Modifying Thermal Switchability of Liquid Crystalline Nanoparticles by Alkyl Ligands Variation
Abstract
:1. Introduction
2. Results and Discussion
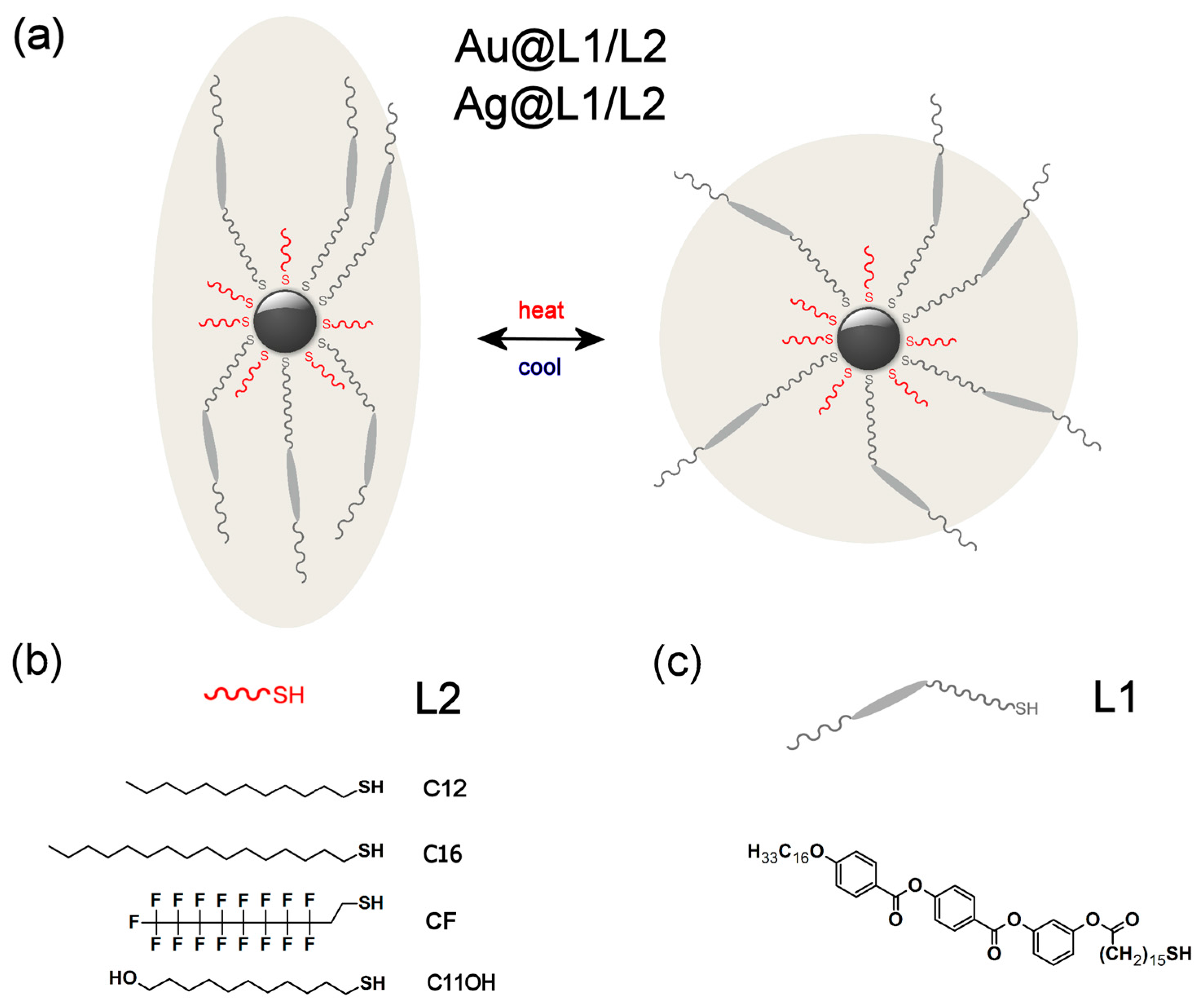
2.1. Designing LC Nanoparticles
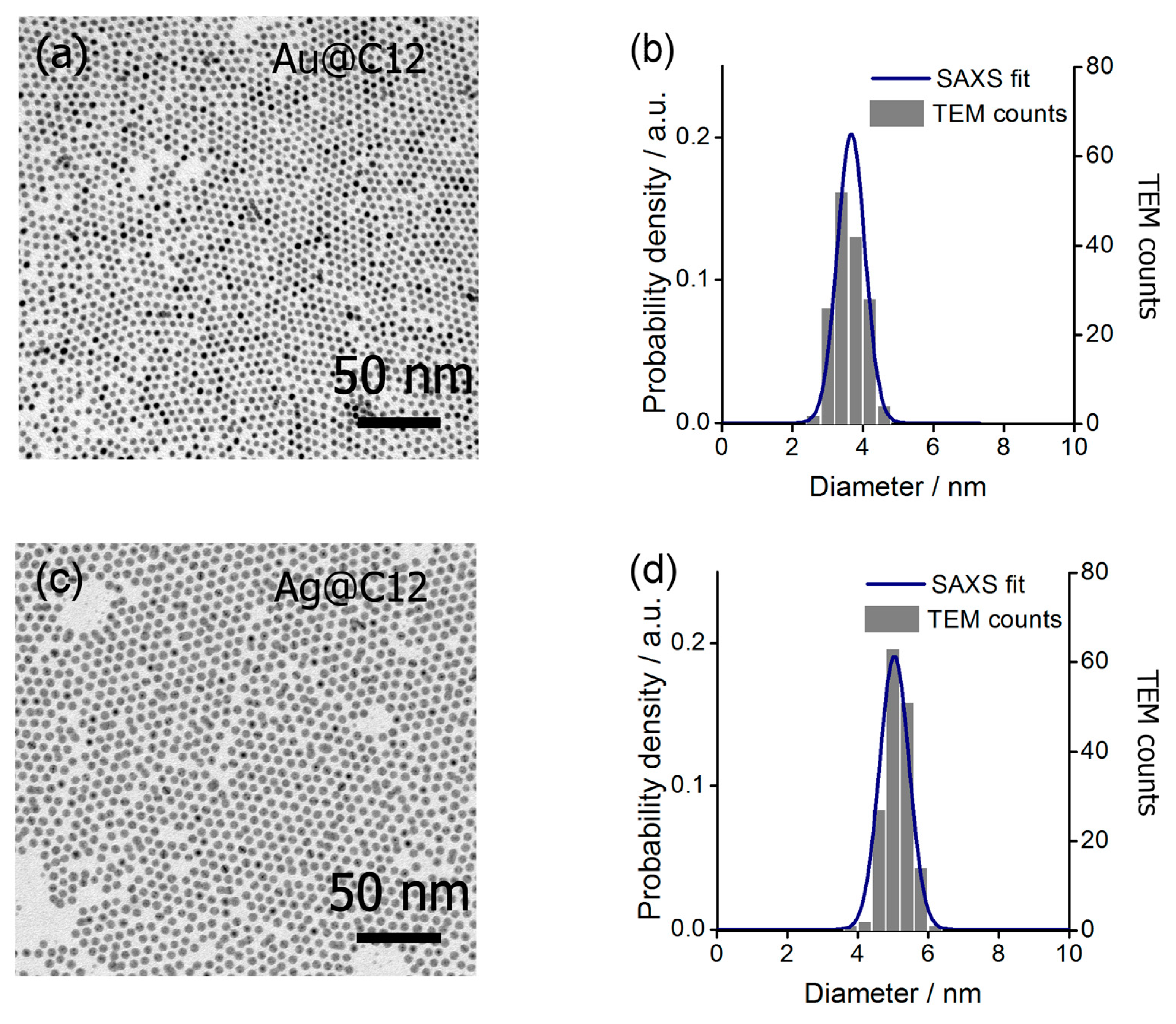
2.2. Characterization of Au@C12 and Ag@C12 Nanoparticles
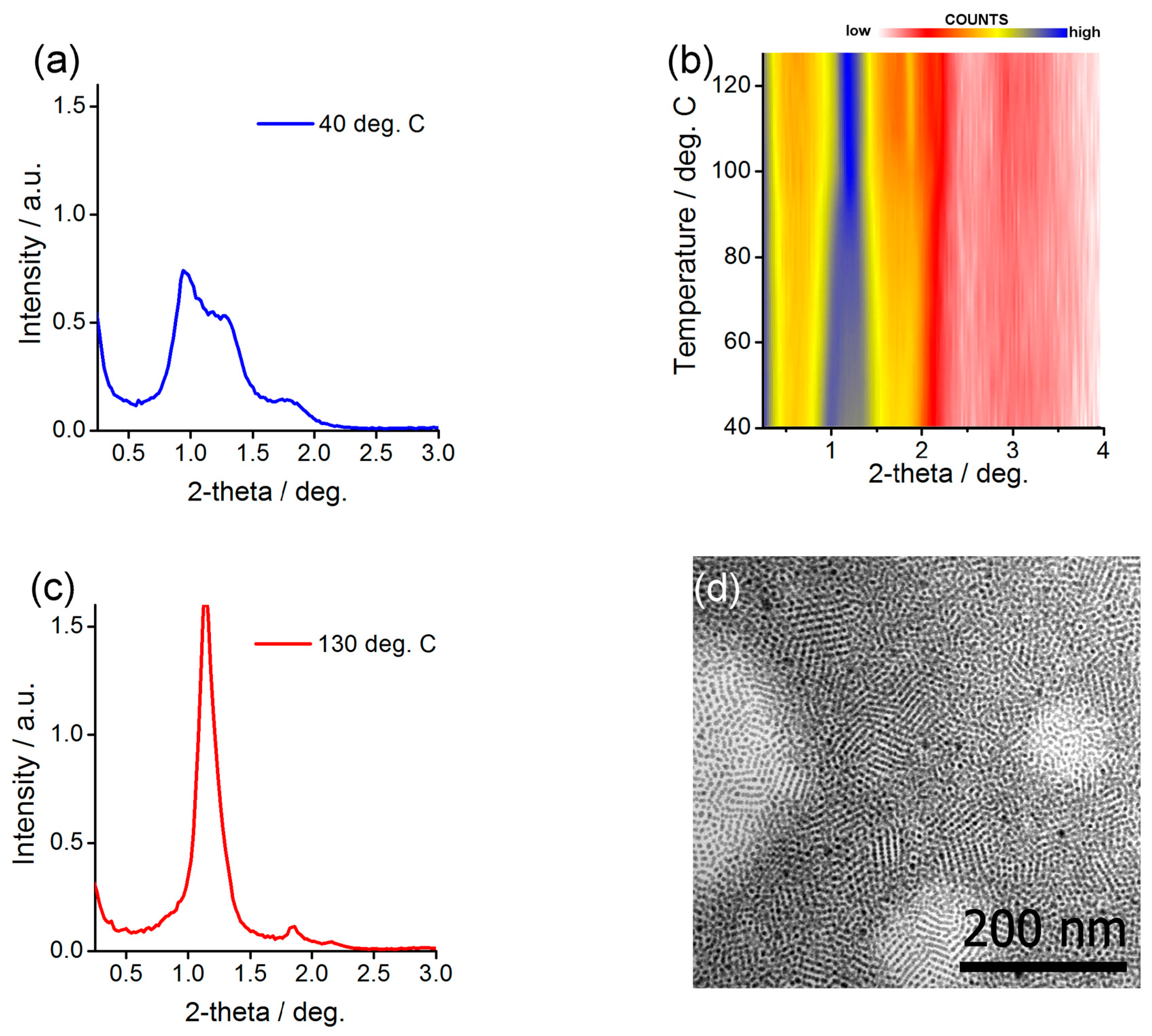
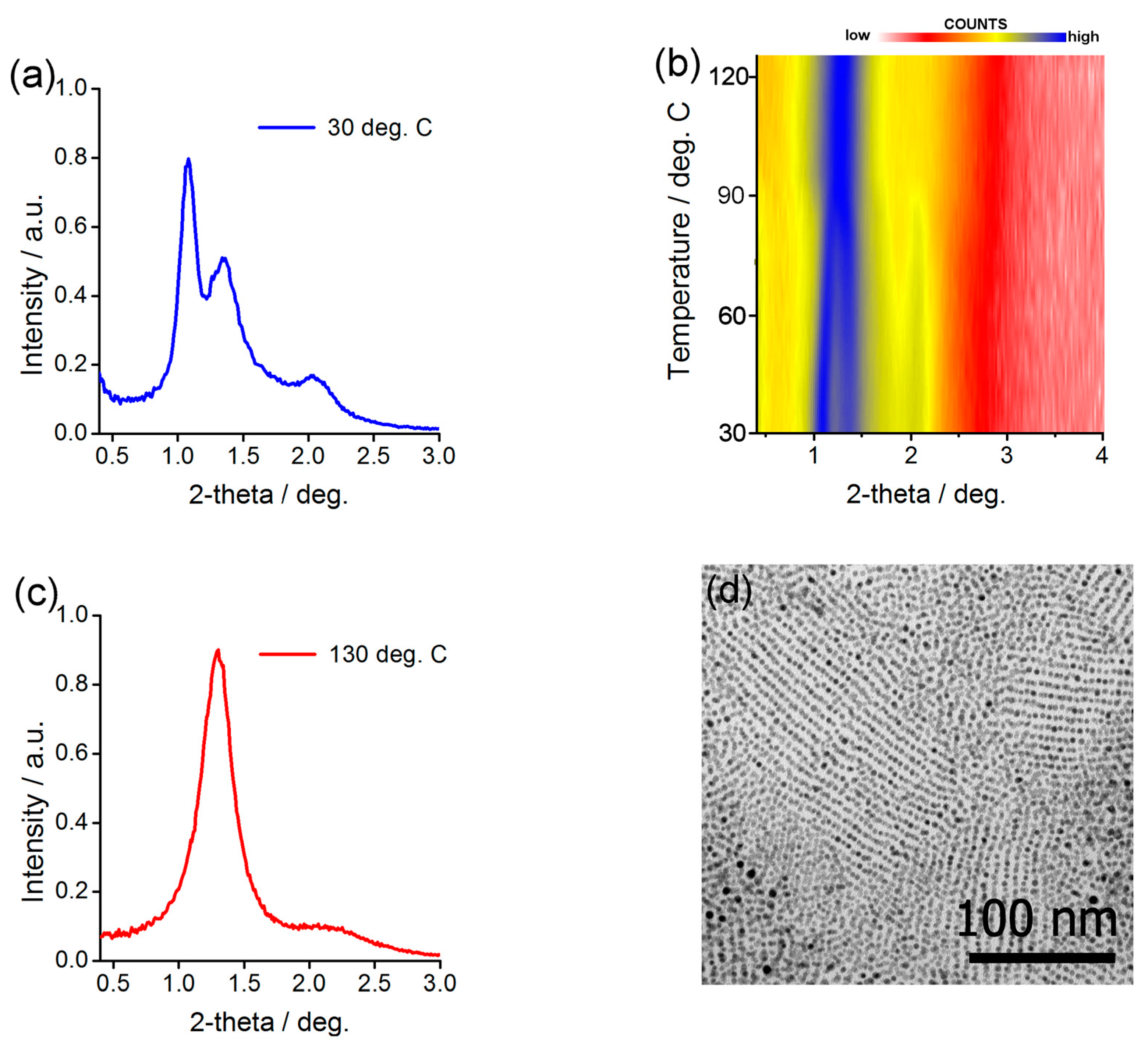
2.3. Preparing and Characterizing Assemblies of Au@L1/C12 and Ag@L1/C12 Nanoparticles
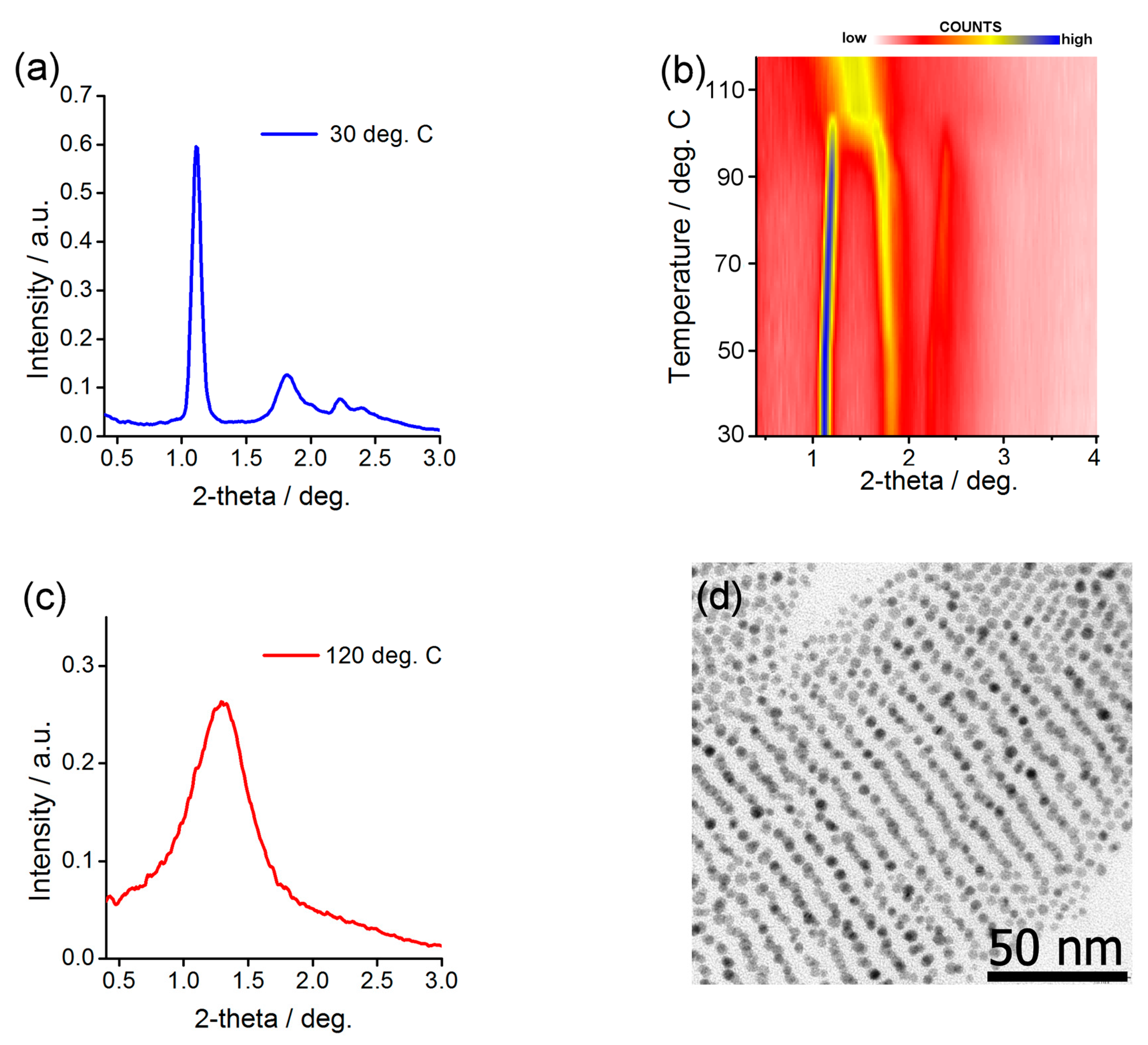
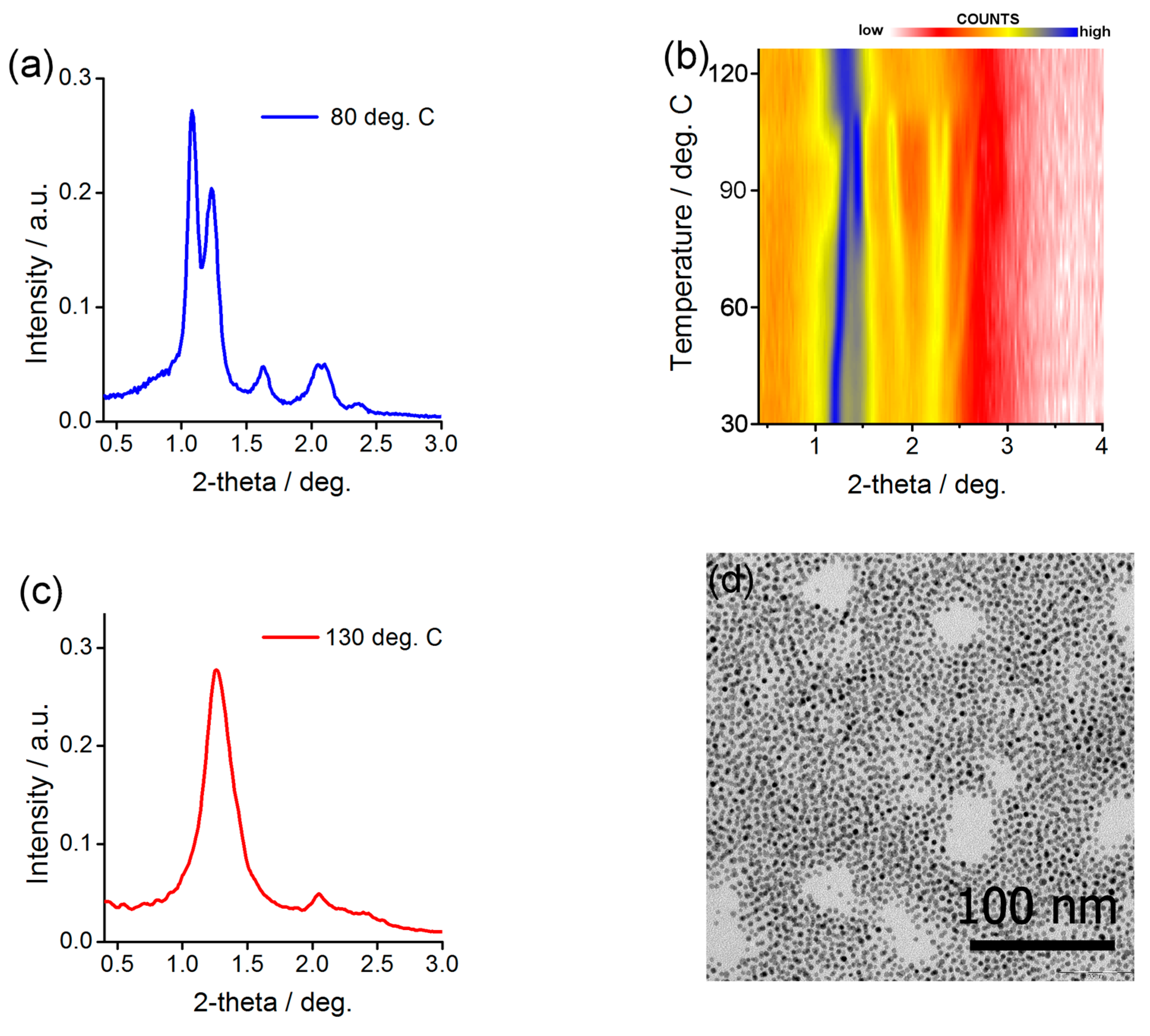
2.4. Preparing and Characterizing Assemblies of Au@L1/C16 Nanoparticles
2.5. Preparing and Characterizing Assemblies of Au@L1/CF Nanoparticles
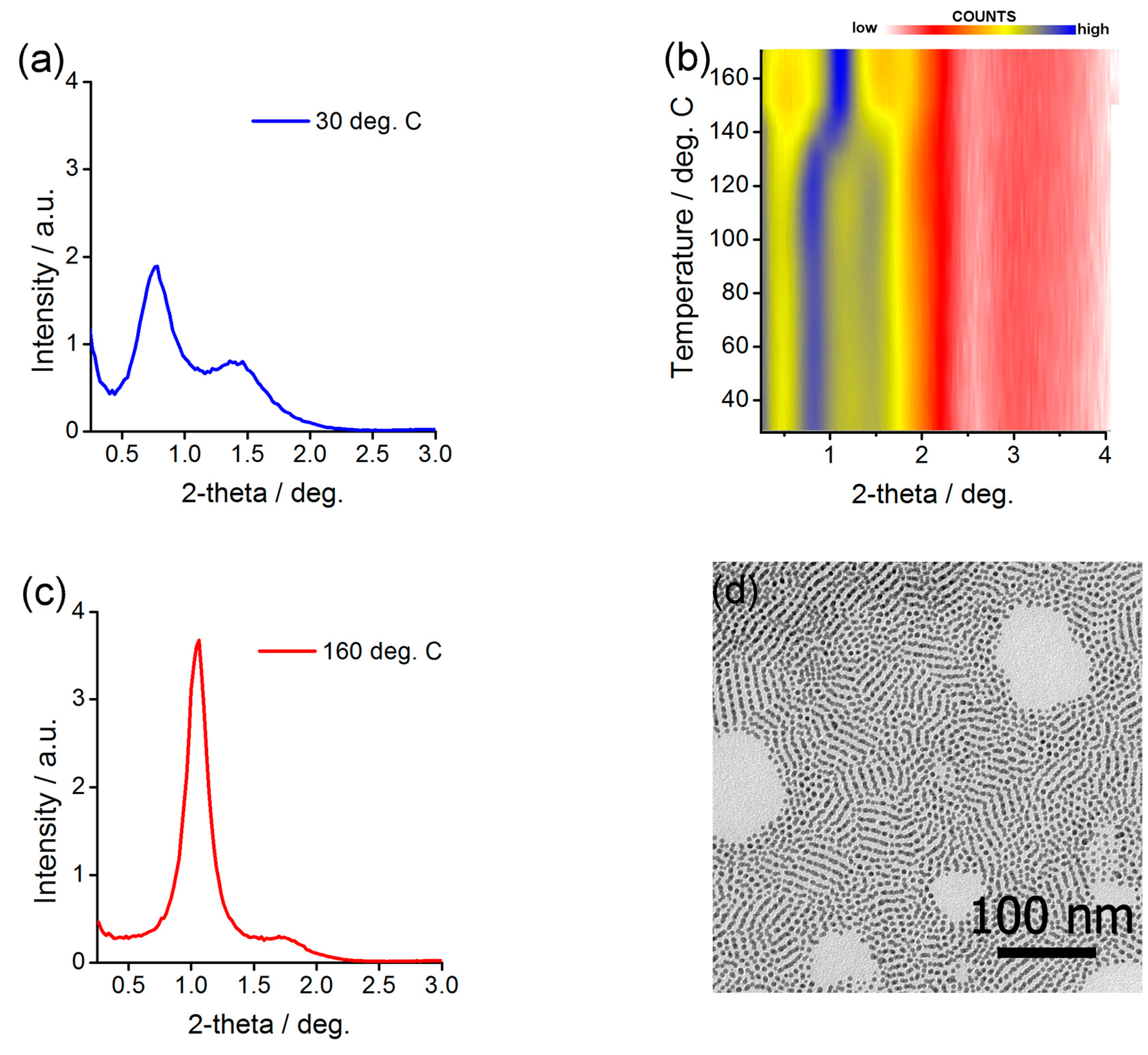
2.6. Preparing and Characterizing Assemblies of Ag@L1/C11OH Nanoparticles
3. Materials and Methods
3.1. Materials
3.2. Preparing LC-NPs Assemblies
3.3. Structural Investigation of LC-NPs Assemblies
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Silva, A.; Monticone, F.; Castaldi, G.; Galdi, V.; Alù, A.; Engheta, N. Performing mathematical operations with metamaterials. Science 2014, 343, 160–163. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, D.; Raab, N.; Noyong, M.; Santhanam, V.; Dittmann, R.; Simon, U. Resistive Switching of Sub-10 nm TiO2 Nanoparticle Self-Assembled Monolayers. Nanomaterials 2017, 7, 370. [Google Scholar] [CrossRef] [PubMed]
- Kagan, C.R.; Lifshitz, E.; Sargent, E.H.; Talapin, D.V. Building devices from colloidal quantum dots. Science 2016, 353, aac5523. [Google Scholar] [CrossRef] [PubMed]
- Urban, J.J. Prospects for thermoelectricity in quantum dot hybrid arrays. Nat. Nanotechnol. 2015, 10, 997–1001. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.; Wong, Z.J.; Mrejen, M.; Wang, Y.; Zhang, X. An ultrathin invisibility skin cloak for visible light. Science 2015, 349, 1310–1314. [Google Scholar] [CrossRef] [PubMed]
- Baron, A.; Aradian, A.; Ponsinet, V.; Barois, P. Self-assembled optical metamaterials. Opt. Laser Technol. 2016, 82, 94–100. [Google Scholar] [CrossRef]
- Hamon, C.; Novikov, S.M.; Scarabelli, L.; Solís, D.M.; Altantzis, T.; Bals, S.; Taboada, J.M.; Obelleiro, F.; Liz-Marzán, L.M. Collective Plasmonic Properties in Few-Layer Gold Nanorod Supercrystals. ACS Photonics 2015, 2, 1482–1488. [Google Scholar] [CrossRef] [PubMed]
