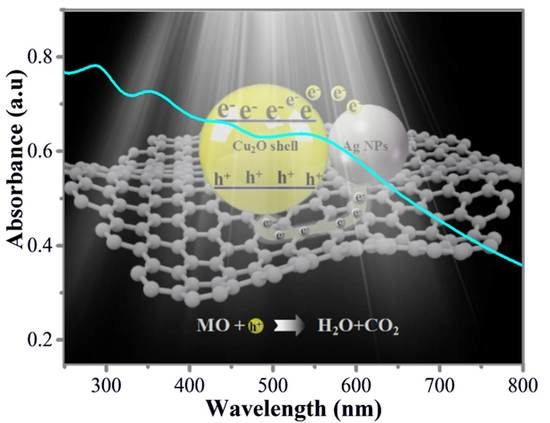
In Situ Synthesis of Ag@Cu2O-rGO Architecture for Strong Light-Matter Interactions
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of the Ag@Cu2O-rGO Composites
2.2.1. Fabrication and Modification of a 2D Graphene Oxide (GO) Nanosheet
2.2.2. Synthesis of the Ag@Cu2O-rGO Composites
2.3. Degradation MO Experiments
2.4. Characterization
3. Results and Discussion
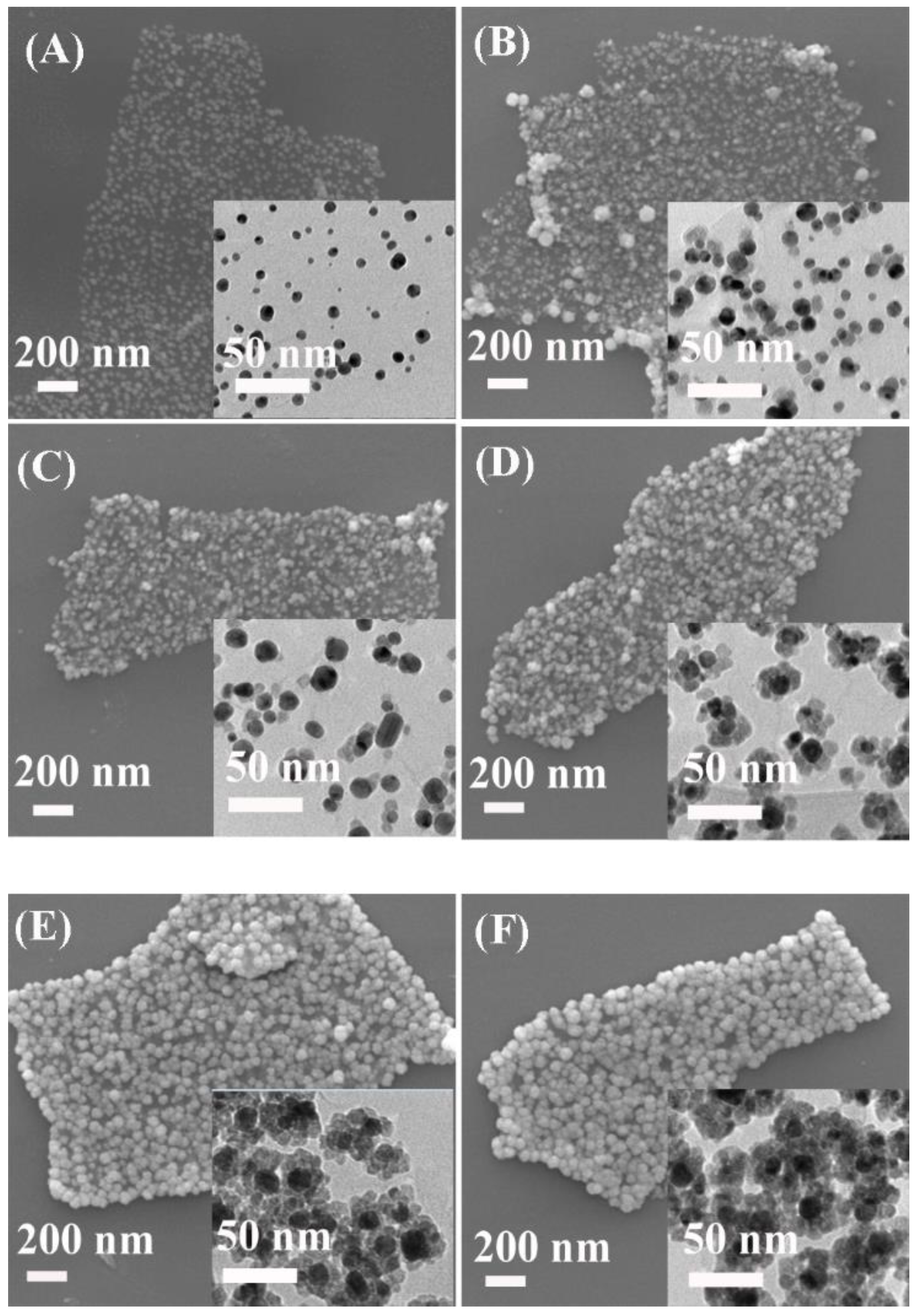
3.1. Structure and Properties of the Ag@Cu2O-rGO Nanocomposites
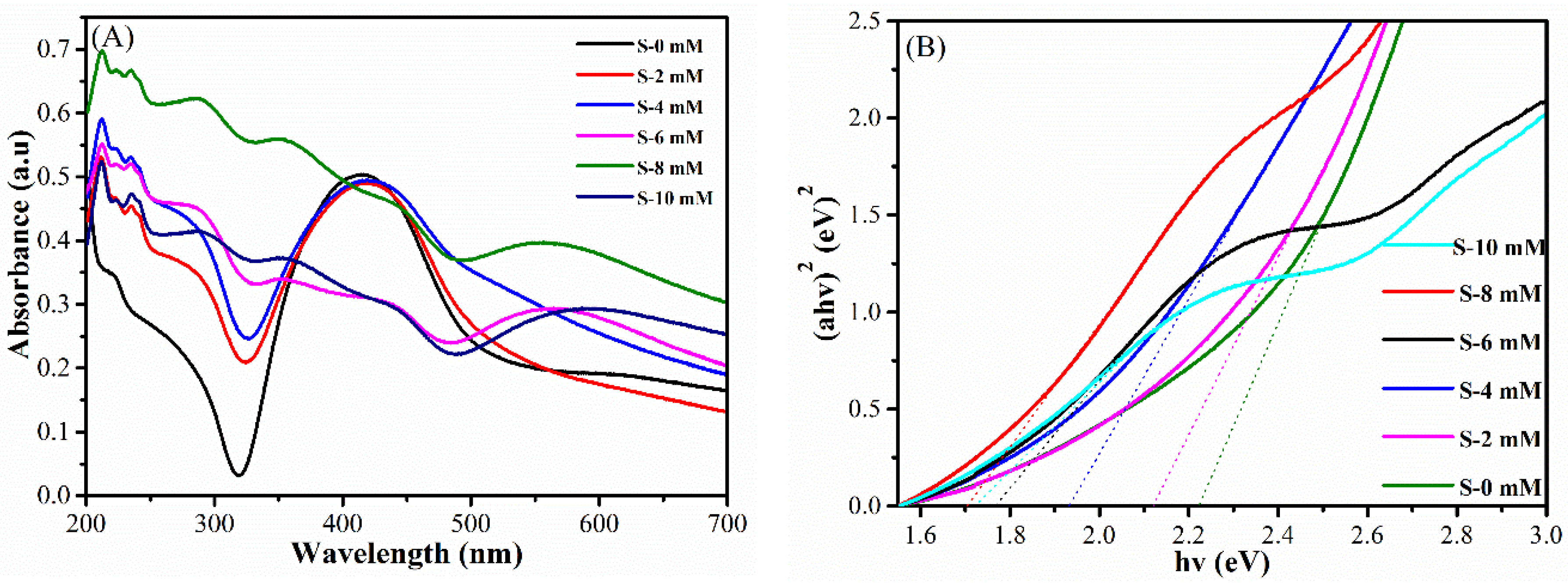
3.2. Optical Properties of the Ag@Cu2O-rGO Nanocomposites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Yury, G. Transition metal carbides go 2D Nature Nanotechnology. Nat. Mater. 2015, 14, 1079–1080. [Google Scholar]
- Tian, H.W.; Liu, M.; Zheng, W.T. Constructing 2D graphitic carbon nitride nanosheets/layered MoS2/graphene ternary nanojunction with enhanced photocatalytic activity. Appl. Catal. B Environ. 2018, 225, 468–476. [Google Scholar] [CrossRef]
- Jariwala, D.; Sangwan, V.K.; Lauhon, L.J.; Marks, T.J.; Hersam, M.C. Emerging device applications for semiconducting two-dimensional transition metal dichalcogenides. ACS Nano 2014, 8, 1102–1122. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Li, H.; An, H.; Hao, W.B.; Wei, J.J.; Da, Y.Z.; Ma, C.S.; Yang, G.D. Preparation of 2D/2D g-C3N4 nanosheet@ZnIn2S4 nanoleaf heterojunctions with well-designed high-speed charge transfer nanochannels towards high-efficiency photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2018, 220, 542–552. [Google Scholar] [CrossRef]
- Lee, Y.T.; Jeon, P.J.; Han, J.H.; Ahn, J.; Lee, H.S.; Lim, J.Y.; Choi, W.K.; Song, J.D.; Park, M.; Lm, S.; et al. Mixed-Dimensional 1D ZnO-2D WSe2 van der Waals Heterojunction Device for Photosensor. Adv. Funct. Mater. 2018, 27, 1703822–1703830. [Google Scholar] [CrossRef]
- Yang, L.; Mao, M.; Zhou, B.B.; Tang, X.H.; Chen, C.; Ge, M.H.; Li, P.; Huang, X.J.; Yang, L.B.; Liu, J.H. Self-Assembled 2D Nanoparticles Arrays of interfacial self-assembled large-scale densely packed monolayer film with ligand exchanged Au nanorods for in situ SERS drugs detection. Chem. A Eur. J. 2018, 24, 1–10. [Google Scholar]
