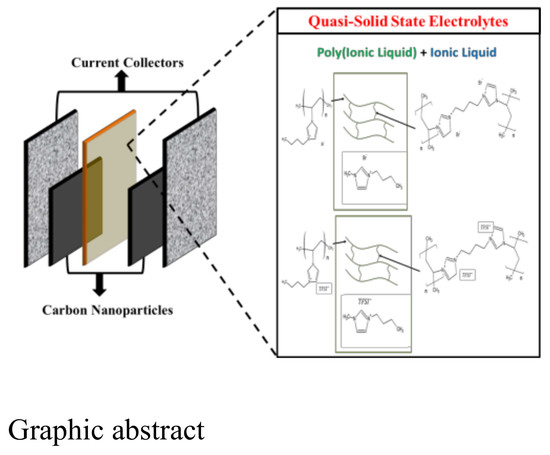
Crosslinked Polymer Ionic Liquid/Ionic Liquid Blends Prepared by Photopolymerization as Solid-State Electrolytes in Supercapacitors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Ionic Liquid
2.2.1. Preparation of Ionic Liquid Monomer
2.2.2. Preparation of PIL
2.3. Electrode Preparation
2.4. Characterization and Measurements
2.5. Electrochemical Characterization
3. Results and Discussion
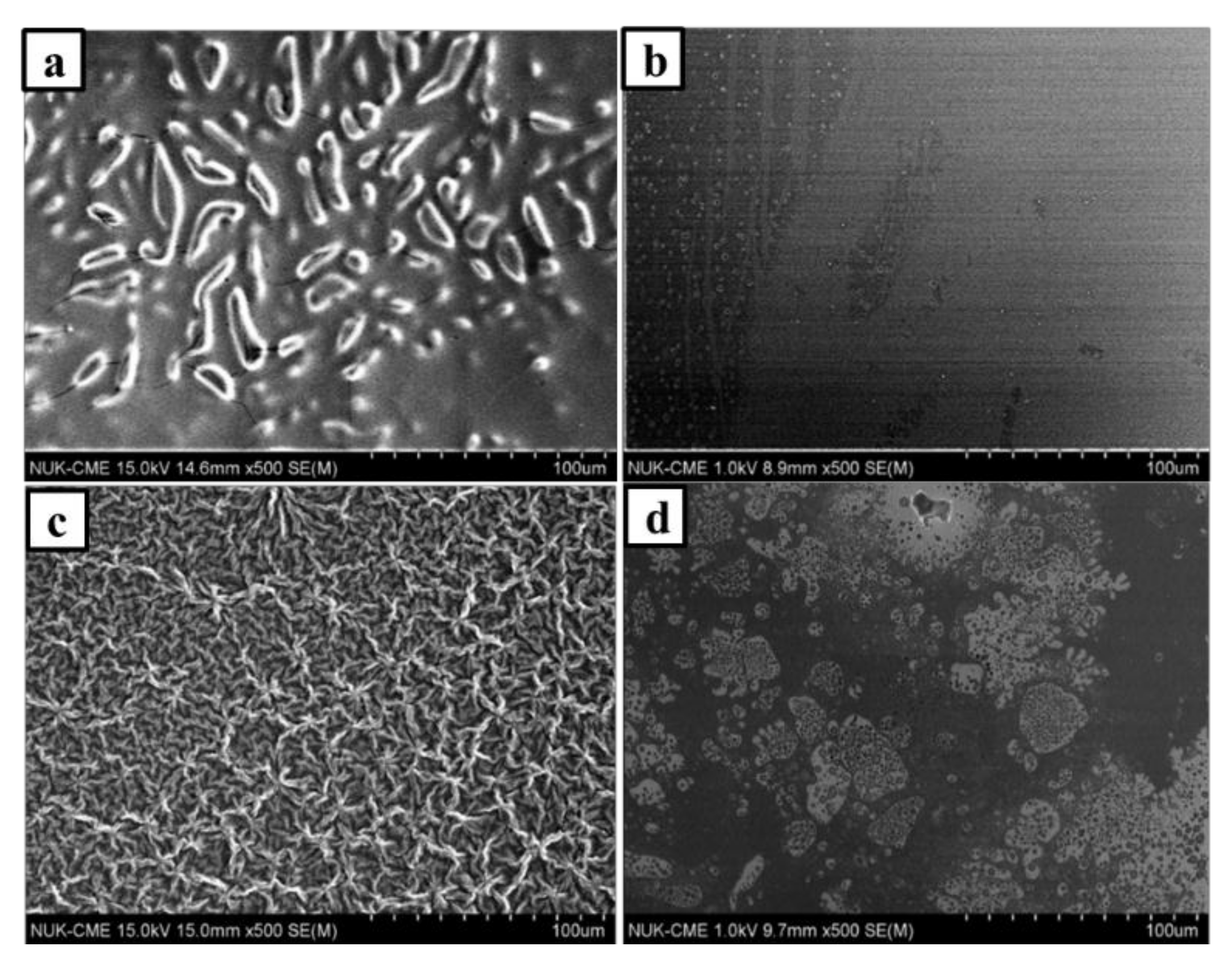
3.1. Morphology of PILs Derived from Photopolymerization
3.2. FTIR Spectra of PILs Derived from Photopolymerization
3.3. Thermal Gravity Analysis of PILs Derived from Photopolymerization
3.4. Ionic Conductivity
3.5. Cyclic Voltammogram
3.6. Electrochemical Impedance Spectroscopy (EIS)
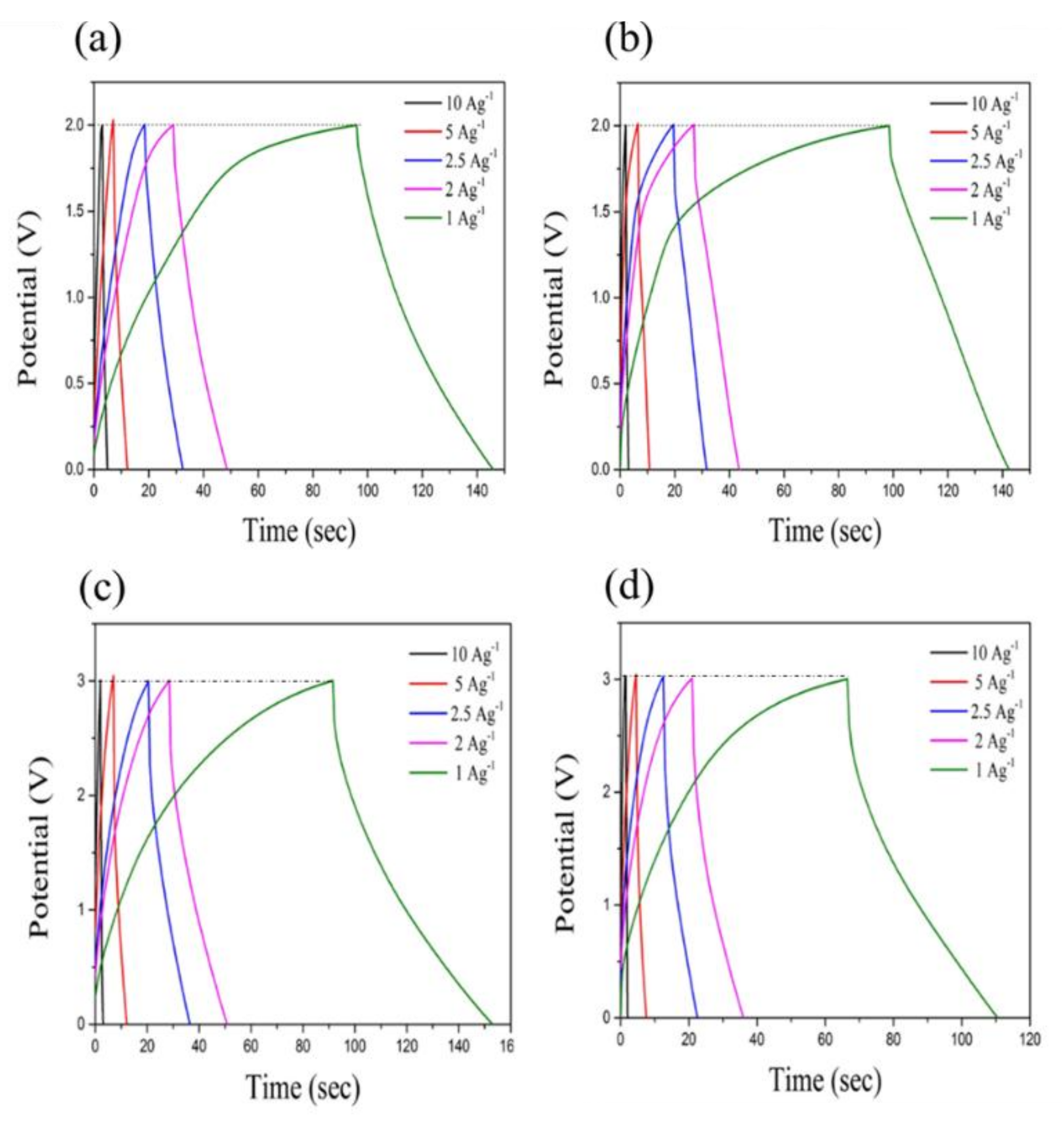
3.7. Galvanic Charge–Discharge (GCD)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Burke, A. Ultracapacitors: Why, how, and where is the technology. J. Power Sources 2000, 91, 37–50. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y.; Dunn, B. Where do batteries end and supercapacitors begin? Mater. Sci. 2014, 343, 1210–1211. [Google Scholar] [CrossRef] [Green Version]
- Schneuwly, A.; Gallay, R. Properties and applications of supercapacitors from the state-of-the-art to future trends. Proc. PCIM 2000, 1–10. [Google Scholar]
- Béguin, F.; Frąckowiak, E. Supercapacitors Materials, Systems, and Applications; John Wiley & Sons: Weinhein, Germany, 2013. [Google Scholar]
- Kötza, R.; Carlenb, M. Principle and applications of electrochemical capacitors. Electrochim. Acta 2000, 45, 2483–2498. [Google Scholar] [CrossRef]
- Ruiz, V.; Santamaría, R.; Granda, M.; Blanco, C. Long-term cycling of carbon-based supercapacitors in aqueous media. Electrochim. Acta 2009, 54, 4481–4486. [Google Scholar] [CrossRef]
