Gadolinium Tagged Osteoprotegerin-Mimicking Peptide: A Novel Magnetic Resonance Imaging Biospecific Contrast Agent for the Inhibition of Osteoclastogenesis and Osteoclast Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Peptides
2.3. Cyclisation of OPG Mimetic Peptides
2.4. Gadolinium Chelation into DOTA-OP3-4
2.5. Chromatographic Separation of the Peptides by LC-MS
2.6. Tandem MS
2.7. MRI Analysis
2.8. Cell Viability Studies
2.9. Monocyte Isolation and Osteoclastogenesis Studies
3. Statistical Analysis
4. Results and Discussion
4.1. Peptide Synthesis and Characterisation
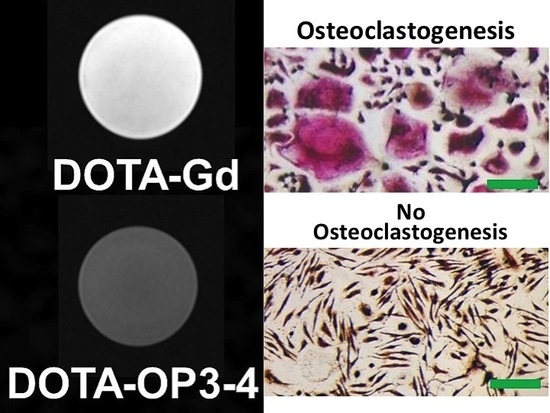
4.2. The Effect of the Peptides on Cell Viability and Osteoclastogenesis
4.3. Osteoclastogenesis Inhibition Studies
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, J.; Xiang, L.; Jiang, X.; Teng, B.; Sun, Y.; Chen, G.; Chen, J.; Zhang, J.V.; Ren, P.-G. Investigation of bioeffects of G protein-coupled receptor 1 on bone turnover in male mice. J. Orthop. Transl. 2017, 10, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, K.; Bollerslev, J.; Everts, V.; Karsdal, M.A. Osteoclast Activity and Subtypes as a Function of Physiology and Pathology—Implications for Future Treatments of Osteoporosis. Endocr. Rev. 2011, 32, 31–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crockett, J.C.; Helfrich, M.H. Chapter 6—Early Onset Pagetic Disorders: Implications for Late-Onset Paget’s Disease of Bone. In Advances in Pathobiology and Management of Paget’s Disease of Bone; Reddy, S.V., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 71–88. ISBN 978-0-12-805083-5. [Google Scholar]
- Sigl, V.; Penninger, J.M. Chapter 8—RANK and RANKL of Bones, T Cells, and the Mammary Glands A2—Lorenzo, Joseph. In Osteoimmunology (Second Edition); Horowitz, M.C., Choi, Y., Takayanagi, H., Schett, G., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 121–142. ISBN 978-0-12-800571-2. [Google Scholar]
- Kohli, S.S.; Kohli, V.S. Role of RANKL–RANK/osteoprotegerin molecular complex in bone remodeling and its immunopathologic implications. Indian J. Endocrinol. Metab. 2011, 15, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Kearns, A.E.; Khosla, S.; Kostenuik, P.J. Receptor Activator of Nuclear Factor κB Ligand and Osteoprotegerin Regulation of Bone Remodeling in Health and Disease. Endocr. Rev. 2008, 29, 155–192. [Google Scholar] [CrossRef] [PubMed]
- Appelman-Dijkstra, N.M.; Papapoulos, S.E. Modulating Bone Resorption and Bone Formation in Opposite Directions in the Treatment of Postmenopausal Osteoporosis. Drugs 2015, 75, 1049–1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Villiers, T. Johannes, Bone health and osteoporosis in postmenopausal women. Best Pract. Res. Clin. Obstet. Gynaecol. 2009, 23, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Weitzmann, M.N. The Role of Inflammatory Cytokines, the RANKL/OPG Axis, and the Immunoskeletal Interface in Physiological Bone Turnover and Osteoporosis. Scientifica 2013, 2013, 29. [Google Scholar] [CrossRef] [PubMed]
- Rizzoli, R.; Yasothan, U.; Kirkpatrick, P. Fresh from the pipeline: Denosumab. Nat. Rev. Drug Discov. 2010, 9, 591–592. [Google Scholar] [CrossRef] [PubMed]
