Facile Strategy for Synthesizing Non-Stoichiometric Monoclinic Structured Tungsten Trioxide (WO3−x) with Plasma Resonance Absorption and Enhanced Photocatalytic Activity
Abstract
:1. Introduction
2. Experimental
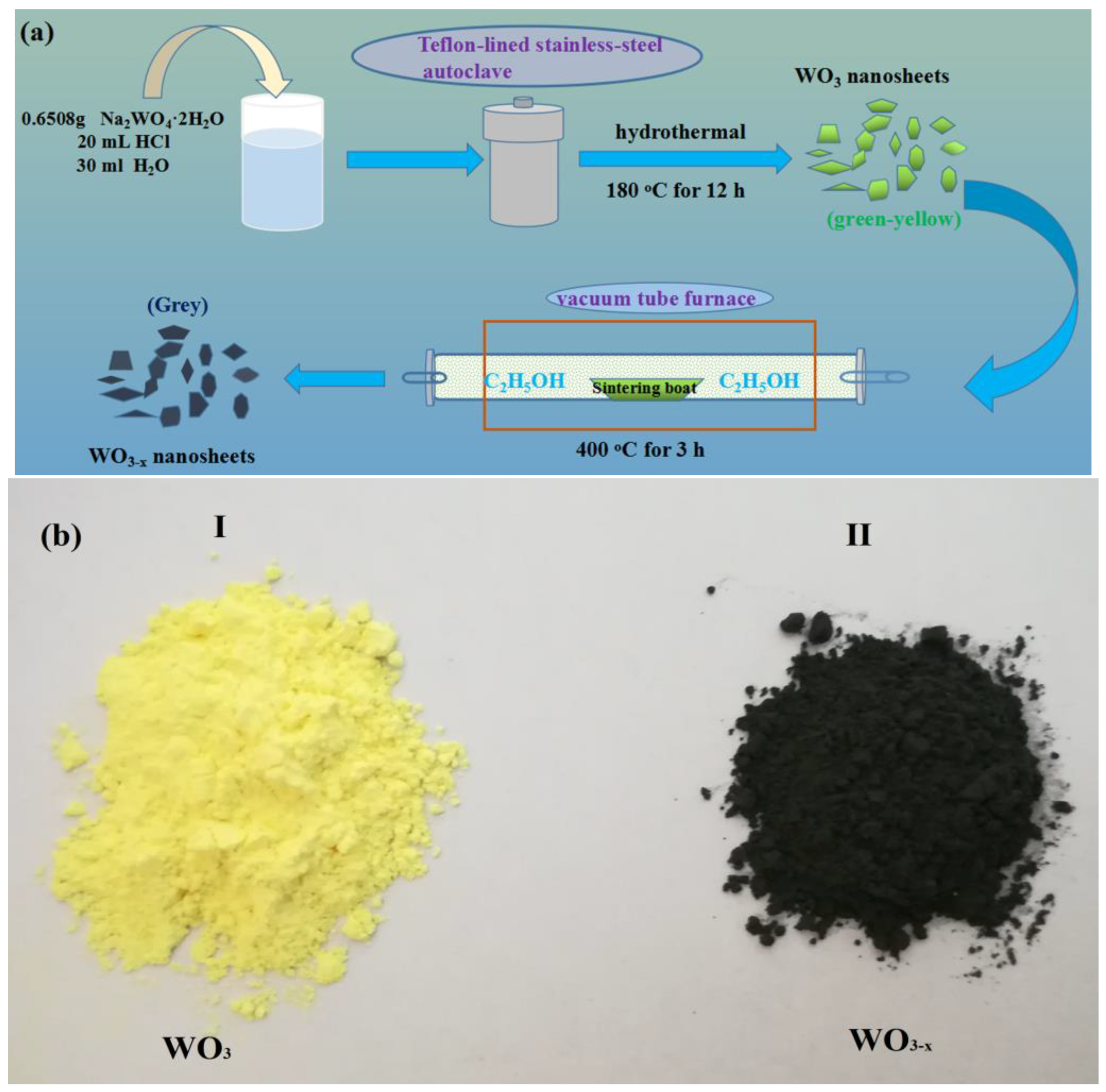
2.1. Preparation of the WO3 Nanosheets
2.2. Preparation of WO3−x Nanosheets
2.3. Characterization
2.4. Photocatalytic Test
2.4.1. UV Light Photocatalytic Degradation
2.4.2. Visible Light Photocatalytic Degradation
3. Results and Discussion
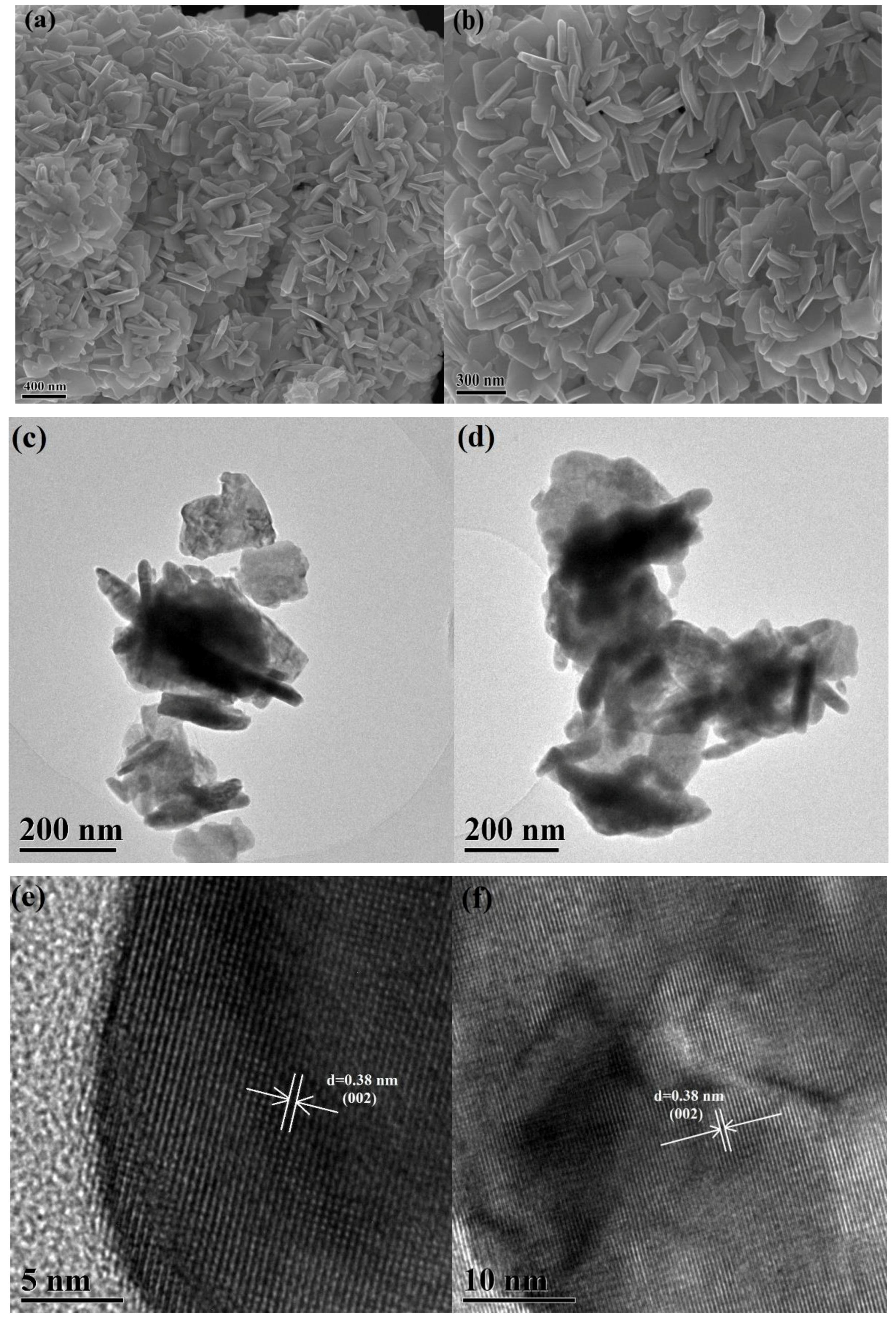
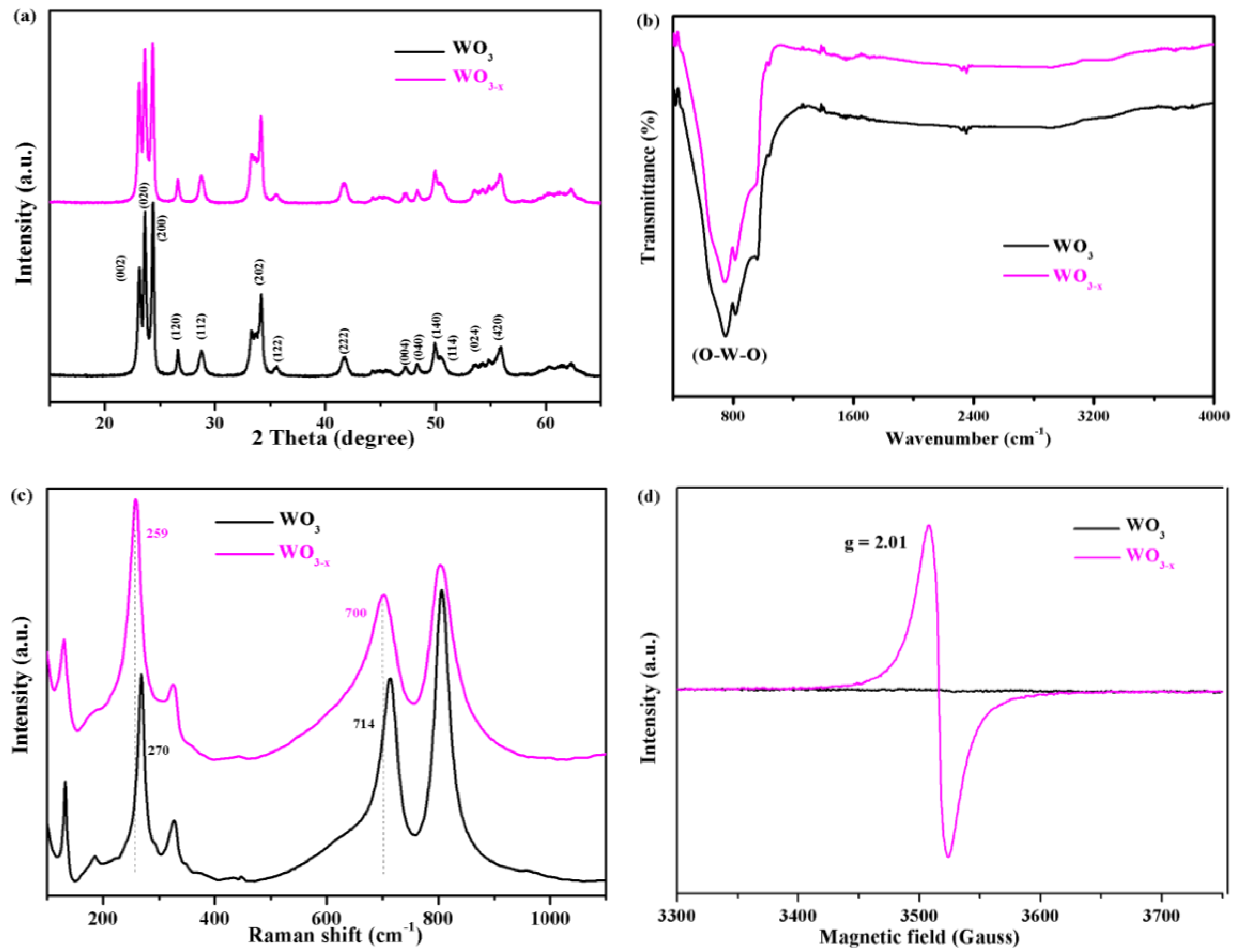
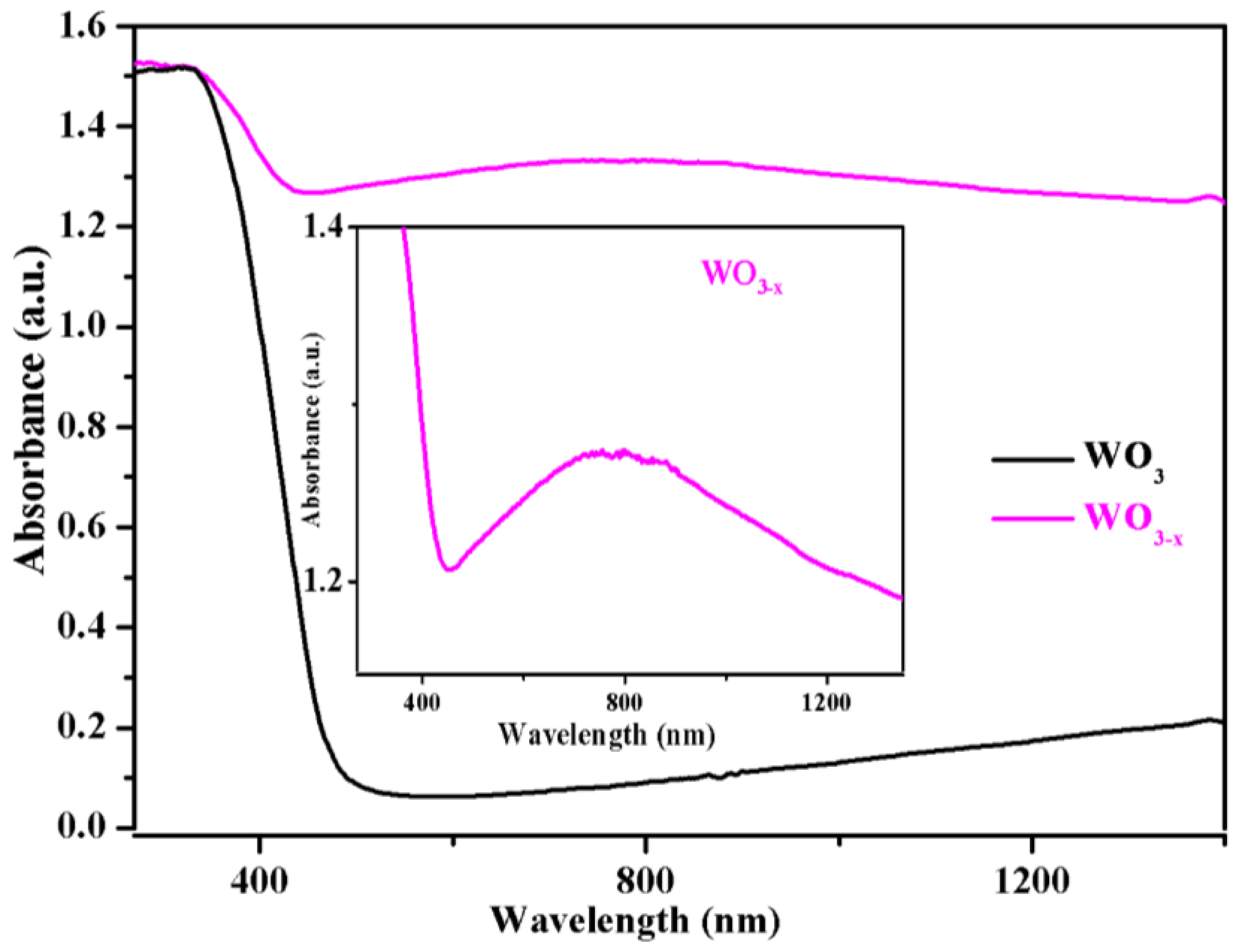
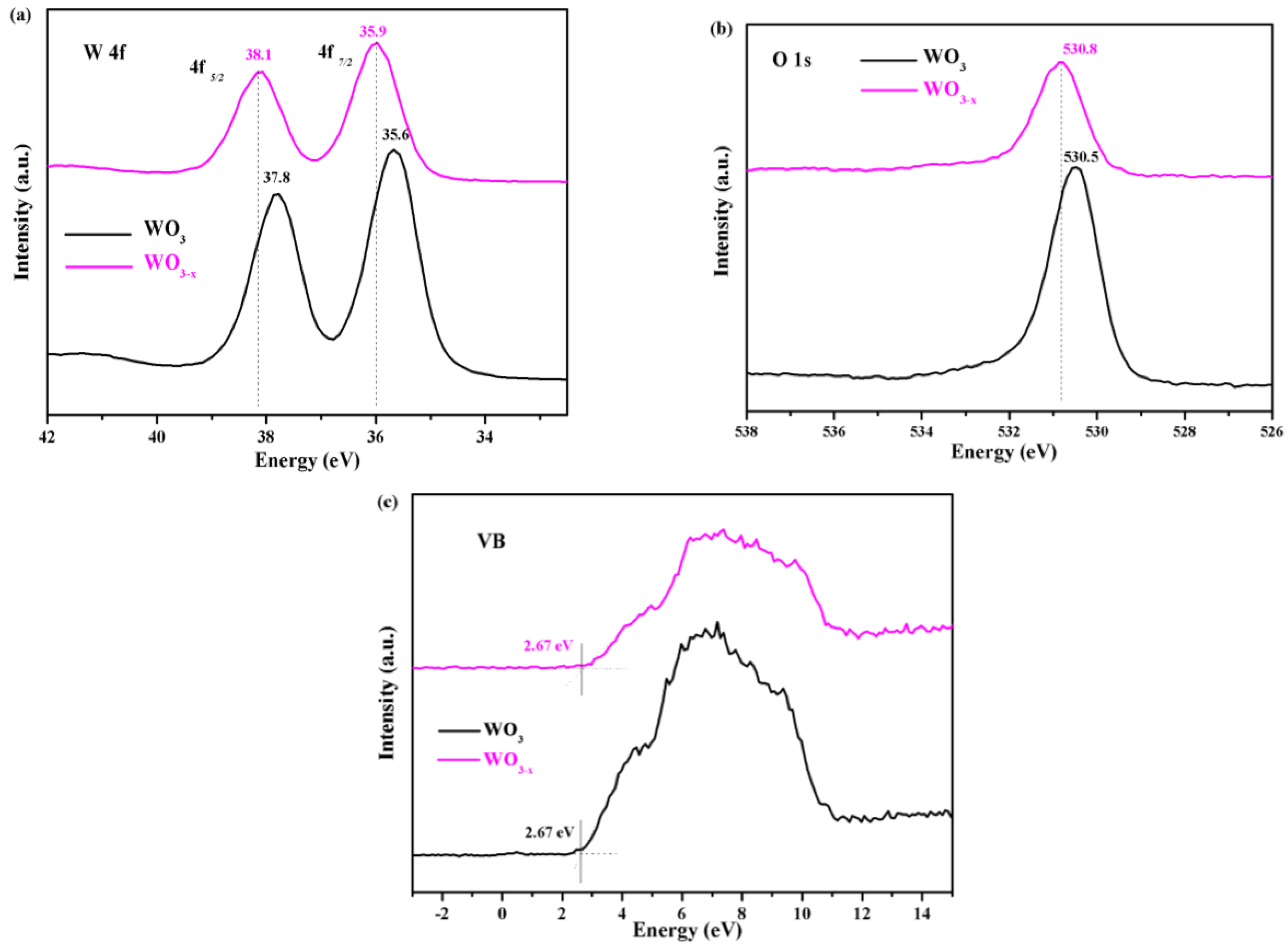
3.1. Characterization of the WO3−x Nanosheets
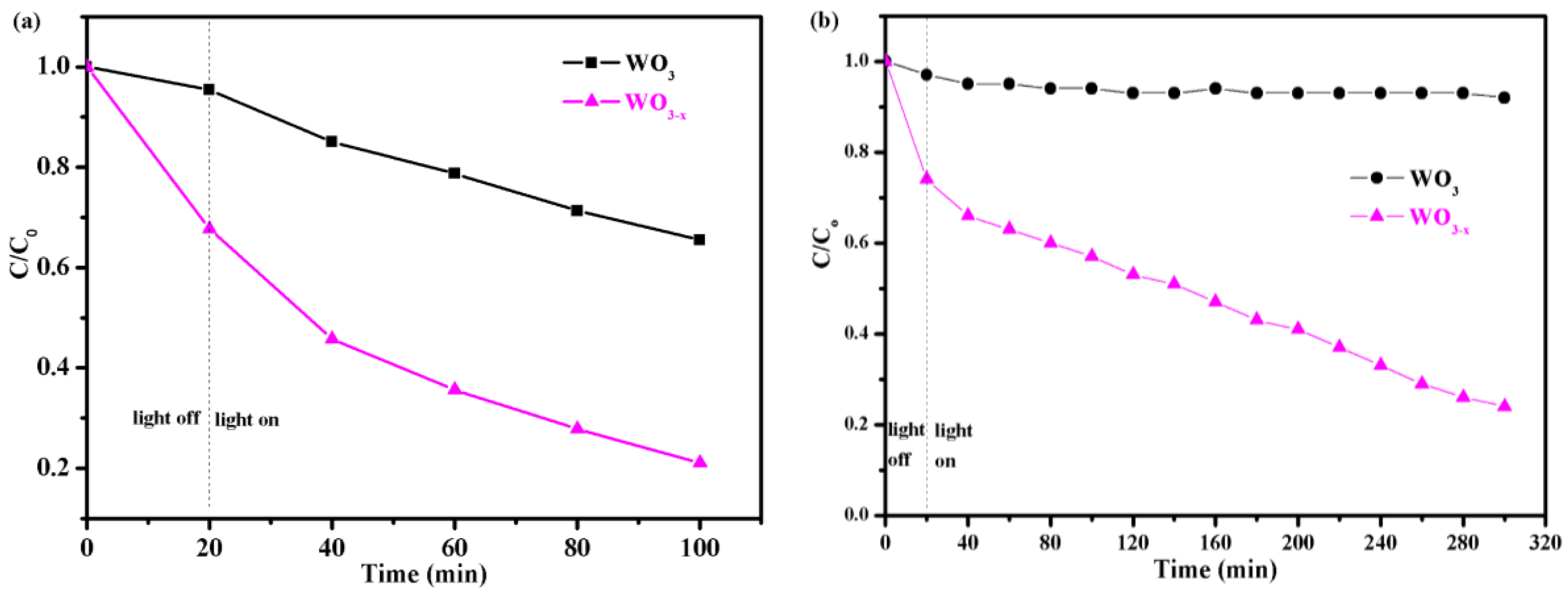
3.2. Photocatalytic Degradation Test
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.B.; Mao, S.S. Titanium Dioxide Nanomaterials: Synthesis, properties, modifications, and applications. Chem. Rev. 2007, 107, 289. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, X.; Jia, Y.; Chen, X.; Han, H.; Li, C. Titanium Dioxide-based nanomaterials for photocatalytic fuel generations. Chem. Rev. 2014, 114, 9987. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Shen, S.; Guo, L.; Mao, S.S. Semiconductor-based photocatalytic hydrogen generation. Chem. Rev. 2010, 110, 6503. [Google Scholar] [CrossRef] [PubMed]
- Tada, H.; Kiyonaga, T.; Naya, S. Rational design and applications of highly efficient reaction systems photocatalyzed by noble metal nanoparticle-loaded titanium(IV) dioxide. Chem. Soc. Rev. 2009, 38, 1849. [Google Scholar] [CrossRef] [PubMed]
