Preparation of TiO2/Carbon Nanotubes/Reduced Graphene Oxide Composites with Enhanced Photocatalytic Activity for the Degradation of Rhodamine B
Abstract
:1. Introduction
2. Experimental Part
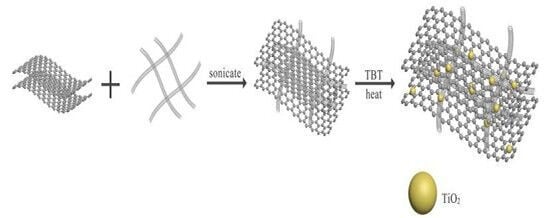
2.1. Sample Preparation
2.2. Characterization
2.3. Photocatalytic Activity
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kaneko, M.; Okura, I. Photocatalysis: Science and Technology; Springer: Berlin, Germany, 2002; pp. 20–25. [Google Scholar]
- Ma, L.; Chen, A.; Zhang, Z.; Lu, J.; He, H.; Li, C. In-situ fabrication of CNT/TiO2 interpenetrating network film on nickel substrate by chemical vapour deposition and application in photoassisted water electrolysis. Catal. Commun. 2012, 21, 27–31. [Google Scholar] [CrossRef]
- Shah, M.S.A.S.; Zhang, K.; Park, A.R.; Kim, K.S.; Park, A.G.; Park, J.H.; Yoo, P.J. Single-stepsolvothermal synthesis of mesoporous Ag-TiO2-reduced graphene oxide ternary composites with enhanced photocatalytic activity. Nanoscale 2013, 5, 5093–5101. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; He, F.; Gai, S.L.; Gao, P.; Chen, Y.J.; Yang, P.P. A novel 3D structured reduced graphene oxide/TiO2 composite: Synthesis and photocatalytic performance. J. Mater. Chem. A 2014, 2, 3605–3612. [Google Scholar] [CrossRef]
- Gu, X.; Chai, T.; Gao, Y.H. Three dimensional TiO2-graphene with improved adsorption capacities and photocatalytic. Appl. Chem. Ind. 2018, 47, 126–130. [Google Scholar]
- Wang, C.; Cao, M.; Wang, P.; Ao, Y.; Hou, J.; Qian, J. Preparation of graphene-carbon nanotube-TiO2 composites with enhanced photocatalytic activity for the removal of dye and Cr (VI). Appl. Catal. A Gen. 2014, 473, 83–89. [Google Scholar] [CrossRef]
- Pan, X.; Zhao, Y.; Liu, S.; Korzeniewski, C.L.; Wang, S.; Fan, Z. Comparing graphene-TiO2 nanowire and graphene-TiO2 nanoparticle composite photocatalysts. ACS Appl. Mater. Interfaces 2012, 4, 3944–3950. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Liu, S.; Fu, X.; Xu, Y.J. Synthesis of M@TiO2 (M = Au, Pd, Pt) core-shell nanocomposites with tunable photoreactivity. J. Phys. Chem. C 2011, 115, 9136–9145. [Google Scholar] [CrossRef]
- Xiao, F. An efficient layer-by-layer self-assembly of metal-TiO2 nanoring/nanotube heterostructures, M/T-NRNT (M = Au, Ag, Pt), for versatile catalytic applications. Chem. Commun. 2012, 48, 6538–6540. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Yang, W.; Zhang, W.; Liu, G.; Yue, P. Improved photocatalytic activity of Sn4+ doped TiO2 nanoparticulate films prepared by plasma-enhanced chemical vapor deposition. New J. Chem. 2004, 28, 218–222. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, R.; Chen, D.; Hu, X.; Chen, P.; Chen, Z.; Li, D. Synthesis and characterization of CNT/TiO2/ZnO composites with high photocatalytic performance. Catalysts 2018, 8, 151. [Google Scholar] [CrossRef]
- Chen, J.S.; Luan, D.; Li, C.M.; Boey, F.Y.; Qiao, S.; Lou, X.W. TiO2 and SnO2@TiO2 hollow spheres assembled from anatase TiO2 nanosheets with enhanced lithium storage properties. Chem. Commun. 2010, 46, 8252–8254. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Chen, D.; Huang, Y.; Hu, X.; Chen, P.; Chen, Z.; Li, D. Controllable deposition of titanium dioxides onto carbon nanotubes in aqueous solutions. Integr. Ferroelectr. 2016, 183, 43–53. [Google Scholar] [CrossRef]
