Facet-Dependent Cuprous Oxide Nanocrystals Decorated with Graphene as Durable Photocatalysts under Visible Light
Abstract
:1. Introduction
2. Experiment
2.1. Photocatalyts Preparation
2.2. Characterizations of Photocatalyts
2.3. Photocatalytic Degradation of Organic Pollutants
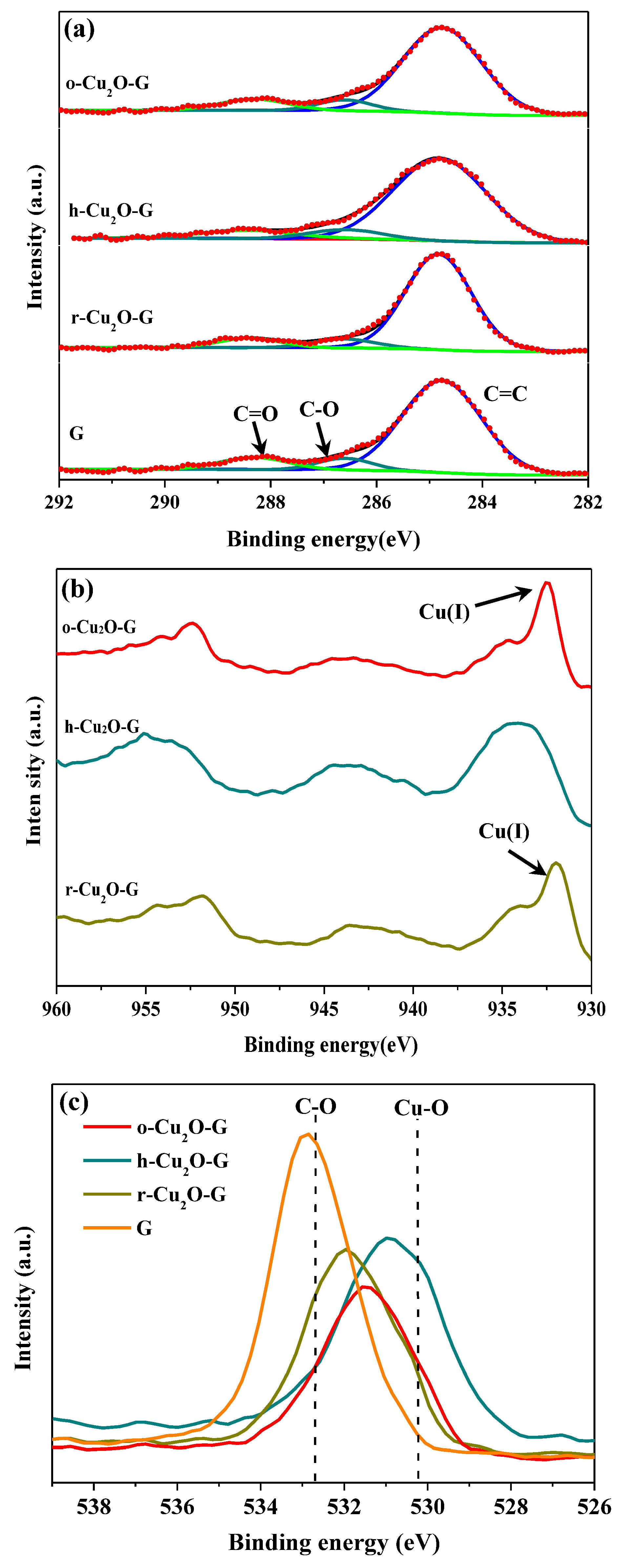
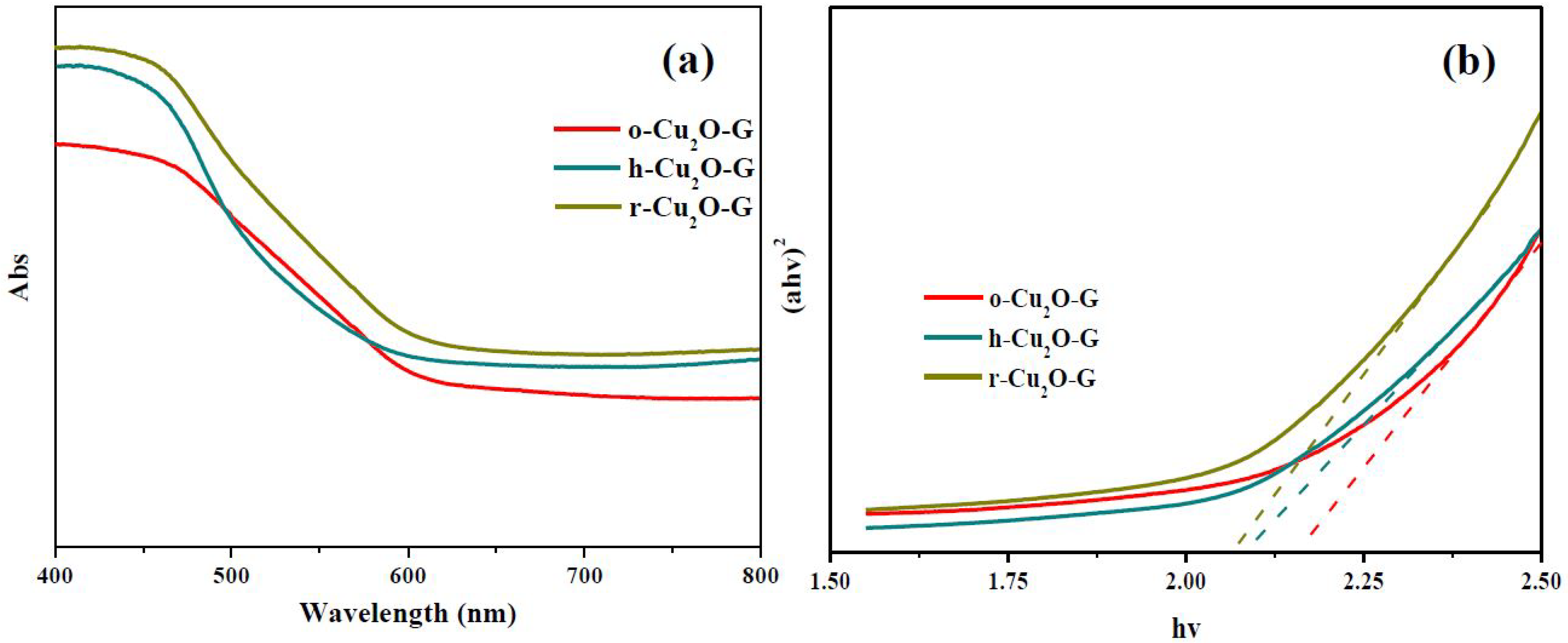
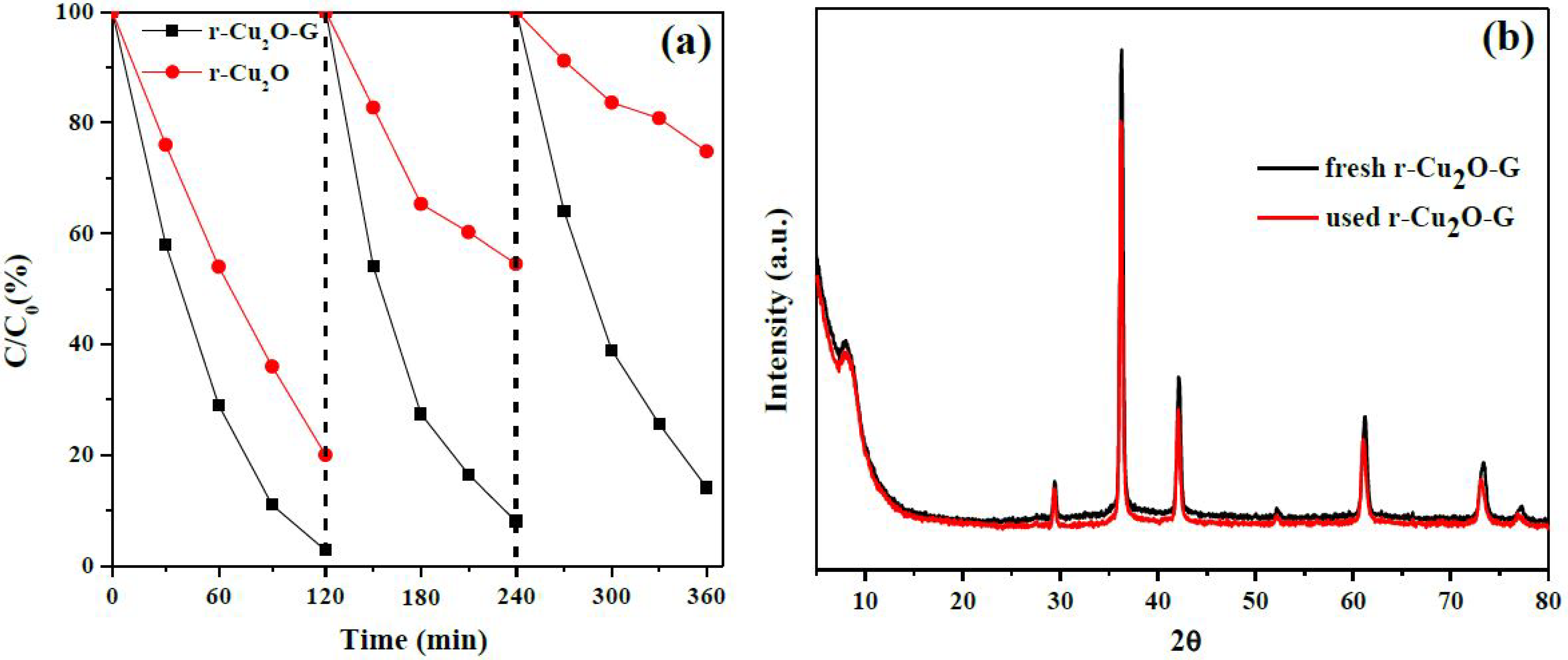
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gilja, V.; Novakovic, K.; Travas-Sejdic, J.; Hrnjak-Murgic, Z.; Rokovic, M.K.; Zic, M. Stability and synergistic effect of polyaniline/TiO2 photocatalysts in degradation of Azo Dye in wastewater. Nanomaterials 2018, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Liao, T.W.; Verbruggen, S.W.; Claes, N.; Yadav, A.; Grandjean, D.; Bals, S.; Lievens, P. TiO2 films modified with Au nanoclusters as self-cleaning surfaces under visible light. Nanomaterials 2018, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.Q.; Su, Y.R.; Jin, X.L.; Xie, H.Q.; Zhang, C. Recent advances in BiOX (X = Cl, Br and I) photocatalysts: Synthesis, modification, facet effects and mechanisms. Environ. Sci. Nano 2014, 1, 90–112. [Google Scholar] [CrossRef]
- Liu, S.-H.; Syu, H.-R. One-step fabrication of N-doped mesoporous TiO2 nanoparticles by self-assembly for photocatalytic water splitting under visible light. Appl. Energy 2012, 100, 48–154. [Google Scholar] [CrossRef]
- Pang, D.D.; Wang, Y.T.; Ma, X.D.; Ouyang, F. Fluorine promoted and silica supported TiO2 for photocatalytic decomposition of acrylonitrile under simulant solar light irradiation. Chem. Eng. J. 2014, 258, 43–50. [Google Scholar] [CrossRef]
- Liu, S.-H.; Syu, H.-R. High visible-light photocatalytic hydrogen evolution of C,N-codoped mesoporous TiO2 nanoparticles prepared via an ionic-liquid template approach. Int. J. Hydrogen Energy 2013, 38, 13856–13865. [Google Scholar] [CrossRef]
- Song, X.L.; Li, Y.Y.; Wei, Z.D.; Ye, S.Y.; Dionysiou, D.D. Synthesis of BiVO4/P25 composites for the photocatalytic degradation of ethylene under visible light. Chem. Eng. J. 2017, 314, 443–452. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Xu, J.A.; Wang, L.Q.; Zhang, H.Y.; Xu, P.; Duan, X.G.; Sun, H.Q.; Wang, S.B. Three-dDimensional BiOI/BiOX (X = Cl or Br) nanohybrids for enhanced visible-light photocatalytic activity. Nanomaterials 2017, 7, 64. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.Y.; Rui, Y.L.; Sun, K.L.; Cui, W.Q.; An, W.J. Surface decoration of ZnWO4 nanorods with Cu2O nanoparticles to bBuild heterostructure with enhanced photocatalysis. Nanomaterials 2018, 8, 33. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumar, A.; Sharma, G.; Al-Muhtaseb, A.H.; Naushad, M.; Ghfar, A.A.; Stadler, F.J. Quaternary magnetic BiOCl/g-C3N4/Cu2O/Fe3O4 nano-junction for visible light and solar powered degradation of sulfamethoxazole from aqueous environment. Chem. Eng. J. 2018, 334, 462–478. [Google Scholar] [CrossRef]
- Singh, M.; Jampaiah, D.; Kandjani, A.E.; Sabri, Y.M.; Della Gaspera, E.; Reineck, P.; Judd, M.; Langley, J.; Cox, N.; van Embden, J.; et al. Oxygen-deficient photostable Cu2O for enhanced visible light photocatalytic activity. Nanoscale 2018, 10, 6039–6050. [Google Scholar] [CrossRef] [PubMed]
- Sakar, M.; Balakumar, S. Reverse Ostwald ripening process induced dispersion of Cu2O nanoparticles in silver-matrix and their interfacial mechanism mediated sunlight driven photocatalytic properties. J. Photochem. Photobiol. A 2018, 356, 150–158. [Google Scholar] [CrossRef]
- Wei, Q.; Wang, Y.; Qin, H.Y.; Wu, J.M.; Lu, Y.F.; Chi, H.Z.; Yang, F.; Zhou, B.; Yu, H.L.; Liu, J.B. Construction of rGO wrapping octahedral Ag-Cu2O heterostructure for enhanced visible light photocatalytic activity. Appl. Catal. B Environ. 2018, 227, 132–144. [Google Scholar] [CrossRef]
- Su, Y.; Li, H.F.; Ma, H.B.; Wang, H.; Robertson, J.; Nathan, A. Dye-assisted transformation of Cu2O nanocrystals to amorphous CuxO nanoflakes for enhanced photocatalytic performance. ACS Omega 2018, 3, 1939–1945. [Google Scholar] [CrossRef]
- Sun, S.D. Recent advances in hybrid Cu2O-based heterogeneous nanostructures. Nanoscale 2015, 7, 10850–10882. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Q.; Wu, Z.S.; Gao, Z.Z.; Ye, B.C. Effect of different activated carbon as carrier on the photocatalytic activity of Ag-N-ZnO photocatalyst for methyl orange degradation under visible light irradiation. Nanomaterials 2018, 7, 258. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Shin, D.; Yeo, B.C.; Song, T.; Han, S.S.; Park, N.; Kim, S. Simultaneously controllable doping sites and the activity of a W-N codoped TiO2 photocatalyst. ACS Catal. 2016, 6, 2745–2753. [Google Scholar] [CrossRef]