- Dahl, M.; Liu, Y.; Yin, Y. Composite titanium dioxide nanomaterials. Chem. Rev. 2014, 114, 9853. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Duan, X. Challenge and perspective of heterogeneous photocatalysts. Chem. Soc. Rev. 2013, 42, 2568. [Google Scholar] [CrossRef] [PubMed]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhao, Y.; Sun, C.; Li, F.; Lu, G.Q.; Cheng, H.M. Synergistic effects of B/N doping on the visible-light photocatalytic activity of mesoporous TiO2. Angew. Chem. Int. Ed. 2008, 47, 4516–4520. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.D.; Jiang, Z.; Shi, H.H.; Xiao, T.C.; Yan, Z.F. Preparation of highly visible-light active N-doped TiO2 photocatalyst. J. Mater. Chem. 2010, 20, 5301–5309. [Google Scholar] [CrossRef]
- Hernández-Alonso, M.D.; Fresno, F.; Suáreza, S.; Coronado, J.M. Development of alternative photocatalysts to TiO2: Challenges and opportunities. Energy Environ. Sci. 2009, 2, 1231. [Google Scholar] [CrossRef]
- Fernández-Domene, R.M.; Sánchez-Tovar, R.; Lucas-Granados, B.; García-Antón, J. Improvement in photocatalytic activity of stable WO3, nanoplatelet globular clusters arranged in a tree-like fashion: influence of rotation velocity during anodization. Appl. Catal. B 2016, 189, 266–282. [Google Scholar] [CrossRef]
- Bai, X.; Sun, C.; Liu, D.; Luo, X.; Li, D.; Wang, J. Photocatalytic degradation of deoxynivalenol using graphene/ZnO hybrids in aqueous suspension. Appl. Catal. B 2017, 204, 11–20. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Ou, J.Z.; Strano, M.S.; Kaner, R.B.; Mitchell, A.; Kalantar-zadeh, K. Nanostructured tungsten oxide-properties synthesis, and applications. Adv. Funct. Mater. 2011, 21, 2175–2196. [Google Scholar] [CrossRef]