- Gwo, S.; Wang, C.-Y.; Chen, H.-Y.; Lin, M.-H.; Sun, L.; Li, X.; Chen, W.-L.; Chang, Y.-M.; Ahn, H. Plasmonic Metasurfaces for Nonlinear Optics and Quantitative SERS. ACS Photonics 2016, 3, 1371–1384. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, J.; Wang, Y.; Chen, H. Emerging chirality in nanoscience. Chem. Soc. Rev. 2013, 42, 2930–2962. [Google Scholar] [CrossRef] [PubMed]
- Bagiński, M.; Szmurło, A.; Andruszkiewicz, A.; Wójcik, M.; Lewandowski, W. Dynamic self-assembly of nanoparticles using thermotropic liquid crystals. Liq. Cryst. 2016, 43, 2391–2409. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Pal, T. Interparticle coupling effect on the surface plasmon resonance of gold nanoparticles: From theory to applications. Chem. Rev. 2007, 107, 4797–4862. [Google Scholar] [CrossRef] [PubMed]
- Rožič, B.; Fresnais, J.; Molinaro, C.; Calixte, J.; Umadevi, S.; Lau-Truong, S.; Felidj, N.; Kraus, T.; Charra, F.; Dupuis, V.; et al. Oriented Gold Nanorods and Gold Nanorod Chains within Smectic Liquid Crystal Topological Defects. ACS Nano 2017, 11, 6728–6738. [Google Scholar] [CrossRef] [PubMed]
- Boles, M.A.; Engel, M.; Talapin, D.V. Self-Assembly of Colloidal Nanocrystals: From Intricate Structures to Functional Materials. Chem. Rev. 2016, 116, 11220–11289. [Google Scholar] [CrossRef] [PubMed]
- Steiner, A.M.; Mayer, M.; Seuss, M.; Nikolov, S.; Harris, K.D.; Alexeev, A.; Kuttner, C.; König, T.A.F.; Fery, A. Macroscopic Strain-Induced Transition from Quasi-infinite Gold Nanoparticle Chains to Defined Plasmonic Oligomers. ACS Nano 2017, 11, 8871–8880. [Google Scholar] [CrossRef] [PubMed]
- Mayer, M.; Tebbe, M.; Kuttner, C.; Schnepf, M.J.; König, T.A.F.; Fery, A. Template-assisted colloidal self-assembly of macroscopic magnetic metasurfaces. Faraday Discuss. 2016, 191, 159–176. [Google Scholar] [CrossRef] [PubMed]
- Tebbe, M.; Mayer, M.; Glatz, B.A.; Hanske, C.; Probst, P.T.; Müller, M.B.; Karg, M.; Chanana, M.; König, T.A.F.; Kuttner, C.; et al. Optically anisotropic substrates via wrinkle-assisted convective assembly of gold nanorods on macroscopic areas. Faraday Discuss. 2015, 181, 243–260. [Google Scholar] [CrossRef] [PubMed]
- Hanske, C.; Tebbe, M.; Kuttner, C.; Bieber, V.; Tsukruk, V.V.; Chanana, M.; König, T.A.F.; Fery, A. Strongly coupled plasmonic modes on macroscopic areas via template-assisted colloidal self-assembly. Nano Lett. 2014, 14, 6863–6871. [Google Scholar] [CrossRef] [PubMed]