- Anastasilakis, A.D.; Toulis, K.A.; Polyzos, S.A.; Anastasilakis, C.D.; Makras, P. Long-term treatment of osteoporosis: Safety and efficacy appraisal of denosumab. Ther. Clin. Risk Manag. 2012, 8, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Miller, S.C.; Kopecková, P.; Kopecek, J. Bone-targeting macromolecular therapeutics. Adv. Drug Deliv. Rev. 2005, 57, 1049–1076. [Google Scholar] [CrossRef] [PubMed]
- Ilvesaro, J.; Tavi, P.; Tuukkanen, J. Connexin-mimetic peptide Gap 27 decreases osteoclastic activity. BMC Musculoskelet. Disord. 2001, 2, 10. [Google Scholar] [CrossRef] [Green Version]
- Hur, J.; Ghosh, A.; Kim, K.; Ta, H.M.; Kim, H.; Kim, N.; Hwang, H.-Y.; Kim, K.K. Design of a RANK-Mimetic Peptide Inhibitor of Osteoclastogenesis with Enhanced RANKL-Binding Affinity. Mol. Cells 2016, 39, 316–321. [Google Scholar] [PubMed] [Green Version]
- Shin, J.; Kim, Y.-M.; Li, S.-Z.; Lim, S.-K.; Lee, W. Structure-Function of the TNF Receptor-like Cysteine-rich Domain of Osteoprotegerin. Mol. Cells 2008, 25, 352–357. [Google Scholar] [PubMed]
- Alles, N.; Soysa, N.S.; Hussain, M.D.A.; Tomomatsu, N.; Saito, H.; Baron, R.; Morimoto, N.; Aoki, K.; Akiyoshi, K.; Ohya, K. Polysaccharide nanogel delivery of a TNF-[alpha] and RANKL antagonist peptide allows systemic prevention of bone loss. Eur. J. Pharm. Sci. 2009, 37, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Aoki, K.; Saito, H.; Itzstein, C.; Ishiguro, M.; Shibata, T.; Blanque, R.; Mian, A.H.; Takahashi, M.; Suzuki, Y.; Yoshimatsu, M.; et al. A TNF receptor loop peptide mimic blocks RANK ligand-induced signaling, bone resorption, and bone loss. J. Clin. Investig. 2006, 116, 1525–1534. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Kinosaki, M.; Takami, M.; Choi, Y.; Zhang, H.; Murali, R. Disabling of Receptor Activator of Nuclear Factor- KappaB (RANK) Receptor Complex by Novel Osteoprotegerin-like Peptidomimetics Restores Bone Loss in vivo. J. Biol. Chem. 2004, 279, 8269–8277. [Google Scholar] [CrossRef] [PubMed]
- Ta, H.M.; Nguyen, G.; Thi, T.; Jin, H.M.; Choi, J.; Park, H.; Kim, N.; Hwang, H.-Y.; Kim, K.K. Structure-Based Development of a Receptor Activator of Nuclear Factor-kB Ligand (RANKL) Inhibitor Peptide and Molecular Basis for Osteoporosis. Proc. Natl. Acad. Sci. USA 2010, 107, 20281–20286. [Google Scholar] [CrossRef] [PubMed]
- Mbundi, L.; Meikle, S.T.; Santin, M. Biospecific Agents for Bone. WO2014122431, 31 January 2014. [Google Scholar]
- Góngora-Benítez, M.; Tulla-Puche, J.; Paradís-Bas, M.; Werbitzky, O.; Giraud, M.; Albericio, F. Optimized Fmoc solid-phase synthesis of the cysteine-rich peptide linaclotide. Pept. Sci. 2011, 96, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Winther, J.R.; Thorpe, C. Quantification of Thiols and Disulfides. Biochim. Biophys. Acta 2014, 1840, 838–846. [Google Scholar] [CrossRef] [PubMed]
- UCSF Mass Spectrometry Facility ProteinProspector v 5.10.0. Proteomics Tools for Mining Sequence Databases in Conjunction with Mass Spectrometry Experiments. Available online: http://prospector.ucsf.edu/prospector/mshome.htm (accessed on 21 October 2014).