- Chan, M.M.S.; Nie, W.; Stoumpos, C.C.; Tsai, H.; Blancon, J.; Liu, F.; Even, J.; Marks, T.J.; Mohite, A.D.; Kanatzidis, M.G. Understanding Film Formation Morphology and Orientation in High Member 2D Ruddlesden–Popper Perovskites for High-Efficiency Solar Cells. Adv. Energy Mater. 2017, 8, 1700979–1700989. [Google Scholar]
- Atkin, P.; Daeneke, T.; Wang, Y.; Carey, B.J.; Berean, K.J.; Clark, R.M.; Ou, J.Z.; Trinchi, A.; Cole, I.S.; Kalantar-Zadeh, K. 2D WS2/carbon dot hybrids with enhanced photocatalytic activity. J. Mater. Chem. A 2016, 4, 13563–13571. [Google Scholar] [CrossRef]
- Ma, D.; Shi, J.W.; Zou, Y.; Zou, Y.J.; Fan, Z.Y.; Ji, X.; Niu, C. Highly Efficient Photocatalyst Based on a CdS Quantum Dots/ZnO Nanosheets 0D/2D Heterojunction for Hydrogen Evolution from Water Splitting. ACS Appl. Mater. Interfaces 2017, 9, 25377–25386. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.J.; Chen, D.; Zhong, J.S.; Yang, L.X.; Wang, J.J.; Liu, M.J.; Tu, W.G.; Yu, Z.T.; Zou, Z.G. Interface engineering of a noble-metal-free 2D–2D MoS2/Cu-ZnIn2S4 photocatalyst for enhanced photocatalytic H2 production. J. Mater. Chem. A 2017, 5, 15771–15779. [Google Scholar] [CrossRef]
- Khan, A.F.; Down, M.P.; Smith, G.C.; Foster, C.W.; Banks, C.E. Surfactant-exfoliated 2D hexagonal boron nitride (2D-hBN): Role of surfactant upon the electrochemical reduction of oxygen and capacitance applications. J. Mater. Chem. A 2017, 5, 4103–4113. [Google Scholar] [CrossRef]
- Oughaddou, H.; Enriquez, H.; Tchalala, M.R.; Yildirim, H.; Mayne, A.J.; Dujardin, A.; Bendounan, G.; Ali, M.A.; Kara, A. Silicene, a promising new 2D material. Prog. Surf. Sci. 2015, 90, 46–83. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Khare, P.; Verma, S.; Bhati, A.; Sonker, A.K.; Tripathi, K.M.; Sonkar, S.K. Pollutant Soot for Pollutant Dye Degradation: Soluble Graphene Nanosheets for Visible Light Induced Photodegradation of Methylene Blue. ACS Sustain. Chem. Eng. 2017, 5, 8860–8869. [Google Scholar] [CrossRef]