- Hanske, C.; González-Rubio, G.; Hamon, C.; Formentín, P.; Modin, E.; Chuvilin, A.; Guerrero-Martínez, A.; Marsal, L.F.; Liz-Marzán, L.M. Large-Scale Plasmonic Pyramidal Supercrystals via Templated Self-Assembly of Monodisperse Gold Nanospheres. J. Phys. Chem. C 2017, 121, 10899–10906. [Google Scholar] [CrossRef]
- Flauraud, V.; Mastrangeli, M.; Bernasconi, G.D.; Butet, J.; Alexander, D.T.L.; Shahrabi, E.; Martin, O.J.F.; Brugger, J. Nanoscale topographical control of capillary assembly of nanoparticles. Nat. Nanotechnol. 2017, 12, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, L.; Kuang, H.; Xu, C.; Kotov, N.A. Dynamic nanoparticle assemblies. Acc. Chem. Res. 2012, 45, 1916–1926. [Google Scholar] [CrossRef] [PubMed]
- Udayabhaskararao, T.; Altantzis, T.; Houben, L.; Coronado-Puchau, M.; Langer, J.; Popovitz-Biro, R.; Liz-Marzán, L.M.; Vuković, L.; Král, P.; Bals, S.; et al. Tunable porous nanoallotropes prepared by post-assembly etching of binary nanoparticle superlattices. Science 2017, 358, 514–518. [Google Scholar] [CrossRef] [PubMed]
- Kuttner, C.; Chanana, M.; Karg, M.; Fery, A. Macromolecular Decoration of Nanoparticles for Guiding Self-Assembly in 2D and 3D. In Macromolecular Self-Assembly; Billon, L., Borisov, O., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 159–192. ISBN 9781118887813. [Google Scholar]
- Diroll, B.T.; Jishkariani, D.; Cargnello, M.; Murray, C.B.; Donnio, B. Polycatenar Ligand Control of the Synthesis and Self-Assembly of Colloidal Nanocrystals. J. Am. Chem. Soc. 2016, 138, 10508–10515. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, P.; Dai, M.; He, J.; Babu, T.; Xu, Y.L.; Deng, R.; Liang, R.; Lu, M.H.; Nie, Z.; et al. Ordering of gold nanorods in confined spaces by directed assembly. Macromolecules 2013, 46, 2241–2248. [Google Scholar] [CrossRef]
- Lin, H.; Lee, S.; Sun, L.; Spellings, M.; Engel, M.; Glotzer, S.C.; Mirkin, C.A. Clathrate colloidal crystals. Science 2017, 355, 931–935. [Google Scholar] [CrossRef] [PubMed]
- Kalsin, A.M.; Fialkowski, M.; Paszewski, M.; Smoukov, S.K.; Bishop, K.J.M.; Grzybowski, B.A. Electrostatic self-assembly of binary nanoparticle crystals with a diamond-like lattice. Science 2006, 312, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Höller, R.P.M.; Dulle, M.; Thomä, S.; Mayer, M.; Steiner, A.M.; Förster, S.; Fery, A.; Kuttner, C.; Chanana, M. Protein-Assisted Assembly of Modular 3D Plasmonic Raspberry-like Core/Satellite Nanoclusters: Correlation of Structure and Optical Properties. ACS Nano 2016, 10, 5740–5750. [Google Scholar] [CrossRef] [PubMed]