- Chen, Q.; Li, J.; Zhou, B.; Long, M.; Chen, H.; Liu, Y.; Cai, W.; Shangguan, W.F. Preparation of well-aligned WO3 nanoflake arrays vertically grown on tungsten substrate as photoanode for photoelectrochemical water splitting. Electrochem. Commun. 2012, 20, 153–156. [Google Scholar] [CrossRef]
- An, X.; Yu, J.C.; Wang, Y.; Hu, Y.; Yu, X.; Zhang, G. WO3 nanorods/graphene nanocomposites for high-efficiency visible-light-driven photocatalysis and NO2 gas sensing. J. Mater. Chem. 2012, 22, 8525–8531. [Google Scholar] [CrossRef]
- Ng, C.; Ng, Y.H.; Iwase, A.; Amal, R. Influence of annealing temperature of WO3 in photoelectrochemical conversion and energy storage for water splitting. ACS Appl. Mater. Interfaces 2013, 5, 5269–5275. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Gil, K.R.; Wiggenhorn, C.; Brunschwig, B.S.; Lewis, N.S. Comparison between the quantum yields of compact and porous WO3 photoanodes. J. Phys. Chem. C 2013, 117, 14947–14957. [Google Scholar] [CrossRef]
- Hu, X.X.; Xu, P.Q.; Gong, H.Y.; Yin, G.T. Synthesis and characterization of WO3/Graphene nanocomposites for enhanced photocatalytic activities by one-step in-situ hydrothermal reaction. Materials 2018, 11, 147. [Google Scholar]