- Annesley, T.M. Ion Suppression in Mass Spectrometry. Clin. Chem. 2003, 49, 1041–1044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perazella, M.A. Current Status of Gadolinium Toxicity in Patients with Kidney Disease. Clin. J. Am. Soc. Nephrol. 2009, 4, 461–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svenson, S.; Tomalia, D.A. Dendrimers in biomedical applications-reflections on the field. Adv. Drug Deliv. Rev. 2005, 57, 2106–2129. [Google Scholar] [CrossRef] [PubMed]
- Rogosnitzky, M.; Branch, S. Gadolinium-based contrast agent toxicity: A review of known and proposed mechanisms. Biometals 2016, 29, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Lu, Z.-R. Gadolinium-Based Contrast Agents for MR Cancer Imaging. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2013, 5, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Stetter, H.; Frank, W. Complex Formation with Tetraazacycloalkane-N,N′,N″,N‴-tetraacetic Acids as a Function of Ring Size. Angew. Chem. Int. Ed. Engl. 1976, 15, 686. [Google Scholar] [CrossRef]
- Viola-Villegas, N.; Doyle, R.P. The coordination chemistry of 1,4,7,10-tetraazacyclododecane-N,N′,N″,N′″-tetraacetic acid (H4DOTA): Structural overview and analyses on structure–stability relationships. Coord. Chem. Rev. 2009, 253, 1906–1925. [Google Scholar] [CrossRef]
- Hathout, G.; Jamshidi, N. Parameter Optimization for Quantitative Signal-Concentration Mapping Using Spoiled Gradient Echo MRI. Radiol. Res. Pract. 2012, 2012, 815729. [Google Scholar] [CrossRef] [PubMed]
- Overoye-Chan, K.; Koerner, S.; Looby, R.J.; Kolodziej, A.F.; Zech, S.G.; Deng, Q.; Chasse, J.M.; McMurry, T.J.; Caravan, P. EP-2104R: A Fibrin-Specific Gadolinium-Based MRI Contrast Agent for Detection of Thrombus. J. Am. Chem. Soc. 2008, 130, 6025–6039. [Google Scholar] [CrossRef] [PubMed]
- Varga-Szemes, A.; Kiss, P.; Rab, A.; Suranyi, P.; Lenkey, Z.; Simor, T.; Bryant, R.G.; Elgavish, G.A. In vitro Longitudinal Relaxivity Profile of Gd(ABE-DTTA), an Investigational Magnetic Resonance Imaging Contrast Agent. PLoS ONE 2016, 11, e0149260. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Goerner, F.L.; Snyder, C.; Morelli, J.N.; Hao, D.; Hu, D.; Li, X.; Runge, V.M. T1 Relaxivities of Gadolinium-Based Magnetic Resonance Contrast Agents in Human Whole Blood at 1.5, 3, and 7 T. Investig. Radiol. 