- Garbovskiy, Y.; Glushchenko, A. Ferroelectric Nanoparticles in Liquid Crystals: Recent Progress and Current Challenges. Nanomaterials 2017, 7, 361. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Macfarlane, R.J.; Jones, M.R.; Mirkin, C.A. Transmutable nanoparticles with reconfigurable surface ligands. Science 2016, 351, 579–582. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Smilgies, D.M.; Price, A.D.; Huber, D.L.; Clem, P.G.; Fan, H. Poly(N-isopropylacrylamide) surfactant-functionalized responsive silver nanoparticles and superlattices. ACS Nano 2014, 8, 4799–4804. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Cáceres, R.; Pacifico, J.; Pastoriza-Santos, I.; Pérez-Juste, J.; Fernández-Barbero, A.; Liz-Marzán, L.M. Au@pNIPAM thermosensitive nanostructures: Control over shell cross-linking, overall dimensions, and core growth. Adv. Funct. Mater. 2009, 19, 3070–3076. [Google Scholar] [CrossRef]
- Wang, K.; Jin, S.M.; Xu, J.; Liang, R.; Shezad, K.; Xue, Z.; Xie, X.; Lee, E.; Zhu, J. Electric-Field-Assisted Assembly of Polymer-Tethered Gold Nanorods in Cylindrical Nanopores. ACS Nano 2016, 10, 4954–4960. [Google Scholar] [CrossRef] [PubMed]
- Zep, A.; Wojcik, M.M.; Lewandowski, W.; Sitkowska, K.; Prominski, A.; Mieczkowski, J.; Pociecha, D.; Gorecka, E. Phototunable liquid-crystalline phases made of nanoparticles. Angew. Chem. Int. Ed. Engl. 2014, 53, 13725–13728. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, W.; Wójcik, M.; Górecka, E. Metal nanoparticles with liquid-crystalline ligands: Controlling nanoparticle superlattice structure and properties. Chemphyschem 2014, 15, 1283–1295. [Google Scholar] [CrossRef] [PubMed]
- Bisoyi, H.K.; Kumar, S. Liquid-crystal nanoscience: An emerging avenue of soft self-assembly. Chem. Soc. Rev. 2011, 40, 306–319. [Google Scholar] [CrossRef] [PubMed]
- Stamatoiu, O.; Mirzaei, J.; Feng, X.; Hegmann, T. Nanoparticles in Liquid Crystals and Liquid Crystalline Nanoparticles. In Topics in Current Chemistry; Springer: Berlin/Heidelberg, Germany, 2011; Volume 318, pp. 331–393. [Google Scholar]
- Qi, H.; Hegmann, T. Liquid crystal–gold nanoparticle composites. Liq. Cryst. Today 2011, 20, 102–114. [Google Scholar] [CrossRef]
- Blanc, C.; Coursault, D.; Lacaze, E. Ordering nano- and microparticles assemblies with liquid crystals. Liq. Cryst. Rev. 2013, 1, 83–109. [Google Scholar] [CrossRef]
- Saliba, S.; Mingotaud, C.; Kahn, M.L.; Marty, J.-D. Liquid crystalline thermotropic and lyotropic nanohybrids. Nanoscale 2013, 5, 6641–6661. [Google Scholar] [CrossRef] [PubMed]