- Székely, I.; Kovács, G.; Baia, L.; Danciu, V.; Pap, Z. Synthesis of shape-tailored WO3 micro-/nanocrystals and the photocatalytic activity of WO3/TiO2 composites. Materials 2016, 9, 258. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Da, P.; Zhang, Y.; Wang, Y.; Lin, X.; Gong, X.; Zheng, G. WO3 Nanoflakes for enhanced photoelectrochemical conversion. ACS Nano 2014, 8, 11770–11777. [Google Scholar] [CrossRef] [PubMed]
- Osterloh, F.E. Cheminform abstract: Inorganic nanostructures for photoelectrochemical and photocatalytic water splitting. Cheminform 2013, 42, 2294–2320. [Google Scholar] [CrossRef]
- Tanaka, A.; Hashimoto, K.; Kominami, H. Visible-light-induced hydrogen and oxygen formation over Pt/Au/WO3 photocatalyst utilizing two types of photoabsorption due to surface plasmon resonance and band-gap excitation. J. Am. Chem. Soc. 2014, 136, 586–589. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Meng, G.F.; Zhao, H.B.; Zhang, Y.; Li, H.; Ma, W.J.; Xu, J.Q. Au nanoparticle modified WO3 nanorods with their enhanced properties for photocatalysis and gas sensing. J. Phys. Chem. C 2010, 114, 2049–2055. [Google Scholar] [CrossRef]
- Sun, S.M.; Wang, W.Z.; Zeng, S.Z.; Shang, M.; Zhang, L. Preparation of ordered mesoporous Ag/WO3 and its highly efficient degradation of acetaldehyde under visible-light irradiation. J. Hazard. Mater. 2010, 178, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.F.; Ding, X.; Wang, Y.G.; Shi, H.C.; Huang, L.H.; Zuo, Y.H.; Kang, S.F. Facile preparation of Z-scheme WO3/g-C3N4 composite photocatalyst with enhanced photocatalytic performance under visible light. Appl. Surf. Sci. 2017, 391, 202–210. [Google Scholar] [CrossRef]