- Tu, W.; Zhou, Y.; Liu, Q.; Yan, S.; Bao, S.; Wang, X.; Xiao, M.; Zou, Z. An in situ simultaneous reduction-hydrolysis technique for fabrication of TiO2-graphene 2D sandwich-like hybrid nanosheets: Graphene-promoted selectivity of photocatalytic-driven hydrogenation and coupling of CO2 into methane and ethane. Adv. Funct. Mater. 2013, 23, 1743–1749. [Google Scholar] [CrossRef]
- Shen, L.; Zhang, X.; Li, H.; Yuan, C.; Cao, G. Design and tailoring of a three-dimensional TiO2-graphene-carbon nanotube nanocomposite for fast lithium storage. J. Phys. Chem. Lett. 2011, 2, 3096–3101. [Google Scholar] [CrossRef]
- Rastogi, M.; Kushwaha, H.S.; Vaish, R. Highly efficient visible light mediated azo dye degradation through barium titanate decorated reduced graphene oxide sheets. Electron. Mater. Lett. 2016, 12, 281–289. [Google Scholar] [CrossRef]
- Compton, O.C.; Nguyen, S.T. Graphene oxide, highly reduced graphene oxide, and graphene: Versatile building blocks for carbon-based materials. Small 2010, 6, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Khare, P.; Verma, S.; Bhati, A.; Sonker, A.K.; Tripathi, K.M.; Sonkar, S.K. Pollutant soot for pollutant dye degradation: soluble graphene nanosheets for visible light induced photodegradation of methylene blue. Acs Sustain. Chem. Eng. 2017, 5, 8860–8869. [Google Scholar]
- Zhang, H.; Xu, P.; Du, G.; Chen, Z.; Oh, K.; Pan, D.; Jiao, Z. A facile one-step synthesis of TiO2/graphene composites for photodegradation of methyl orange. Nano Res. 2011, 4, 274–283. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Shiota, S.; Hirakawa, H.; Tanaka, S.; Ichikawa, S.; Hirai, T. Titanium dioxide/reduced graphene oxide hybrid photocatalysts for efficient and selective partial oxidation of cyclohexane. ACS Catal. 2016, 7, 293–300. [Google Scholar] [CrossRef]
- Mishra, Y.K.; Adelung, R. ZnO tetrapod materials for functional applications. Mater. Today 2017. [Google Scholar] [CrossRef]
- Malekshoar, G.; Pal, K.; He, Q.; Yu, A.; Ray, A.K. Enhanced solar photocatalytic degradation of phenol with coupled graphene-based titanium dioxide and zinc oxideo. Ind. Eng. Chem. Res. 2014, 53, 18824–18832. [Google Scholar] [CrossRef]
- Fu, S. Preparation of ZnO/TiO2 Coupled Films and Its Photocatalytic Properties. Master’s Thesis, Zhejiang University, Hangzhou, China, 2015. [Google Scholar]
- Tyagi, A.; Tripathi, K.M.; Singh, N.; Choudhary, S.; Gupta, R.K. Green synthesis of carbon quantum dots from lemon peel waste: Applications in sensing and photocatalysis. RSC. Adv. 2016, 6, 72423–72432. [Google Scholar] [CrossRef]
- Gogoi, S.; Karak, N. Solar-driven fydrogen peroxide production using polymer-supported carbon bots as heterogeneous catalyst. Nano Micro. Lett. 2017, 9, 40–51. [Google Scholar] [CrossRef]
- Muhulet, A.; Miculescu, F.; Voicu, S.; Fabian Schütt, F.; Thakur, V.K.; Mishra, Y.K. Fundamentals and scopes of doped carbon nanotubes towards energy and biosensing applications. Mater. Today Energy 2018, 9, 154–186. [Google Scholar] [CrossRef]
- Xiong, H.; Motchelaho, M.A.; Moyo, M.; Jewell, L.L.; Coville, N.J. Fischere-tropsch synthesis: Iron-based catalysts supported on nitrogen-doped carbon nanotubes synthesized by post-doping. Appl. Catal. A Gen. 2014, 482, 377–386. [Google Scholar] [CrossRef]
- Jiang, S.; Handberg, E.S.; Liu, F.; Liao, Y.; Wang, H.; Li, Z.; Song, S. Effect of doping the nitrogen into carbon nanotubes on the activity of NiO catalysts for the oxidation removal of toluene. Appl. Catal. B Environ. 2014, 160, 716–721. [Google Scholar] [CrossRef]