- Nealon, G.L.; Greget, R.; Dominguez, C.; Nagy, Z.T.; Guillon, D.; Gallani, J.-L.; Donnio, B. Liquid-crystalline nanoparticles: Hybrid design and mesophase structures. Beilstein J. Org. Chem. 2012, 8, 349–370. [Google Scholar] [CrossRef] [PubMed]
- Hegmann, T.; Qi, H.; Marx, V.M. Nanoparticles in Liquid Crystals: Synthesis, Self-Assembly, Defect Formation and Potential Applications. J. Inorg. Organomet. Polym. Mater. 2007, 17, 483–508. [Google Scholar] [CrossRef]
- Matsubara, M.; Stevenson, W.; Yabuki, J.; Zeng, X.; Dong, H.; Kojima, K.; Chichibu, S.F.; Tamada, K.; Muramatsu, A.; Ungar, G.; et al. A Low-Symmetry Cubic Mesophase of Dendronized CdS Nanoparticles and Their Structure-Dependent Photoluminescence. Chem 2017, 2, 860–876. [Google Scholar] [CrossRef]
- Cseh, L.; Mang, X.; Zeng, X.; Liu, F.; Mehl, G.H.; Ungar, G.; Siligardi, G. Helically Twisted Chiral Arrays of Gold Nanoparticles Coated with a Cholesterol Mesogen. J. Am. Chem. Soc. 2015, 137, 12736–12739. [Google Scholar] [CrossRef] [PubMed]
- Wolska, J.M.; Pociecha, D.; Mieczkowski, J.; Górecka, E. Control of sample alignment mode for hybrid lamellar systems based on gold nanoparticles. Chem. Commun. 2014, 50, 7975. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, W.; Constantin, D.; Walicka, K.; Pociecha, D.; Mieczkowski, J.; Górecka, E. Smectic mesophases of functionalized silver and gold nanoparticles with anisotropic plasmonic properties. Chem. Commun. 2013, 49, 7845–7847. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, W.; Jatczak, K.; Pociecha, D.; Mieczkowski, J. Control of gold nanoparticle superlattice properties via mesogenic ligand architecture. Langmuir 2013, 29, 3404–3410. [Google Scholar] [CrossRef] [PubMed]
- Mang, X.; Zeng, X.; Tang, B.; Liu, F.; Ungar, G.; Zhang, R.; Cseh, L.; Mehl, G.H. Control of anisotropic self-assembly of gold nanoparticles coated with mesogens. J. Mater. Chem. 2012, 22, 11101–11106. [Google Scholar] [CrossRef]
- Kumar, S.; Pal, S.K.; Kumar, P.S.; Lakshminarayanan, V. Novel conducting nanocomposites: Synthesis of triphenylene-covered gold nanoparticles and their insertion into a columnar matrix. Soft Matter 2007, 3, 896–900. [Google Scholar] [CrossRef]
- Demortière, A.; Buathong, S.; Pichon, B.P.; Panissod, P.; Guillon, D.; Bégin-Colin, S.; Donnio, B. Nematic-like organization of magnetic mesogen-hybridized nanoparticles. Small 2010, 6, 1341–1346. [Google Scholar] [CrossRef] [PubMed]