- Jin, T.; Diao, P.; Wu, Q.Y.; Xu, D.; Hu, D.Y.; Xie, Y.H.; Zhang, M. WO3 nanoneedles/α-Fe2O3/cobalt phosphate composite photoanode for efficient photoelectrochemical water splitting. Appl. Catal. B 2014, 148–149, 304–310. [Google Scholar] [CrossRef]
- Momeni, M.M.; Ghayeb, Y.; Davarzadeh, M. Single-step electrochemical anodization for synthesis of hierarchical WO3-TiO2 nanotube arrays on titanium foil as a good photoanode for water splitting with visible light. J. Electroanal. Chem. 2015, 739, 149–155. [Google Scholar] [CrossRef]
- Bazarjani, M.S.; Hojamberdiev, M.; Morita, K.; Zhu, G.Q.; Cherkashinin, G.; Fasel, C.; Herrmann, T.; Breitzke, H.; Gurlo, A.; Riedel, R. Visible light photocatalysis with c-WO3−x/WO3·H2O nanoheterostructures in situ formed in mesoporous polycarbosilane-siloxane polymer. J. Am. Chem. Soc. 2013, 135, 4467–4475. [Google Scholar] [CrossRef] [PubMed]
- Song, J.J.; Huang, Z.F.; Pan, L.; Zou, J.J.; Zhang, X.W.; Wang, L. Oxygen-deficient tungsten oxide as versatile and efficient hydrogenation catalyst. ACS Catal. 2015, 5, 6594–6599. [Google Scholar] [CrossRef]
- Yan, M.; Li, G.L.; Guo, C.S.; Guo, W.; Ding, D.D.; Zhang, S.H.; Liu, S.Q. WO3−x sensitized TiO2 spheres with full-spectrum-driven photocatalytic activities from UV to near infrared. Nanoscale 2016, 8, 17828–17835. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.Z.; Xue, C. In situ growth of WO3−x nanowires on g-C3N4 nanosheets: 1D/2D heterostructures with enhanced photocatalytic activity. Cryst Eng Comm 2016, 18, 8406. [Google Scholar] [CrossRef]