2015, 50, 330–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bagher-Ebadian, H.; Paudyal, R.; Nagaraja, T.N.; Croxen, R.L.; Fenstermacher, J.D.; Ewing, J.R. MRI estimation of gadolinium and albumin effects on water proton. NeuroImage 2011, 54, S176–S179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noordin, S.; Winalski, C.S.; Shortkroff, S.; Mulkern, R.V. Factors affecting paramagnetic contrast enhancement in synovial fluid: Effects of electrolytes, protein concentrations, and temperature on water proton relaxivities from Mn ions and Gd chelated contrast agents. Osteoarthr. Cartil. 2010, 18, 964–970. [Google Scholar] [CrossRef] [PubMed]
- Jansen, I.; Vermeer, J.; Bloemen, V.; Stap, J.; Everts, V. Osteoclast Fusion and Fission. Calcif. Tissue Int. 2012, 90, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Susa, M.; Luong-Nguyen, N.-H.; Cappellen, D.; Zamurovic, N.; Gamse, R. Human primary osteoclasts: In vitro generation and applications as pharmacological and clinical assay. J. Transl. Med. 2004, 2, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zambonin, Z.A.; Teti, A.; Primavera, M.V. Monocytes from circulating blood fuse in vitro with purified osteoclasts in primary. J. Cell Sci. 1984, 66, 335–342. [Google Scholar]
- Naismith, J.H.; Sprang, S.R. Modularity in the TNF-receptor family. Trends Biochem. Sci. 1998, 23, 74–79. [Google Scholar] [CrossRef]
- Jones, E.Y.; Stuart, D.I.; Walker, N.P.C. Structure of tumour necrosis factor. Nature 1989, 338, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Banner, D.W.; D’Arcy, A.; Janes, W.; Gentz, R.; Schoenfeld, H.-J.; Broger, C.; Loetscher, H.; Lesslauer, W. Crystal structure of the soluble human 55 kd TNF receptor-human TNF[beta] complex: Implications for TNF receptor activation. Cell 1993, 73, 431–445. [Google Scholar] [CrossRef]
- Ito, S.; Wakabayashi, K.; Ubukata, O.; Hayashi, S.; Okada, F.; Hata, T. Crystal Structure of the Extracellular Domain of Mouse RANK Ligand at 2.2-Ã Resolution. J. Biol. Chem. 2002, 277, 6631–6636. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.; Nelson, C.A.; Ross, F.P.; Teitelbaum, S.L.; Fremont, D.H. Crystal structure of the TRANCE/RANKL cytokine reveals determinants of receptor-ligand specificity. J. Clin. Investig. 2001, 108, 971–979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naismith, J.H.; Devine, T.Q.; Kohno, T.; Sprang, S.R. Structures of the extracellular domain of the type I tumor necrosis factor receptor. Structure 1996, 4, 1251–1262. [Google Scholar] [CrossRef]