- Kanie, K.; Matsubara, M.; Zeng, X.; Liu, F.; Ungar, G.; Nakamura, H.; Muramatsu, A. Simple cubic packing of gold nanoparticles through rational design of their dendrimeric corona. J. Am. Chem. Soc. 2012, 134, 808–811. [Google Scholar] [CrossRef] [PubMed]
- Wójcik, M.M.; Olesińska, M.; Sawczyk, M.; Mieczkowski, J.; Górecka, E. Controlling the Spatial Organization of Liquid Crystalline Nanoparticles by Composition of the Organic Grafting Layer. Chemistry 2015, 21, 10082–10088. [Google Scholar] [CrossRef] [PubMed]
- Wojcik, M.; Lewandowski, W.; Matraszek, J.; Mieczkowski, J.; Borysiuk, J.; Pociecha, D.; Gorecka, E. Liquid-crystalline phases made of gold nanoparticles. Angew. Chem. Int. Ed. Engl. 2009, 48, 5167–5169. [Google Scholar] [CrossRef] [PubMed]
- Houston, J.E.; Kim, H.I. Adhesion, friction, and mechanical properties of functionalized alkanethiol self-assembled monolayers. Acc. Chem. Res. 2002, 35, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Esplandiú, M.J.; Hagenström, H.; Kolb, D.M. Functionalized self-assembled alkanethiol monolayers on Au(111) electrodes: 1. Surface structure and electrochemistry. Langmuir 2001, 17, 828–838. [Google Scholar] [CrossRef]
- Vericat, C.; Vela, M.E.; Benitez, G.; Carro, P.; Salvarezza, R.C. Self-assembled monolayers of thiols and dithiols on gold: New challenges for a well-known system. Chem. Soc. Rev. 2010, 39, 1805–1834. [Google Scholar] [CrossRef] [PubMed]
- Techane, S.D.; Gamble, L.J.; Castner, D.G. Multi-technique Characterization of Self-assembled Carboxylic Acid Terminated Alkanethiol Monolayers on Nanoparticle and Flat Gold Surfaces. J. Phys. Chem. C Nanomater. Interfaces 2011, 115, 9432–9441. [Google Scholar] [CrossRef] [PubMed]
- Yong, J.; Chen, F.; Yang, Q.; Huo, J.; Hou, X. Superoleophobic surfaces. Chem. Soc. Rev. 2017, 46, 4168–4217. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.; Nguyen, T.L.A.; Deschenaux, R. Designing liquid-crystalline gold nanoparticles via the olefin cross-metathesis reaction. J. Porphyr. Phthalocyanines 2016, 20, 1060–1064. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Albert, S.; Nguyen, T.L.A.; Deschenaux, R. Liquid-crystalline fullerene-gold nanoparticles. RSC Adv. 2015, 5, 27224–27228. [Google Scholar] [CrossRef]
- Mischler, S.; Guerra, S.; Deschenaux, R. Design of liquid-crystalline gold nanoparticles by click chemistry. Chem. Commun. 2012, 48, 2183–2185. [Google Scholar] [CrossRef] [PubMed]
