Synthesis of Pt–Pd Bimetallic Porous Nanostructures as Electrocatalysts for the Methanol Oxidation Reaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Synthesis of Pt–Pd NPs
2.3. Structural Characterization
2.4. Electrochemical Measurements
3. Results
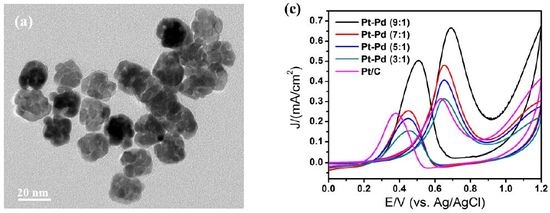
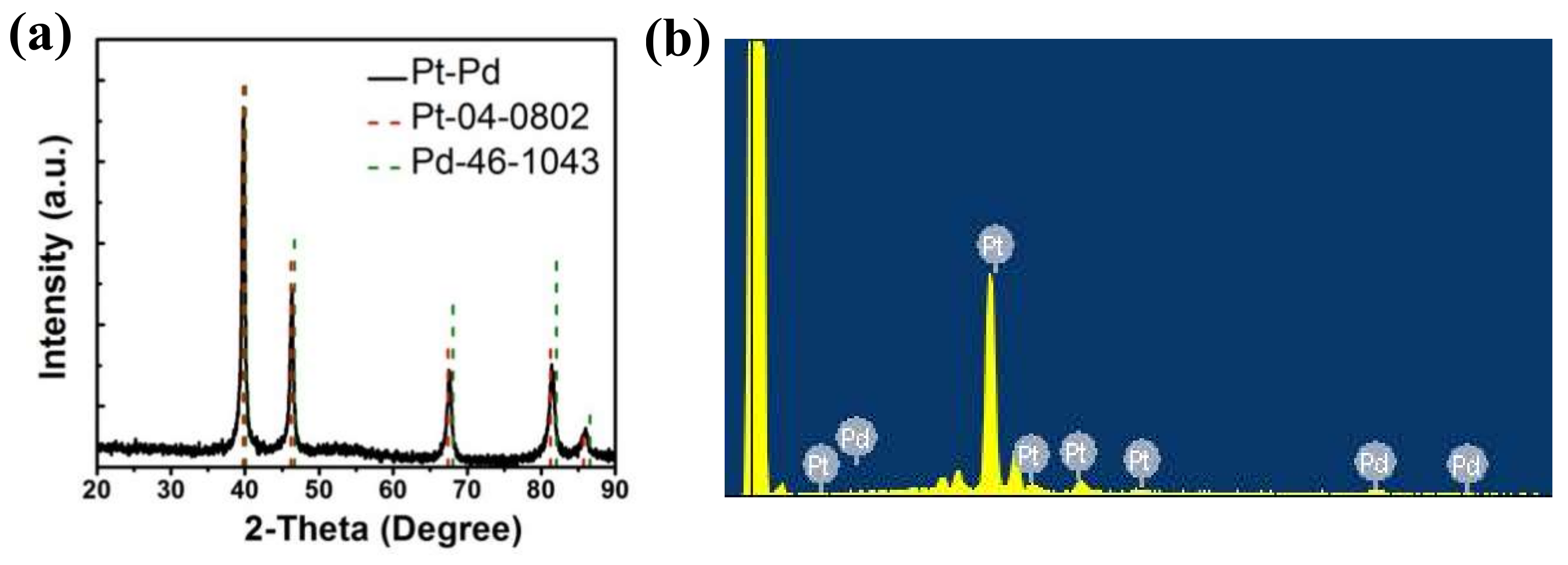
3.1. Structural Characterization of Porous Pt–Pd Nanoparticles
3.2. Controllable Synthesis of Pt–Pd NPs
3.3. Electrocatalytic Properties
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hong, J.W.; Kang, S.W.; Choi, B.-S.; Kim, D.; Lee, S.B.; Han, S.W. Controlled Synthesis of Pd–Pt Alloy Hollow Nanostructures with Enhanced Catalytic Activities for Oxygen Reduction. ACS Nano 2012, 6, 2410–2419. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jin, M.; Liu, H.; Wang, J.; Kim, M.J.; Yang, D.; Xie, Z.; Liu, J.; Xia, Y. Facile Synthesis of Pd–Pt Alloy Nanocages and Their Enhanced Performance for Preferential Oxidation of CO in Excess Hydrogen. ACS Nano 2011, 5, 8212–8222. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.; Jiang, M.; Camargo, P.H.; Cho, E.C.; Tao, J.; Lu, X.; Zhu, Y.; Xia, Y. Pd–Pt bimetallic nanodendrites with high activity for oxygen reduction. Science 2009, 324, 1302–1305. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.Q.; Yang, Y.; Shan, Y.; Fu, C.; Long, N.V.; Huang, Z.; Guo, X.; Nogami, M. Large-scale template-free synthesis of ordered mesoporous platinum nanocubes and their electrocatalytic property. Nanoscale 2015, 7, 19461–19467. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, H.; Wu, H.; Sun, Z.; Qian, L. Facile synthesis of Pt nanoparticles loaded porous graphene towards oxygen reduction reaction. Mater. Des. 2016, 96, 323–328. [Google Scholar] [CrossRef]
- Long, N.V.; Yang, Y.; Thi, C.M.; Van Minh, N.; Cao, Y.Q.; Nogami, M. The development of mixture, alloy, and core-shell nanocatalysts with nanomaterial supports for energy conversion in low-temperature fuel cells. Nano Energy 2013, 2, 636–676. [Google Scholar] [CrossRef]
- Cao, Y.Q.; Yang, Y.; Shan, Y.; Huang, Z. One-Pot and Facile Fabrication of Hierarchical Branched Pt−Cu Nanoparticles as Excellent Electrocatalysts for Direct Methanol Fuel Cells. ACS Appl. Mater. Interfaces 2016, 8, 5998–6003. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.-H.; Jang, Y.; Chung, D.Y.; Seo, P.; Jun, S.W.; Lee, J.E.; Oh, M.H.; Shokouhimehr, M.; Jung, N.; Yoo, S.J.; et al. A simple synthesis of urchin-like Pt–Ni bimetallic nanostructures as enhanced electrocatalysts for the oxygen reduction reaction. Chem. Commun. 2016, 52, 597–600. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y.; Zhang, J.; Shi, S.; Feng, P.; Wang, T. Observation of surface structural changes of Pt octahedron nanoparticles and its effect in electrocatalysis oxidation of methanol. Catal. Commun. 2009, 10, 1244–1247. [Google Scholar] [CrossRef]
