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010. [Google Scholar]
- Zhang, J.; Song, F.; He, Z.; Liu, Y.; Chen, Z.; Lin, Y.; Huang, L.; Huang, W. Wide-Range Tunable Fluorescence Lifetime and Ultrabright Luminescence of Eu-Grafted Plasmonic Core-Shell Nanoparticles for Multiplexing. Small 2016, 12, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Yu, Q.; Zhang, X.; Du, X.; Gong, H.; Jiang, H. Synthesis and application of surface enhanced Raman scattering (SERS) tags of Ag@SiO2 core/shell nanoparticles in protein detection. J. Mater. Chem. 2012, 22, 7767–7774. [Google Scholar] [CrossRef]
- Lee, J.E.; Bera, S.; Choi, Y.S.; Lee, W.I. Size-dependent plasmonic effects of M and M@SiO2 (M = Au or Ag) deposited on TiO2 in photocatalytic oxidation reactions. Appl. Catal. B 2017, 214, 15–22. [Google Scholar] [CrossRef]
- Mosselhy, D.A.; Granbohm, H.; Hynönen, U.; Ge, Y.; Palva, A.; Nordström, K.; Hannula, S.-P. Nanosilver–Silica Composite: Prolonged Antibacterial Effects and Bacterial Interaction Mechanisms for Wound Dressings. Nanomaterials 2017, 7, 261. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Li, Y.; Chen, Y.-N.; Wang, A.-L.; Yue, B.; Qu, Y.-R.; Zhao, Y.-L.; Chu, H.-B. Core-shell Ag@SiO2 nanoparticles of different silica shell thicknesses: Preparation and their effects on photoluminescence of lanthanide complexes. Mater. Res. Bull. 2015, 71, 116–121. [Google Scholar] [CrossRef]
- Qu, Y.-R.; Lin, X.-M.; Wang, A.-L.; Wang, Z.-X.; Kang, J.; Chu, H.-B.; Zhao, Y.-L. Study on silicon oxide coated on silver nanocrystal to enhance fluorescence intensity of rare earth complexes. J. Lumin. 2014, 154, 402–409. [Google Scholar] [CrossRef]
- Zhou, D.; Lin, X.-M.; Wang, A.-L.; Li, J.-J.; Qu, Y.-R.; Chu, H.-B.; Zhao, Y.-L. Fluorescence enhancement of Tb3+ complexes by adding silica-coated silver nanoparticles. Sci. China Chem. 2015, 58, 979–985. [Google Scholar] [CrossRef]
- Haes, A.J.; Zou, S.L.; Schatz, G.C.; Duyne, R.P.V. Nanoscale Optical Biosensor: Short Range Distance Dependence of the Localized Surface Plasmon Resonance of Noble Metal Nanoparticles. J. Phys. Chem. B 2004, 108, 6961–6968. [Google Scholar] [CrossRef]
- Li, D.; Li, D.-W.; Li, Y.; Fossey, J.-S.; Long, Y.-T. Cyclic electroplating and stripping of silver on Au@SiO2 core/shell nanoparticles for sensitive and recyclable substrate of surface-enhanced Raman scattering. J. Mater. Chem. 2010, 20, 3688–3693. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Z.; Han, M. Gram-scale synthesis and biofunctionalization of silica-coated silver nanoparticles for fast colorimetric DNA detection. Anal. Chem. 2005, 77, 2595–2600. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Fu, Y.; Liang, D.; Zhao, R.Y.; Lakowicz, J.-R. Enhanced Fluorescence Images for Labeled Cells on Silver Island Films. Langmuir 2008, 24, 12452–12457. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.-D.; Carter, C.-P.; Wynter, C.-I. The infra-red spectra and structure of the rare-earth benzoates Original. J. Inorg. Nucl. Chem. 1968, 30, 1503–1511. [Google Scholar] [CrossRef]