- Wojcik, M.M.; Gora, M.; Mieczkowski, J.; Romiszewski, J.; Gorecka, E.; Pociecha, D. Temperature-controlled liquid crystalline polymorphism of gold nanoparticles. Soft Matter 2011, 7, 10561. [Google Scholar] [CrossRef]
- Yu, C.H.; Schubert, C.P.J.; Welch, C.; Tang, B.J.; Tamba, M.-G.; Mehl, G.H. Design, synthesis, and characterization of mesogenic amine-capped nematic gold nanoparticles with surface-enhanced plasmonic resonances. J. Am. Chem. Soc. 2012, 134, 5076–5079. [Google Scholar] [CrossRef] [PubMed]
- Henry, A.-I.; Courty, A.; Pileni, M.-P.; Albouy, P.; Israelachvili, J. Tuning of solid phase in supracrystals made of silver nanocrystals. Nano Lett. 2008, 8, 2000–2005. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Schaeffer, N.; Pileni, M.-P. Ag Nanocrystals: 1. Effect of Ligands on Plasmonic Properties. J. Phys. Chem. B 2014, 118, 14070–14075. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Schaeffer, N.; Albouy, P.-A.; Pileni, M.-P. Surface Plasmon Resonance Properties of Silver Nanocrystals Differing in Size and Coating Agent Ordered in 3D Supracrystals. Chem. Mater. 2015, 27, 5614–5621. [Google Scholar] [CrossRef]
- Chapus, L.; Aubertin, P.; Joiret, S.; Lucas, I.T.; Maisonhaute, E.; Courty, A. Tunable SERS Platforms from Small Nanoparticle 3D Superlattices: A Comparison between Gold, Silver, and Copper. ChemPhysChem 2017, 18, 3066–3075. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Lin, M.H.; Wang, C.Y.; Chang, Y.M.; Gwo, S. Large-Scale Hot Spot Engineering for Quantitative SERS at the Single-Molecule Scale. J. Am. Chem. Soc. 2015, 137, 13698–13705. [Google Scholar] [CrossRef] [PubMed]
- Young, K.L.; Ross, M.B.; Blaber, M.G.; Rycenga, M.; Jones, M.R.; Zhang, C.; Senesi, A.J.; Lee, B.; Schatz, G.C.; Mirkin, C.A. Using DNA to Design Plasmonic Metamaterials with Tunable Optical Properties. Adv. Mater. 2014, 26, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, W.; Łojewska, T.; Szustakiewicz, P.; Mieczkowski, J.; Pociecha, D. Reversible switching of structural and plasmonic properties of liquid-crystalline gold nanoparticle assemblies. Nanoscale 2016, 8, 2656–2663. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, W.; Fruhnert, M.; Mieczkowski, J.; Rockstuhl, C.; Górecka, E. Dynamically self-assembled silver nanoparticles as a thermally tunable metamaterial. Nat. Commun. 2015, 6, 6590. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Ulman, A.; Fanfan, S.; Korniakov, A.; Loos, K. Mixed self-assembled monolayers of alkanethiolates on ultrasmooth gold do not exhibit contact-angle hysteresis. J. Am. Chem. Soc. 2005, 127, 4–5. [Google Scholar] [CrossRef] [PubMed]