- Li, Y.S.; Tang, Z.L.; Zhang, J.Y.; Zhang, Z.T. Defect engineering of air-treated WO3 and its enhanced visible light-driven photocatalytic and electrochemical performance. J. Phys. Chem. C 2016, 120, 9750–9763. [Google Scholar] [CrossRef]
- Yan, J.; Wang, T.; Wu, G.; Dai, W.; Guan, N.; Li, L.; Gong, J. Tungsten oxide single crystal nanosheets for enhanced multichannel solar light harvesting. Adv. Mater. 2015, 27, 1580. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Jiang, Y.; Gao, X.; Wang, X.; Chen, W. Strongly coupled Pd nanotetrahedron/tungsten oxide nanosheet hybrids with enhanced catalytic activity and stability as oxygen reduction electrocatalysts. J. Am. Chem. Soc. 2014, 136, 11687. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.M.; Ling, Y.C.; Wang, H.Y.; Yang, X.Y.; Wang, C.C.; Zhang, J.Z.; Li, Y. Hydrogen-treated WO3 nanoflakes show enhanced photostability. Energy Environ. Sci. 2012, 5, 6180. [Google Scholar] [CrossRef]
- Chen, X.B.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.F.; Wen, M.C.; Ma, X.C.; Kuwahara, Y.; Mori, K.; Dai, Y.; Huang, B.B.; Yamashita, H. Hydrogen doped metal oxide semiconductors with exceptional and tunable localized surface plasmon resonances. J. Am. Chem. Soc. 2016, 138, 9316. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.H. A highly efficient photocatalyst-hydrogenated black TiO2 for the photocatalytic splitting of water. Angew. Chem. Int. Ed. 2012, 51, 12410–12412. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.L.; Yang, J.Q.; Li, D.; Guo, W.; Qin, Q.; Zhua, L.J.; Zheng, W.J. Template-free facile preparation of monoclinic WO3 nanoplates and their high photocatalytic activities. Appl. Surf. Sci. 2014, 305, 274–280. [Google Scholar] [CrossRef]