- Zhang, H.; Jin, M.; Xia, Y. Enhancing the catalytic and electrocatalytic properties of Pt-based catalysts by forming bimetallic nanocrystals with Pd. Chem. Soc. Rev. 2012, 41, 8035–8049. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hao, F.; Wang, Y.; Zhang, Y.; Ge, C.; Lu, T. Facile synthesis of octahedral Pt–Pd nanoparticles stabilized by silsesquioxane for the electrooxidation of formic acid. Electrochim. Acta 2014, 133, 302–307. [Google Scholar] [CrossRef]
- Chu, Y.Y.; Wang, Z.B.; Dai, Z.; Gu, D.M.; Yin, G.P. Synthesis of Truncated-Octahedral Pt–Pd Nanocrystals Supported on Carbon Black as a Highly Efficient Catalyst for Methanol Oxidation. Fuel Cells 2014, 14, 49–55. [Google Scholar] [CrossRef]
- Scott, R.W.J.; Datye, A.K.; Crooks, R.M. Bimetallic Palladium-Platinum Dendrimer-Encapsulated Catalysts. J. Am. Chem. Soc. 2003, 125, 3708–3709. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chi, M.; Mazumder, V.; More, K.L.; Soled, S.; Henao, J.D.; Sun, S. Composition-Controlled Synthesis of Bimetallic PdPt Nanoparticles and Their Electro-oxidation of Methanol. Chem. Mater. 2011, 23, 4199–4203. [Google Scholar] [CrossRef]
- Yin, A.X.; Min, X.Q.; Zhang, Y.W.; Yan, C.H. Shape-selective synthesis and facet-dependent enhanced electrocatalytic activity and durability of mondisperse sub-10 nm Pt–Pd tetrahedrons cubes. J. Am. Chem. Soc. 2011, 133, 3816–3819. [Google Scholar] [CrossRef] [PubMed]
- Yin, A.X.; Min, X.Q.; Zhu, W.; Wu, H.S.; Zhang, Y.W.; Yan, C.H. Multiply twinned Pt–Pd nanoicosahedrons as highly active electrocatalysts for methanol oxidation. Chem. Commun. 2012, 48, 543–545. [Google Scholar] [CrossRef] [PubMed]
- Li, S.S.; Zheng, J.N.; Ma, X.; Hu, Y.Y.; Wang, A.J.; Chen, J.R.; Feng, J.J. Facile synthesis of hierarchical dendritic Pt-Pd nanogarlands supported on reduced graphene oxide with enhanced electrocatalytic properties. Nanoscale 2014, 6, 5708–5713. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-W.; Ko, A.R.; Kim, D.-Y.; Han, S.-B.; Park, K.-W. Octahedral Pt–Pd alloy catalysts with enhanced oxygen reduction activity and stability in proton exchange membrane fuel cells. RSC Adv. 2012, 2, 1119–1125. [Google Scholar] [CrossRef]