- Karpusas, M.; Hsu, Y.-M.; Wang, J.-H.; Thompson, J.; Lederman, S.; Chess, L.; Thomas, D. 2 å crystal structure of an extracellular fragment of human CD40 ligand. Structure 1995, 3, 1031–1039. [Google Scholar] [CrossRef]
- Mongkolsapaya, J.; Grimes, J.M.; Chen, N.; Xu, X.-N.; Stuart, D.I.; Jones, E.Y.; Screaton, G.R. Structure of the TRAIL-DR5 complex reveals mechanisms conferring specificity in apoptotic initiation. Nat. Struct. Mol. Biol. 1999, 6, 1048–1053. [Google Scholar]


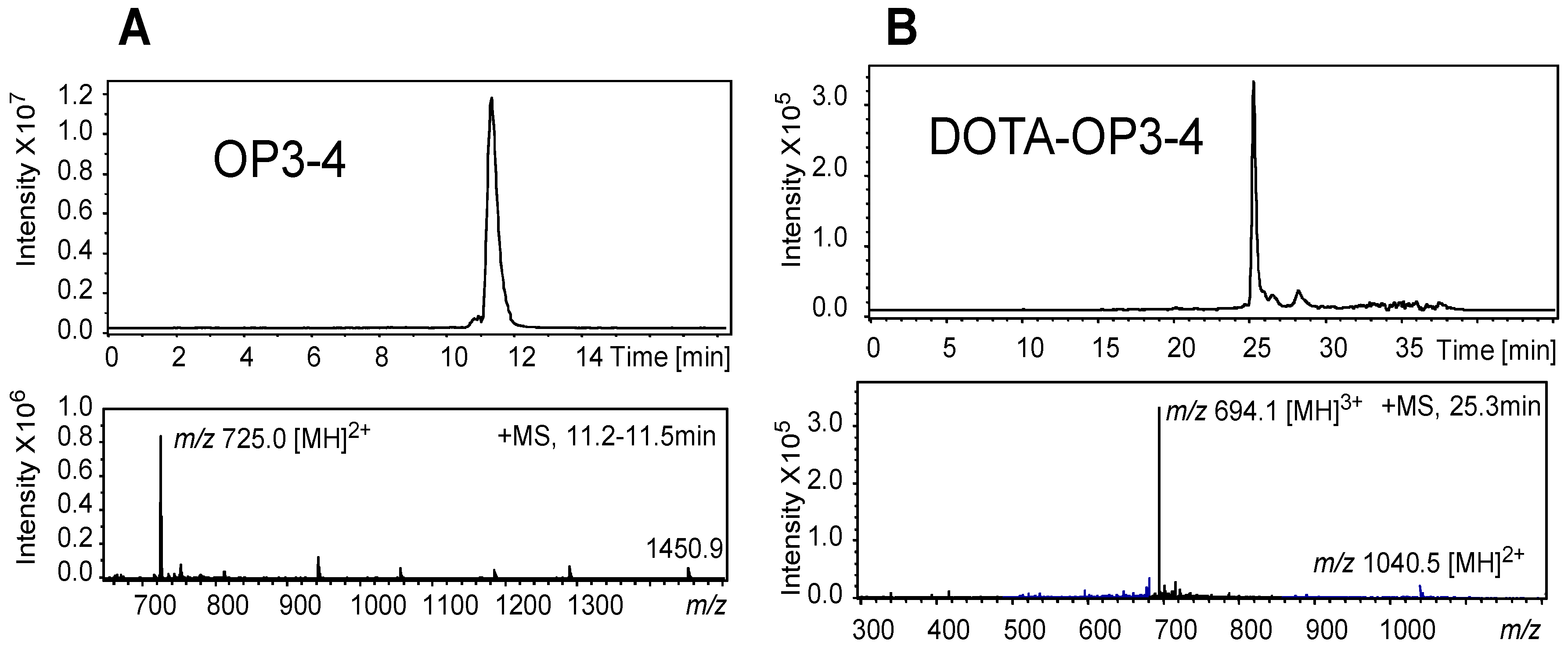




| TOF-MS (Full Scan MS) | ITMS MS-MS Product Ions (Ion Trap) | |||||
|---|---|---|---|---|---|---|
| Peptide | (m/z) | Tentative Assignment | SIM (m/z) | MS/MS Product Ion (m/z) | Tentative Assignment | MS/MS Scan Range |
| OP3-4 | 1451.6960 | [M + H]+ | 500–1500 | |||
| 726.377808 | [M + 2H]2+ | 726 | 886 | b7-2H | 300–1200 | |
| 733 | b6-H2O | |||||
| 715 | b6-2H2O | |||||
| 564 | y4 | |||||
| DOTA-OP3-4 | 1040.52070 | [M + 2H]2+ | ||||
| 694.01938 | [M + 3H]3+ | 694 | 987 | 300–1200 | ||
| 691 | b11-2H2O-NH32+ | |||||
| 596 | ||||||
| 514 | y72+ | |||||
| 458 | y62+ | |||||
| 401 | y52+ | |||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mbundi, L.; Meikle, S.T.; Busquets, R.; Dowell, N.G.; Cercignani, M.; Santin, M. Gadolinium Tagged Osteoprotegerin-Mimicking Peptide: A Novel Magnetic Resonance Imaging Biospecific Contrast Agent for the Inhibition of Osteoclastogenesis and Osteoclast Activity. Nanomaterials 2018, 8, 399. https://doi.org/10.3390/nano8060399
Mbundi L, Meikle ST, Busquets R, Dowell NG, Cercignani M, Santin M. Gadolinium Tagged Osteoprotegerin-Mimicking Peptide: A Novel Magnetic Resonance Imaging Biospecific Contrast Agent for the Inhibition of Osteoclastogenesis and Osteoclast Activity. Nanomaterials. 2018; 8(6):399. https://doi.org/10.3390/nano8060399
Chicago/Turabian StyleMbundi, Lubinda, Steve T. Meikle, Rosa Busquets, Nicholas G. Dowell, Mara Cercignani, and Matteo Santin. 2018. "Gadolinium Tagged Osteoprotegerin-Mimicking Peptide: A Novel Magnetic Resonance Imaging Biospecific Contrast Agent for the Inhibition of Osteoclastogenesis and Osteoclast Activity" Nanomaterials 8, no. 6: 399. https://doi.org/10.3390/nano8060399