- Armand, M.; Endres, F.; MacFarlane, D.R.; Ohno, H.; Scrosati, B. Ionic-liquid materials for the electrochemical challenges of the future. Nat. Mater. 2009, 8, 621–629. [Google Scholar] [CrossRef]
- Yamazaki, S.; Ito, T.; Yamagata, M.; Ishikawa, M. Non-aqueous electrochemical capacitor utilizing electrolytic redox reactions of bromide species in ionic liquid. Electrochim. Acta 2012, 86, 294–297. [Google Scholar] [CrossRef]
- Brachet, M.; Brousse, T.; Bideaua, J.L. All Solid-state symmetrical activated carbon electrochemical double layer capacitors designed with ionogel electrolyte. ECS Electrochem. Lett. 2014, 3, 112–115. [Google Scholar] [CrossRef]
- Ahn, Y.K.; Kim, B.; Ko, J.; You, D.J.; Yin, Z.; Kim, H.; Shin, D.; Cho, S.; Yoo, J.; Kim, Y.S. All solid state flexible supercapacitors operating at 4 V with a cross-linked polymer–ionic liquid electrolyte. J. Mater. Chem. A 2016, 4, 4386–4439. [Google Scholar] [CrossRef]
- Tiruye, G.A.; Munoz-Torrero, D.; Palma, J.; Anderson, M.; Marcilla, R. All-solid state supercapacitors operating at 3.5 V by using ionic liquid based polymer electrolytes. J. Power Sources 2016, 279, 472–480. [Google Scholar] [CrossRef]
- Tehfe, M.A.; Louradour, F.; Lalevée, J.; Fouassier, J.P. Photopolymerization reactions: On the way to a green and sustainable chemistry. Appl. Sci. 2013, 3, 490–514. [Google Scholar] [CrossRef]
- Bowers, D.I.; Maddams, W.F. Radiation curing of polymeric materials. Anal. Chim. 1990, 62, 668. [Google Scholar]
- Chen, S.M.; Wang, T.L.; Chang, P.Y.; Yang, C.H.; Lee, Y.C. Poly(ionic liquid) prepared by photopolymerization of ionic liquid monomers as quasi-solid-state electrolytes for dye-sensitized solar cells. React. Funct. Polym. 2016, 108, 103–112. [Google Scholar] [CrossRef]
- Yuan, J.; Antonietti, M. Poly(ionic liquid) latexes prepared by dispersion polymerization of ionic liquid monomers. Macromolecules 2011, 44, 744–750. [Google Scholar] [CrossRef]