- Miller, O.D.; Ilic, O.; Christensen, T.; Reid, M.T.H.; Atwater, H.A.; Joannopoulos, J.D.; Soljačić, M.; Johnson, S.G. Limits to the Optical Response of Graphene and Two-Dimensional Materials. Nano Lett. 2017, 17, 5408–5415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, X.; Tian, G.; Chen, Y.; Zhou, J.; Zhou, W.; Tian, C.; Fu, H. Hierarchical composites of TiO2 nanowire arrays on reduced graphene oxide nanosheets with enhanced photocatalytic hydrogen evolution performance. J. Mater. Chem. A 2014, 2, 4366–4374. [Google Scholar] [CrossRef]
- Vidyasagar, D.; Ghugal, S.G.; Kulkarni, A.; Mishra, P.; Shende, A.G.; Jagannath; Umare, S.S.; Sasikala, R. Silver/Silver(II) oxide (Ag/AgO) loaded graphitic carbon nitride microspheres: An effective visible light active photocatalyst for degradation of acidic dyes and bacterial inactivation. Appl. Catal. B Environ. 2018, 221, 339–348. [Google Scholar] [CrossRef]
- Giannakoudakis, D.A.; Arcibar-Orozco, J.A.; Bandosz, T.J. Effect of GO phase in Zn(OH)2/GO composite on the extent of photocatalytic reactive adsorption of mustard gas surrogate. Appl. Catal. B Environ. 2016, 183, 37–46. [Google Scholar] [CrossRef]
- Mady, A.H.; Baynosa, M.L.; Tuma, D.; Shim, J.J. Facile microwave-assisted green synthesis of Ag-ZnFe2O4@rGO nanocomposites for efficient removal of organic dyes under UV- and visible-light irradiation. Appl. Catal. B Environ. 2017, 203, 416–427. [Google Scholar] [CrossRef]
- Tyagi, A.; Tripathi, K.M.; Singh, N.; Choudhary, S.; Gupta, R.K. Green synthesis of carbon quantum dots from lemon peel waste: Applications in sensing and photocatalysis. RSC Adv. 2016, 6, 72423–72432. [Google Scholar] [CrossRef]
- Muhulet, A.; Miculescu, F.; Voicu, S.I.; Schütt, F.; Thakur, V.K.; Mishra, Y.M. Fundamentals and scopes of doped carbon nanotubes towards energy and biosensing applications. Mater. Today Energy 2018, 9, 154–186. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, H.; Ma, X.W.; Jin, J.; Ji, W.; Wang, X.; Song, W.; Zhao, B.; He, C.Y. Fabrication of Ag-Cu2O/Reduced Graphene Oxide Nanocomposites as Surface-Enhanced Raman Scattering Substrates for in Situ Monitoring of Peroxidase-Like Catalytic Reaction and Biosensing. ACS Appl. Mater. Interfaces 2017, 9, 19074–19081. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Shin, K.; Lee, Y.J.; Jung, C.; Lee, H.M. Effects of shell thickness on Ag-Cu2O core-shell nanoparticles with bumpy structures for enhancing photocatalytic activity and stability. Catal. Today 2017, 303, 313–319. [Google Scholar] [CrossRef]
- Mishra, Y.K.; Adelung, R. ZnO tetrapod materials for functional applications. Mater. Today 2017. [Google Scholar] [CrossRef]
- Cheng, G.Q.; Wang, Y.; Liu, K.; Yu, J.H. Entropy-driven self-assembly of chiral nematic liquid crystalline phases of AgNR@Cu2O hyper branched coaxial nanorods and thickness-dependent handedness transition. Nano Res. 2017, 11, 1018–1028. [Google Scholar] [CrossRef]
- Wei, Y.J.; Wang, T.; Li, C.C.; Gong, J.J. A Low-Cost NiO Hole Transfer Layer for Ohmic Back Contact to Cu2O for Photoelectrochemical Water Splitting. Small 2017, 13, 1702007–1702013. [Google Scholar] [CrossRef] [PubMed]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4820. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, M.M.; Zhao, Y.; Kou, Q.W.; Wang, Y.X.; Liu, Y.; Zhang, Y.J.; Yang, J.H.; Young, M.J. Enhanced Catalyst Activity by Decorating of Au on Ag@Cu2O Nanoshell. Appl. Surf. Sci. 2018, 435, 72–78. [Google Scholar] [CrossRef]
- Li, Y.; Cain, J.D.; Hanson, E.D.; Murthy, A.A.; Hao, S.Q.; Shi, F.G.; Li, Q.Q.; Wolverton, C.; Chen, X.Q.; Dravid, V.P. Au@MoS2 Core−Shell Heterostructures with Strong Light−Matter Interactions. Nano Lett. 2016, 16, 7696–7702. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.S.; Li, R.Y.; Zhang, Z.Y.; Zhang, W.W.; Yan, X.L.; Wu, X.L.; Cheng, G.; Zheng, R.T. Novel Au/Cu2O multi-shelled porous heterostructures for enhanced efficiency of photoelectrochemical water splitting. J. Mater. Chem. A 2017, 5, 14415–14421. [Google Scholar] [CrossRef]
- Liang, X.; You, T.T.; Liu, D.P.; Lang, X.F.; Tan, E.Z.; Shi, J.H.; Yin, P.G.; Guo, L. Direct observation of enhanced plasmon-driven catalytic reaction activity of Au nanoparticles supported on reduced graphene oxides by SERS. Phys. Chem. 2015, 17, 10176–10181. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Liang, B.L.; Pan, Z.H.; Lang, X.F.; Zhang, Y.J.; Wang, G.S.; Yin, P.G.; Guo, L. Tuning plasmonic and chemical enhancement for SERS detection on graphene-based Au hybrids. Nanoscale 2015, 7, 20188–20196. [Google Scholar] [CrossRef] [PubMed]