- Zhang, Q.; Fan, W.; Gao, L. Anatase TiO2, nanoparticles immobilized on ZnO tetrapods as a highly efficient and easily recyclable photocatalyst. Appl. Catal. B Environ. 2007, 76, 168–173. [Google Scholar] [CrossRef]
- Liu, B.; Zeng, H.C. Carbon nanotubes supported mesoporous mesocrystals of anatase TiO2. Chem. Mater. 2008, 20, 2711–2718. [Google Scholar] [CrossRef]
- Tetty, K.E.; Yee, M.Q.; Lee, D. Photocatalytic and conductive MWCNT/TiO2 nanocomposite thin films. ACS Appl. Mater. Interfaces 2010, 2, 2646–2652. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.Y.; Lin, Y.F.; Hung, C.H.; Tseng, Y.H.; Ma, C.C.; Chang, M.C.; Shao, H. The effects of synthesis procedures on the morphology and photocatalytic activity of multi-walled carbon nanotubes/TiO2 nanocomposites. Nanotechnology 2008, 19, 045604–045614. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, Y.; Pan, D.; Li, Y.; Yan, Z.; Xie, J. Nitrogen-doped 3D graphene/MWNTs nano-frameworks embedded Co3O4 for high electrochemical performance supercapacitors. ACS Sustain. Chem. Eng. 2017, 5, 5099–5107. [Google Scholar] [CrossRef]
- Wang, C.; Xu, J.; Yuen, M.; Zhang, J.; Li, Y.; Chen, X.; Zhang, W. Hierarchical composite electrodes of nickel oxide nanoflake 3D graphene for high-performance pseudocapacitors. Adv. Funct. Mater. 2015, 24, 6372–6380. [Google Scholar] [CrossRef]
- Fan, Z.; Yan, J.; Zhi, L.; Zhang, Q.; Wei, T.; Feng, J.; Zhang, M.; Qian, W.; Wei, F. A three-dimensional carbon nanotube/graphene sandwich and its application as electrode in supercapacitors. Adv. Mater. 2010, 22, 3723–3728. [Google Scholar] [CrossRef] [PubMed]
- Min, S.; Lu, G. Advance in Photocatalyst Based on Graphene. In Analysis and Testing Technology and Instruments; CNKI: Beijing, China, 2014; pp. 215–229. [Google Scholar]
- Zhou, B. First-Principles Study of Chemisorption and Diffusion of Small Molecules on Carbon Nanotubes. Doctoral Thesis, Nanjing University of Aeronautics and Astronautics, Nanjing, China, 2008. [Google Scholar]
- Sampaio, M.J.; Benyounes, A.; Serp, P.; Faria, J.L.; Silva, C.G. Photocatalytic synthesis of vanillin using N-doped carbon nanotubes/ZnO catalysts under UV-LED irradiation. Appl. Catal. A Gen. 2017, 551, 71–78. [Google Scholar] [CrossRef]
- Lv, R.; Cruzsilva, E.; Terrones, M. Building complex hybrid carbon architectures by covalent interconnections: Graphene-nanotube hybrids and more. ACS Nano 2014, 8, 4061–4069. [Google Scholar] [CrossRef] [PubMed]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Chen, D.; Hu, X.; Qian, Y.; Li, D. Preparation of TiO2/Carbon Nanotubes/Reduced Graphene Oxide Composites with Enhanced Photocatalytic Activity for the Degradation of Rhodamine B. Nanomaterials 2018, 8, 431. https://doi.org/10.3390/nano8060431
Huang Y, Chen D, Hu X, Qian Y, Li D. Preparation of TiO2/Carbon Nanotubes/Reduced Graphene Oxide Composites with Enhanced Photocatalytic Activity for the Degradation of Rhodamine B. Nanomaterials. 2018; 8(6):431. https://doi.org/10.3390/nano8060431
Chicago/Turabian StyleHuang, Yanzhen, Dongping Chen, Xinling Hu, Yingjiang Qian, and Dongxu Li. 2018. "Preparation of TiO2/Carbon Nanotubes/Reduced Graphene Oxide Composites with Enhanced Photocatalytic Activity for the Degradation of Rhodamine B" Nanomaterials 8, no. 6: 431. https://doi.org/10.3390/nano8060431