- Bain, C.D.; Evall, J.; Whitesides, G.M. Formation of Monolayers by the Coadsorption of Thiols on Gold: Valiation in the Head Group, Tail Group, and Solvent. J. Am. Chem. Soc. 1989, 111, 7155–7164. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X. Novel phase-transfer preparation of monodisperse silver and gold nanoparticles at room temperature. Mater. Lett. 2008, 62, 2215–2218. [Google Scholar] [CrossRef]
- Yu, Y.; Guillaussier, A.; Voggu, V.R.; Houck, D.W.; Smilgies, D.-M.; Korgel, B.A. Bubble Assemblies of Nanocrystals: Superlattices without a Substrate. J. Phys. Chem. Lett. 2017, 4865–4871. [Google Scholar] [CrossRef] [PubMed]
- Elbert, K.C.; Jishkariani, D.; Wu, Y.; Lee, J.D.; Donnio, B.; Murray, C.B. Design, Self-Assembly, and Switchable Wettability in Hydrophobic, Hydrophilic, and Janus Dendritic Ligand–Gold Nanoparticle Hybrid Materials. Chem. Mater. 2017, 29, 8737–8746. [Google Scholar] [CrossRef]
- Ong, Q.; Luo, Z.; Stellacci, F. Characterization of Ligand Shell for Mixed-Ligand Coated Gold Nanoparticles. Acc. Chem. Res. 2017, 50, 1911–1919. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yu, M.; Kim, H.; Mameli, M.; Stellacci, F. Determination of monolayer-protected gold nanoparticle ligand–shell morphology using NMR. Nat. Commun. 2012, 3, 1182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grzelak, J.; Żuk, M.; Tupikowska, M.; Lewandowski, W. Modifying Thermal Switchability of Liquid Crystalline Nanoparticles by Alkyl Ligands Variation. Nanomaterials 2018, 8, 147. https://doi.org/10.3390/nano8030147
Grzelak J, Żuk M, Tupikowska M, Lewandowski W. Modifying Thermal Switchability of Liquid Crystalline Nanoparticles by Alkyl Ligands Variation. Nanomaterials. 2018; 8(3):147. https://doi.org/10.3390/nano8030147
Chicago/Turabian StyleGrzelak, Jan, Maciej Żuk, Martyna Tupikowska, and Wiktor Lewandowski. 2018. "Modifying Thermal Switchability of Liquid Crystalline Nanoparticles by Alkyl Ligands Variation" Nanomaterials 8, no. 3: 147. https://doi.org/10.3390/nano8030147
APA StyleGrzelak, J., Żuk, M., Tupikowska, M., & Lewandowski, W. (2018). Modifying Thermal Switchability of Liquid Crystalline Nanoparticles by Alkyl Ligands Variation. Nanomaterials, 8(3), 147. https://doi.org/10.3390/nano8030147