- Yagci, Y.; Yilmaz, F.; Kiralp, S.; Toppare, L. Photoinduced polymerization of thiophene using iodonium salt. Macromol. Chem. Phys. 2005, 206, 1180–1182. [Google Scholar] [CrossRef]
- Ryu, K.S.; Lee, Y.; Han, K.S.; Parka, Y.J.; Kang, M.G.; Park, N.G.; Chang, S.H. Electrochemical supercapacitor based on polyaniline doped with lithium salt and active carbon electrodes. Solid State Ionics 2004, 175, 765–768. [Google Scholar] [CrossRef]
- Jang, H.S.; Raj, C.J.; Lee, W.G.; Kim, B.C.; Yu, K.H. Enhanced supercapacitive performances of functionalized activated carbon in novel gel polymer electrolytes with ionic liquid redox-mediated poly(vinyl alcohol)/phosphoric acid. RSC Adv. 2016, 6, 75376–75383. [Google Scholar] [CrossRef]
- Mecerreyes, D. Polymeric ionic liquids: Broadening the properties and applications of polyelectrolytes. Prog. Polym. Sci. 2011, 36, 1629–1648. [Google Scholar] [CrossRef]
- Chang, P.Y.; Yang, C.H. Photopolymerization of electroactive film applied to full polymer electrochromic device. Express Polym. Lett. 2017, 11, 176–186. [Google Scholar] [CrossRef]
- Liew, C.W.; Ramesh, S.; Arof, A.K. Good prospect of ionic liquid based-poly(vinyl alcohol) polymer electrolytes for supercapacitors with excellent electrical, electrochemical and thermal properties. Int. J. Hydrogen Energy 2014, 39, 2953–2963. [Google Scholar] [CrossRef]
- Liew, C.W.; Ramesh, S.; Arof, A.K. Characterization of ionic liquid added poly (vinyl alcohol)-based proton conducting polymer electrolytes and electrochemical studies on the supercapacitors. Int. J. Hydrogen Energy 2015, 40, 852–862. [Google Scholar] [CrossRef]
- Jannasch, P. Ion conducting electrolytes based on aggregating comblike poly(propylene oxide). Polymer 2001, 42, 8629–8635. [Google Scholar] [CrossRef]











| Component | Reaction Scheme |
|---|---|
| MBIB | 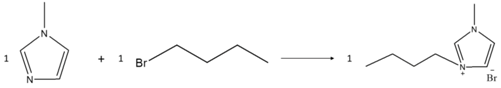 |
| BVIB |  |
| BDVIB | 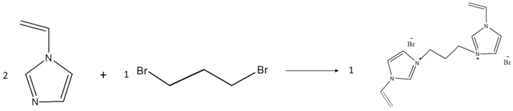 |
| MBIT |  |
| BVIT |  |
| BDVIT |  |