- Leong, K.H.; Lan, C.S.; Bahnemann, D.; Jang, M.; Lbrahim, S.; Saravanan, P. Reduced graphene oxide and Ag wrapped TiO2 photocatalyst for enhanced visible light photocatalysis. APL Mater. 2015, 6, 20085–20092. [Google Scholar] [CrossRef] [Green Version]
- Xiong, X.; Li, L.; Chen, J.; Zhang, S.; Wang, L.; Dou, S. Facile synthesis of highly efficient one-dimensional plasmonic photocatalysts through Ag@Cu2O core-shell hetero-nanowires. ACS Appl. Mater. Interfaces 2014, 6, 15716–15725. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Yao, B.; Lu, Y.M.; Wei, Z.P.; Gai, Y.Q.; Zheng, C.J.; Li, B.H.; Shen, D.Z.; Fan, X.W.; Tang, Z.K. Realization of p-type conduction in undoped MgxZn1−xO thin films by controlling Mg content. Appl. Phys. Lett. 2007, 91, 232115–232117. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, F.; Song, X.; Yin, Z.; Bu, Y. Construction of reduced graphene oxide-supported Ag–Cu2O composites with hierarchical structures for enhanced photocatalytic activities and recyclability. J. Mater. Chem. A 2015, 11, 5923–5933. [Google Scholar] [CrossRef]







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, S.; Wang, Y.; Zhang, F.; Gao, R.; Liu, M.; Dong, L.; Liu, Y.; Zhang, Y.; Chen, L. In Situ Synthesis of Ag@Cu2O-rGO Architecture for Strong Light-Matter Interactions. Nanomaterials 2018, 8, 444. https://doi.org/10.3390/nano8060444
Guo S, Wang Y, Zhang F, Gao R, Liu M, Dong L, Liu Y, Zhang Y, Chen L. In Situ Synthesis of Ag@Cu2O-rGO Architecture for Strong Light-Matter Interactions. Nanomaterials. 2018; 8(6):444. https://doi.org/10.3390/nano8060444
Chicago/Turabian StyleGuo, Shuang, Yaxin Wang, Fan Zhang, Renxian Gao, Maomao Liu, Lirong Dong, Yang Liu, Yongjun Zhang, and Lei Chen. 2018. "In Situ Synthesis of Ag@Cu2O-rGO Architecture for Strong Light-Matter Interactions" Nanomaterials 8, no. 6: 444. https://doi.org/10.3390/nano8060444