- Luster, E.; Avisar, D.; Horovitz, I.; Lozzi, L.; Baker, M.A.; Grilli, R.; Mamane, H. N-doped TiO2-coated ceramic membrane for carbamazepine degradation in different water qualities. Nanomaterials 2017, 7, 206. [Google Scholar] [CrossRef] [PubMed]
- Bailón-García, E.; Elmouwahidi, A.; Álvarez, M.A.; Carrasco-Marín, F.; Pérez-Cadenas, A.F.; Maldonado-Hóda, F.J. New carbon xerogel-TiO2 composites with high performance as visible-light photocatalysts for dye mineralization. Appl. Catal. B Environ. 2017, 201, 29–40. [Google Scholar] [CrossRef]
- Klaysri, R.; Ratova, M.; Praserthdam, P.; Kelly, P.J. Deposition of visible light-active C-doped titania films via magnetron sputtering using CO2 as a source of carbon. Nanomaterials 2017, 7, 113. [Google Scholar] [CrossRef] [PubMed]
- Nica, I.C.; Stan, M.S.; Dinischiotu, A.; Popa, M.; Chifiriuc, M.C.; Lazar, V.; Pircalabioru, G.G.; Bezirtzoglou, E.; Iordache, O.G.; Varzaru, E.; et al. Innovative self-cleaning and biocompatible polyester textiles nano-decorated with Fe-N-doped titanium dioxide. Nanomaterials 2016, 6, 214. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.A.K.; Reddy, P.V.L.; Kwon, E.; Kim, K.H.; Akter, T.; Kalagara, S. Recent advances in photocatalytic treatment of pollutants in aqueous media. Environ. Int. 2016, 91, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.L.; Hu, H.W.; Zhang, Y.Y.; Chen, D.C.; Wu, L.P.; Li, X.J. Improving visible light-absorptivity and photoelectric conversion efficiency of a TiO2 nanotube anode film by sensitization with Bi2O3 Nanoparticles. Nanomaterials 2017, 7, 104. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Dutta, S. A review on H2 production through photocatalytic reactions using TiO2/TiO2-assisted catalysts. Fuel 2018, 220, 607–620. [Google Scholar] [CrossRef]
- Hu, J.L.; Tu, J.H.; Li, X.Y.; Wang, Z.Y.; Li, Y.; Li, Q.S.; Wang, F.P. Enhanced UV-Visible light photocatalytic activity by constructing appropriate heterostructures between mesopore TiO2 nanospheres and Sn3O4 nanoparticles. Nanomaterials 2017, 7, 336. [Google Scholar] [CrossRef] [PubMed]
- Petronella, F.; Truppi, A.; Ingrosso, C.; Placido, T.; Striccoli, M.; Curri, M.L.; Agostiano, A.; Comparelli, R. Nanocomposite materials for photocatalytic degradation of pollutants. Catal. Today 2017, 281, 85–100. [Google Scholar] [CrossRef]