- Wu, H.Y.; Xu, M.; Da, P.M.; Li, W.J.; Jia, D.S.; Zheng, G.F. WO3-reduced graphene oxide composites with enhanced charge transfer for photoelectrochemical conversion. Phys. Chem. Chem. Phys. 2013, 15, 16138. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Wang, C.H.; Zheng, H.; Wan, F.X.; Yu, F.; Zhang, X.T.; Liu, Y.C. Surface oxygen vacancies on WO3 contributed to enhanced photothermo-synergistic effect. Appl. Surf. Sci. 2017, 391, 654–661. [Google Scholar] [CrossRef]
- Hilaire, S.; Süess, M.J.; Kränzlin, N.; Bieńkowski, K.; Solarska, R.; Augustyński, J.; Niederberger, M. Microwave-assisted nonaqueous synthesis of WO3 nanoparticles for crystallographically oriented photoanodes for water splitting. J. Mater. Chem. A 2014, 48, 20530–20537. [Google Scholar] [CrossRef]
- Cheng, H.F.; Klapproth, M.; Sagaltchik, A.; Li, S.; Thomas, A. Ordered mesoporous WO2.83: Selective reduction synthesis, exceptional localized surface plasmon resonance and enhanced hydrogen evolution reaction activity. J. Mater. Chem. A 2018, 6, 2249. [Google Scholar] [CrossRef]
- Manthiram, K.; Alivisatos, A.P. Tunable localized surface plasmon resonances in tungsten oxide nanocrystals. J. Am. Chem. Soc. 2012, 134, 3995–3998. [Google Scholar] [CrossRef] [PubMed]
- Janotti, A.; Varley, J.B.; Rinke, P.; Umezawa, N.; Kresse, G.; Van de Walle, C.G. Hybrid functional studies of the oxygen vacancy in TiO2. Phys. Rev. B 2010, 81, 085212. [Google Scholar] [CrossRef]