- Zhang, Z.C.; Hui, J.F.; Guo, Z.G.; Yu, Q.Y.; Xu, B.; Zhang, X.; Liu, Z.C.; Xu, C.M.; Gao, J.S.; Wang, X. Solvothermal synthesis of Pt–Pd alloys with selective shapes and their enhanced electrocatalytic activities. Nanoscale 2012, 4, 2633–2639. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.W.; Gan, Y.L.; Du, J.J.; Tian, D.; Zhang, R.; Yang, C.; Dai, Z. A review of hollow Pt-based nanocatalysts applied in proton exchange membrane fuel cells. J. Power Sources 2013, 232, 310–322. [Google Scholar] [CrossRef]
- Zhang, H.; Jin, M.; Wang, J.; Li, W.; Camargo, P.H.C.; Kim, M.J.; Yang, D.; Xie, Z.; Xia, Y. Synthesis of Pd–Pt bimettallic Nanocrystals with a concave structure through a bromide-induced galvanic replacement reaction, J. Am. Chem. Soc. 2011, 133, 6078–6089. [Google Scholar] [CrossRef] [PubMed]
- Esfahani, H.A.; Imura, M.; Yamaguchi, Y. All-metal mesoporous nanocolloids: Solution-phase synthesis pf core-shell Pd@Pt nanoparticles with a designed concave surface. Angew. Chem. Int. Ed. 2013, 125, 1–6. [Google Scholar]
- Zhang, G.; Lu, W.; Cao, L.; Qin, X.; Ding, F.; Tang, S.; Shao, Z.-G.; Yi, B. Large faceted Pd nanocrystals supported small Pt nanoparticles as highly durable electrocatalysts for oxygen reduction. J. Power Sources 2016, 326, 23–34. [Google Scholar] [CrossRef]
- Yin, Y.; Yang, Y.; Zhang, L.; Li, Z.; Ma, Y.; Huang, Z. Facile synthesis of Au/Pd nano-dogbones and their plasmon-enhanced visible-to-NIR light photocatalytic performance. RSC Adv. 2017, 7, 36923–36928. [Google Scholar] [CrossRef]





| Catalysts | ECSA (m2/g) | Mass Activity (mA/mg) | Specific Activity (mA/cm2) |
|---|---|---|---|
| Pt–Pd-190 °C | 31.59 | 210.35 | 0.67 |
| Pt–Pd-180 °C | 10.54 | 51.48 | 0.49 |
| Pt–Pd-170 °C | 35.48 | 65.85 | 0.19 |
| Pt/C | 85.63 | 268.3 | 0.31 |
| Catalysts | ECSA (m2/g) | Mass Activity (mA/mg) | Specific Activity (mA/cm2) | If/Ib |
|---|---|---|---|---|
| Pt–Pd (9:1) | 31.59 | 210.35 | 0.67 | 1.32 |
| Pt–Pd (7:1) | 23.51 | 112.95 | 0.48 | 1.89 |
| Pt–Pd (5:1) | 20.70 | 84.20 | 0.41 | 1.89 |
| Pt–Pd (3:1) | 20.90 | 65.60 | 0.31 | 2.02 |
| Pt/C | 85.63 | 268.3 | 0.31 | 1.29 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Cao, Y.; Yang, L.; Huang, Z.; Long, N.V. Synthesis of Pt–Pd Bimetallic Porous Nanostructures as Electrocatalysts for the Methanol Oxidation Reaction. Nanomaterials 2018, 8, 208. https://doi.org/10.3390/nano8040208
Yang Y, Cao Y, Yang L, Huang Z, Long NV. Synthesis of Pt–Pd Bimetallic Porous Nanostructures as Electrocatalysts for the Methanol Oxidation Reaction. Nanomaterials. 2018; 8(4):208. https://doi.org/10.3390/nano8040208
Chicago/Turabian StyleYang, Yong, Yanqin Cao, Lili Yang, Zhengren Huang, and Nguyen Viet Long. 2018. "Synthesis of Pt–Pd Bimetallic Porous Nanostructures as Electrocatalysts for the Methanol Oxidation Reaction" Nanomaterials 8, no. 4: 208. https://doi.org/10.3390/nano8040208