- Huang, W.-C.; Lyu, L.-M.; Yang, Y.-C.; Huang, M.H. Synthesis of Cu2O nanocrystals from cubic to rhombic dodecahedral structures and their comparative photocatalytic activity. J. Am. Chem. Soc. 2012, 134, 1261–1267. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Guo, L. Facet-controlled synthetic strategy of Cu2O-based crystals for catalysis and sensing. Adv. Sci. 2015, 2, 1500140. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.-Z.; Hsia, C.-F.; Lin, Z.-W.; Chiang, C.; Chiang, Y.-W.; Huang, M.H. Highly facet-dependent photocatalytic properties of Cu2O crystals established through the formation of Au-decorated Cu2O heterostructures. Chem. Eur. J. 2016, 22, 12548–12556. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.S.; Yu, W.B.; Deng, Z.; Liu, J.; Jin, J.; Li, Y.; Wu, M.; Chen, L.H.; Su, B.L. Hollow Cu2O microspheres with two active {111} and {110} facets for highly selective adsorption and photodegradation of anionic dye. RSC Adv. 2015, 5, 55520–55526. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, B.; Zhang, T.R.; Gao, D.M.; Xu, A.W. Shape effects of Cu2O polyhedral microcrystals on photocatalytic activity. J. Phys. Chem. C 2010, 114, 5073–5079. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, F.Y.; Song, X.Y.; Yin, Z.L.; Bu, Y.X. Construction of reduced graphene oxide-supported Ag-Cu2O composites with hierarchical structures for enhanced photocatalytic activities and recyclability. J. Mater. Chem. A 2015, 3, 5923–5933. [Google Scholar] [CrossRef]
- Babu, S.G.; Vinoth, R.; Narayana, P.S.; Bahnemann, D.; Neppolian, B.S. Reduced graphene oxide wrapped Cu2O supported on C3N4: An efficient visible light responsive semiconductor photocatalyst. APL Mater. 2015, 3, 104415. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Li, G.J.; Zhang, X.S.; Ba, X.; Shi, G.D.; Li, Y.; Wong, P.K.; Yu, J.C.; Yu, Y. Enhanced activity and stability of carbon-decorated cuprous oxide mesoporous nanorods for CO2 reduction in artificial photosynthesis. ACS Catal. 2016, 6, 6444–6454. [Google Scholar] [CrossRef]
- Liu, S.-H.; Wei, Y.-S.; Lu, J.-S. Visible-light-driven photodegradation of sulfamethoxazole and methylene blue by Cu2O/rGO photocatalysts. Chemosphere 2016, 154, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, R.K.; Soin, N.; Roy, S.S. Role of graphene/metal oxide composites as photocatalysts, adsorbents and disinfectants in water treatment: A review. RSC Adv. 2014, 4, 3823–3851. [Google Scholar] [CrossRef]