| PIL-(Br) | 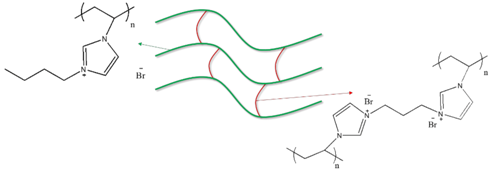 |
| PIL-(TFSI) |  |
| No. | PIL-(Br) | PIL-M-(Br) | Assignment | PIL-(TFSI) | PIL-M-(TFSI) | Assignment |
|---|---|---|---|---|---|---|
| 1 | 3077 | C–H bending | 3141 | C–H bending | ||
| 2 | 2981 2932 2869 | C–H streching | 2981 2854 | C–H streching | ||
| 3 | 1650 | C=C strehing | 1654 | C=N streching | ||
| 4 | 1546 | C=N bending | 1552 | C=N bending | ||
| 5 | 1457 | C–H bending | 1460 | C–H bending | ||
| 6 | 1151 | C–N bending | 1345 1126 | O=S=O streching | ||
| 7 | 600–900 | bromide | 1187 | C–N streching | ||
| 8 | 1046 | CF bending | ||||
| 9 | 849 | S–N–S | ||||
| Substance | ESR (Ω) | |
|---|---|---|
| PIL-M-(Br) | 16.2 | 18.1 |
| PIL-M-(TFSI) | 19.0 | 10.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.-H.; Wang, T.-L.; Lin, W.-C.; Lin, H.-Y.; Lee, M.-H.; Yang, C.-H. Crosslinked Polymer Ionic Liquid/Ionic Liquid Blends Prepared by Photopolymerization as Solid-State Electrolytes in Supercapacitors. Nanomaterials 2018, 8, 225. https://doi.org/10.3390/nano8040225
Wang P-H, Wang T-L, Lin W-C, Lin H-Y, Lee M-H, Yang C-H. Crosslinked Polymer Ionic Liquid/Ionic Liquid Blends Prepared by Photopolymerization as Solid-State Electrolytes in Supercapacitors. Nanomaterials. 2018; 8(4):225. https://doi.org/10.3390/nano8040225
Chicago/Turabian StyleWang, Po-Hsin, Tzong-Liu Wang, Wen-Churng Lin, Hung-Yin Lin, Mei-Hwa Lee, and Chien-Hsin Yang. 2018. "Crosslinked Polymer Ionic Liquid/Ionic Liquid Blends Prepared by Photopolymerization as Solid-State Electrolytes in Supercapacitors" Nanomaterials 8, no. 4: 225. https://doi.org/10.3390/nano8040225
APA StyleWang, P.-H., Wang, T.-L., Lin, W.-C., Lin, H.-Y., Lee, M.-H., & Yang, C.-H. (2018). Crosslinked Polymer Ionic Liquid/Ionic Liquid Blends Prepared by Photopolymerization as Solid-State Electrolytes in Supercapacitors. Nanomaterials, 8(4), 225. https://doi.org/10.3390/nano8040225