- Morgan, B.J.; Watson, G.W. Intrinsic n-type defect formation in TiO2: a comparison of rutile and anatase from GGA+U calculations. J. Phys. Chem. C 2010, 114, 2321. [Google Scholar] [CrossRef]
- Pan, X.Y.; Yang, M.Q.; Fu, X.Z.; Zhang, N.; Xu, Y.J. Defective TiO2 with oxygen vacancies: synthesis, properties and photocatalytic applications. Nanoscale 2013, 5, 3601–3614. [Google Scholar] [CrossRef] [PubMed]
- Naldoni, A.; Allieta, M.; Santangelo, S.; Marelli, M.; Fabbri, F.; Cappelli, S.; Bianchi, C.L.; Psaro, R.; Dal Santo, V. Effect of nature and location of defects on bandgap narrowing in black TiO2 nanoparticles. J. Am. Chem. Soc. 2012, 134, 7600. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Xiao, Y.; Xie, W.; Wang, Y.; Hu, Z.; Zhang, W.; Zhao, H. Facile Strategy for Synthesizing Non-Stoichiometric Monoclinic Structured Tungsten Trioxide (WO3−x) with Plasma Resonance Absorption and Enhanced Photocatalytic Activity. Nanomaterials 2018, 8, 553. https://doi.org/10.3390/nano8070553
Chen S, Xiao Y, Xie W, Wang Y, Hu Z, Zhang W, Zhao H. Facile Strategy for Synthesizing Non-Stoichiometric Monoclinic Structured Tungsten Trioxide (WO3−x) with Plasma Resonance Absorption and Enhanced Photocatalytic Activity. Nanomaterials. 2018; 8(7):553. https://doi.org/10.3390/nano8070553
Chicago/Turabian StyleChen, Shihao, Yang Xiao, Wei Xie, Yinhai Wang, Zhengfa Hu, Wei Zhang, and Hui Zhao. 2018. "Facile Strategy for Synthesizing Non-Stoichiometric Monoclinic Structured Tungsten Trioxide (WO3−x) with Plasma Resonance Absorption and Enhanced Photocatalytic Activity" Nanomaterials 8, no. 7: 553. https://doi.org/10.3390/nano8070553