- Stoller, M.D.; Park, S.; Zhu, Y.; An, J.; Ruoff, R.S. Graphene-based ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef] [PubMed]
- Mayorov, A.S.; Gorbachev, R.V.; Morozov, S.V.; Britnell, L.; Jalil, R.; Ponomarenko, L.A.; Blake, P.; Novoselov, K.S.; Watanabe, K.; Taniguchi, T.; et al. Micrometer-scale ballistic transport in encapsulated graphene at room temperature. Nano Lett. 2011, 11, 2396–2399. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Sun, H.Q.; Peng, W.C. 2D transition metal dichalcogenides and graphene-based ternary composites for photocatalytic hydrogen evolution and pollutants degradation. Nanomaterials 2017, 7, 62. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Sun, Z.H.; Zhang, Y.G.; Wang, X.; Bakenov, Z.; Yin, F.X. Micro-spherical sulfur/graphene oxide composite via spray drying for high performance lithium sulfur batteries. Nanomaterials 2018, 8, 50. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.X.; Wang, Y.; Sun, G.; Jia, T.K.; Jia, L.; Zhang, F.M.; Lin, L.; Zhang, B.Q.; Cao, J.L.; Zhang, Z.Y. Carbon nitride decorated ball-flower like Co3O4 hybrid composite: Hydrothermal synthesis and ethanol gas sensing application. Nanomaterials 2018, 8, 132. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.X.; Jia, L.P.; Wang, T.X.; Du, Y.L.; Wang, C.M. Preparation of carbon nanotube and graphene doped polyphenylene sulfide flexible film electrodes and the electrodeposition of Cu2O nanocrystals for hydrogen-generation. Int. J. Hydrogen Energy 2018, 43, 7356–7365. [Google Scholar] [CrossRef]
- Sharma, K.; Maiti, K.; Kim, N.H.; Hui, D.; Lee, J.H. Green synthesis of glucose-reduced graphene oxide supported Ag-Cu2O nanocomposites for the enhanced visible-light photocatalytic activity. Compos. Part B 2018, 138, 35–44. [Google Scholar] [CrossRef]
- González, J.A.; Villanueva, M.E.; Piehl, L.L.; Copello, G.J. Development of a chitin/graphene oxide hybrid composite for the removal of pollutant dyes: Adsorption and desorption study. Chem. Eng. J. 2015, 280, 42–48. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Liu, S.-H.; Yang, S.-W. Highly efficient cuprous oxide nanocrystals assisted with graphene for decolorization using visible light. Water Air Soil Pollut. 2018, 229, 67. [Google Scholar] [CrossRef]