- Geary, W.-J. The use of conductivity measurements in organic solvents for the characterisation of coordination compounds. Coord. Chem. Rev. 1971, 7, 81–122. [Google Scholar] [CrossRef]
- Nawrota, I.; Machuraa, B.; Kruszynskib, R. Thiocyanate cadmium(II) complexes of 2,4,6-tri(2-pyridyl)-1,3,5-triazine—Synthesis, structure and luminescence properties. J. Lumin. 2014, 156, 240–254. [Google Scholar] [CrossRef]
- Deng, Z.-P.; Kang, W.; Huo, L.-H.; Zhao, H.; Gao, S. Rare-earth organic frameworks involving three types of architecture tuned by the lanthanide contraction effect: Hydrothermal syntheses, structures and luminescence. Dalton Trans. 2010, 39, 6276–6284. [Google Scholar] [CrossRef] [PubMed]
- Gabr, I.M.; El-Asmy, H.A.; Emmam, M.S.; Mostafa, S.I. Synthesis, characterization and anticancer activity of 3-aminopyrazine-2-carboxylic acid transition metal complexes. Trans. Metal. Chem. 2009, 34, 409–418. [Google Scholar] [CrossRef]
- Tamer, Ö.; Avcı, D.; Atalay, Y. Synthesis, X-Ray crystal structure, photophysical characterization and nonlinear optical properties of the unique manganese complex with picolinate and 1,10 phenantroline: Toward the designing of new high NLO response crystal. J. Phys. Chem. Solids 2016, 99, 124–133. [Google Scholar] [CrossRef]
- Varaprasad, K.; Pariguana, M.; Raghavendra, G.-M.; Jayaramudu, T.; Sadiku, E.-R. Development of biodegradable metaloxide/polymer nanocomposite films based on poly-ε-caprolactone and terephthalic acid. Mater. Sci. Eng. C 2017, 70, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-H. Rare Earth Coordination Chemistry: Fundamentals and Applications; John Wiley & Sons (Asia) Pte Ltd.: Singapore, 2010. [Google Scholar]
- Zhao, Y.-F.; Zhao, Y.-L.; Bai, F.; Wei, X.-Y.; Zhou, Y.-S.; Shan, M.-N.; Li, H.-H.; Ma, R.-J.; Fu, X.-T.; Du, Y. Fluorescent Property of the Gd3+-Doped Terbium Complexes and Crystal Structure of [Tb(TPTZ)(H2O)6]Cl3·3H2O. J. Fluoresc. 2010, 20, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Bünzli, J.C.-G. On the design of highly luminescent lanthanide complexes. Coord. Chem. Rev. 2015, 293–294, 19–47. [Google Scholar] [CrossRef]
- Li, D.; Chen, F.-F.; Bian, Z.-Q.; Liu, Z.-W.; Zhao, Y.-L.; Huang, C.-H. Sensitized near-infrared emission of YbIII from an IrIII–YbIII bimetallic complex. Polyhedron 2009, 28, 897–902. [Google Scholar] [CrossRef]
- Kong, L.J.; Zhao, Y.F.; Kong, K.; Zhao, Y.L.; Chu, H.B. Fluorescence enhancement of europium nitrobenzoates by Ag@SiO2 nanoparticles in solution. J. Lumin. 2017, 186, 255–261. [Google Scholar] [CrossRef]