- Pu, Y.-C.; Chou, H.-Y.; Kuo, W.-S.; Wei, K.-H.; Hsu, Y.-J. Interfacial charge carrier dynamics of cuprous oxide-reduced graphene oxide (Cu2O-rGO) nanoheterostructures and their related visible-light-driven photocatalysis. Appl. Catal. B Environ. 2017, 204, 21–32. [Google Scholar] [CrossRef]
- Gao, Z.; Liu, J.; Xu, F.; Wu, D.; Wu, Z.; Jiang, K. One-pot synthesis of graphene-cuprous oxide composite with enhanced photocatalytic activity. Solid State Sci. 2012, 14, 276–280. [Google Scholar] [CrossRef]
- Chang, X.F.; Gondal, M.A.; Al-Saadi, A.A.; Ali, M.A.; Shen, H.; Zhou, Q.; Zhang, J.; Du, M.; Liu, Y.; Ji, G. Photodegradation of Rhodamine B over unexcited semiconductor compounds of BiOCl and BiOBr. J. Colloid Interface Sci. 2012, 377, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.T.W.; Ball, J.M.; Barea, E.M.; Abate, A.; Alexander-Webber, J.A.; Huang, J.; Saliba, M.; Mora-Sero, I.; Bisquert, J.; Snaith, H.J.; et al. Low-temperature processed electron collection layers of graphene/TiO2 nanocomposites in thin film perovskite solar cells. Nano Lett. 2014, 14, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.C.; Li, Z.S.; Zou, Z.G. Photodegradation of Rhodamine B and methyl orange over boron-doped g-C3N4 under visible light irradiation. Langmuir 2010, 26, 3894–3901. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhou, X.; Wang, R.; Hu, C.; Qu, J. Characterization and photostability of Cu2O-Ag-AgBr/Al2O3 for the degradation of toxic pollutants with visible-light irradiation. Appl. Catal. B 2014, 154–155, 44–50. [Google Scholar] [CrossRef]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.-H.; Lu, J.-S. Facet-Dependent Cuprous Oxide Nanocrystals Decorated with Graphene as Durable Photocatalysts under Visible Light. Nanomaterials 2018, 8, 423. https://doi.org/10.3390/nano8060423
Liu S-H, Lu J-S. Facet-Dependent Cuprous Oxide Nanocrystals Decorated with Graphene as Durable Photocatalysts under Visible Light. Nanomaterials. 2018; 8(6):423. https://doi.org/10.3390/nano8060423
Chicago/Turabian StyleLiu, Shou-Heng, and Jun-Sheng Lu. 2018. "Facet-Dependent Cuprous Oxide Nanocrystals Decorated with Graphene as Durable Photocatalysts under Visible Light" Nanomaterials 8, no. 6: 423. https://doi.org/10.3390/nano8060423