| Complex | C(%) | H(%) | N(%) | Conductivity (S·cm2·mol−1) |
|---|---|---|---|---|
| Eu(phen)(ampca)3·3H2O | 40.03(40.48) | 3.56(3.50) | 18.92(19.24) | 11.2 |
| Eu(TPTZ)(BA)3·3H2O | 53.46(53.18) | 4.22(4.07) | 9.98(9.55) | 13.1 |
| Eu(TPTZ)(ampca)3·3H2O | 42.88(42.46) | 3.67(3.22) | 22.90(22.52) | 12.6 |
| Eu(phen)(BA)3·3H2O | 52.47(52.62) | 4.47(4.52) | 3.79(3.72) | 13.2 |
| Eu(phen)(PTA)1.5·3H2O | 45.47(45.33) | 3.46(3.17) | 3.97(4.41) | 14.2 |
| Tb(phen)(ampca)3·3H2O | 40.57(40.14) | 3.59(3.25) | 19.47(19.08) | 14.2 |
| Tb(TPTZ)(BA)3·3H2O | 52.07(52.49) | 4.03(4.04) | 9.78(9.42) | 18.3 |
| Tb(TPTZ)(ampca)3·3H2O | 42.57(42.17) | 3.59(3.22) | 22.70(22.37) | 17.4 |
| Tb(phen)(PTA)1.5·3H2O | 45.37(45.14) | 3.47(3.73) | 4.48(4.38) | 15.9 |
| Complex | λex (nm) | Emission Intensity (a.u.) (105) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||
| Eu(phen)(ampca)3·3H2O | 386 | 2.7 | 5.7(2.1) | 11.4(4.2) | 15.8(5.9) | 25.0(9.3) | 13.2(4.9) | 39.9(14.8) | 57.6(21.3) |
| Eu(phen)(BA)3·3H2O | 291 | 1.7 | 3.4(2.0) | 7.1(4.2) | 11.3(6.6) | 15.6(9.2) | 9.1(5.4) | 18.5(10.9) | 25.0(14.7) |
| Eu(phen)(PTA)1.5·3H2O | 276 | 2.3 | 3.8(1.7) | 7.3(3.2) | 12.6(5.5) | 15.6(6.8) | 10.0(4.4) | 18.6(8.1) | 36.1(15.7) |
| Eu(TPTZ)(ampca)3·3H2O | 366 | 2.9 | 5.4(1.9) | 11.1(3.8) | 15.0(5.2) | 18.7(6.4) | 12.1(4.2) | 24.8(8.6) | 48.2(16.6) |
| Eu(TPTZ)(BA)3·3H2O | 310 | 2.0 | 3.8(1.9) | 6.8(3.4) | 11.3(5.7) | 14.6(7.3) | 8.1(4.1) | 18.2(9.1) | 25.0(12.5) |
| Tb(phen)(ampca)3·3H2O | 330 | 0.62 | 2.5(4.0) | 3.3(5.3) | 5.9(9.5) | 7.7(12.4) | 5.2(8.4) | 9.6(15.5) | 11.9(19.2) |
| Tb(phen)(PTA)1.5·3H2O | 306 | 0.60 | 2.3(3.8) | 3.1(5.2) | 6.8(11.3) | 7.6(12.7) | 5.1(8.5) | 8.6(14.3) | 9.7(16.2) |
| Tb(TPTZ)(ampca)3·3H2O | 273 | 0.64 | 1.8(2.8) | 2.8(4.4) | 4.6(7.2) | 6.6(10.3) | 3.3(5.2) | 7.8(12.2) | 13.3(20.8) |
| Tb(TPTZ)(BA)3·3H2O | 312 | 0.63 | 2.0(3.2) | 2.5(4.0) | 6.4(10.2) | 8.1(12.9) | 4.6(7.3) | 9.3(14.8) | 11.9(18.9) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.-J.; Qu, Y.-R.; Zhao, Y.-L.; Chu, H.-B. Effect of the Composition of Lanthanide Complexes on Their Luminescence Enhancement by Ag@SiO2 Core-Shell Nanoparticles. Nanomaterials 2018, 8, 98. https://doi.org/10.3390/nano8020098
Wang X-J, Qu Y-R, Zhao Y-L, Chu H-B. Effect of the Composition of Lanthanide Complexes on Their Luminescence Enhancement by Ag@SiO2 Core-Shell Nanoparticles. Nanomaterials. 2018; 8(2):98. https://doi.org/10.3390/nano8020098
Chicago/Turabian StyleWang, Xiao-Jing, Yan-Rong Qu, Yong-Liang Zhao, and Hai-Bin Chu. 2018. "Effect of the Composition of Lanthanide Complexes on Their Luminescence Enhancement by Ag@SiO2 Core-Shell Nanoparticles" Nanomaterials 8, no. 2: 98. https://doi.org/10.3390/nano8020098