High-Aspect Ratio β-Ga2O3 Nanorods via Hydrothermal Synthesis
Abstract
:1. Introduction
2. Experimental
2.1. Precipitated α-GaOOH, Hydrothermal-Synthesized α-GaOOH, and β-Ga2O3 Nanorods
2.2. Material Characterization
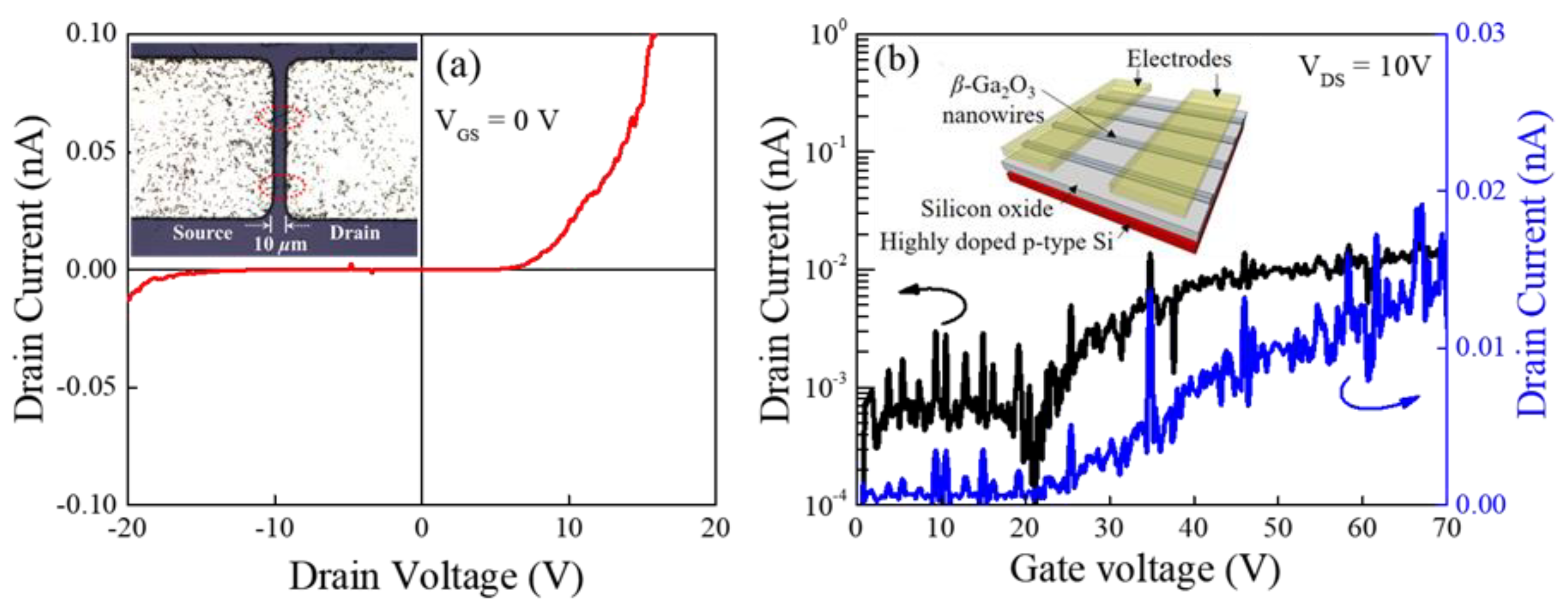
2.3. Fabrication of the Ga2O3 Nanorods Field-Effect Transistor (FET)
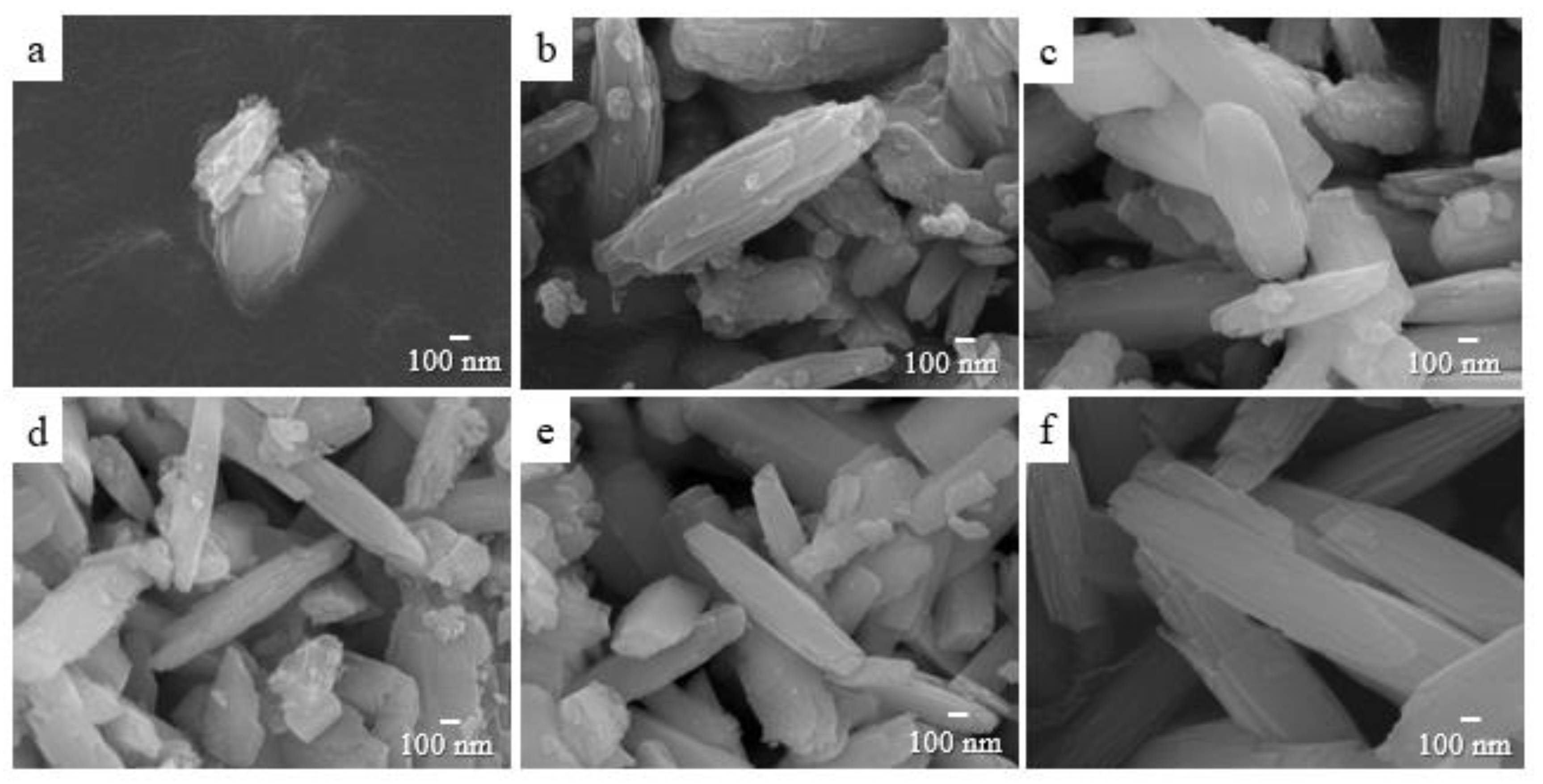
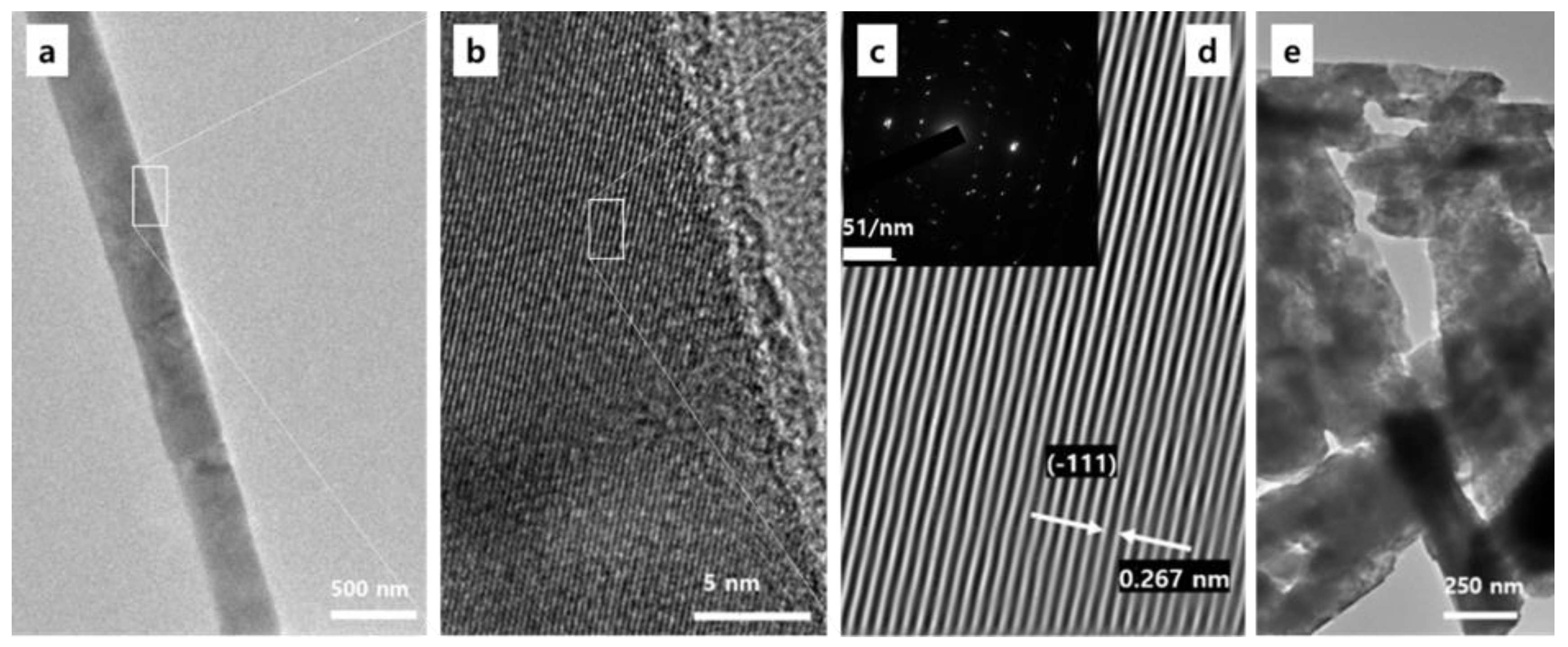
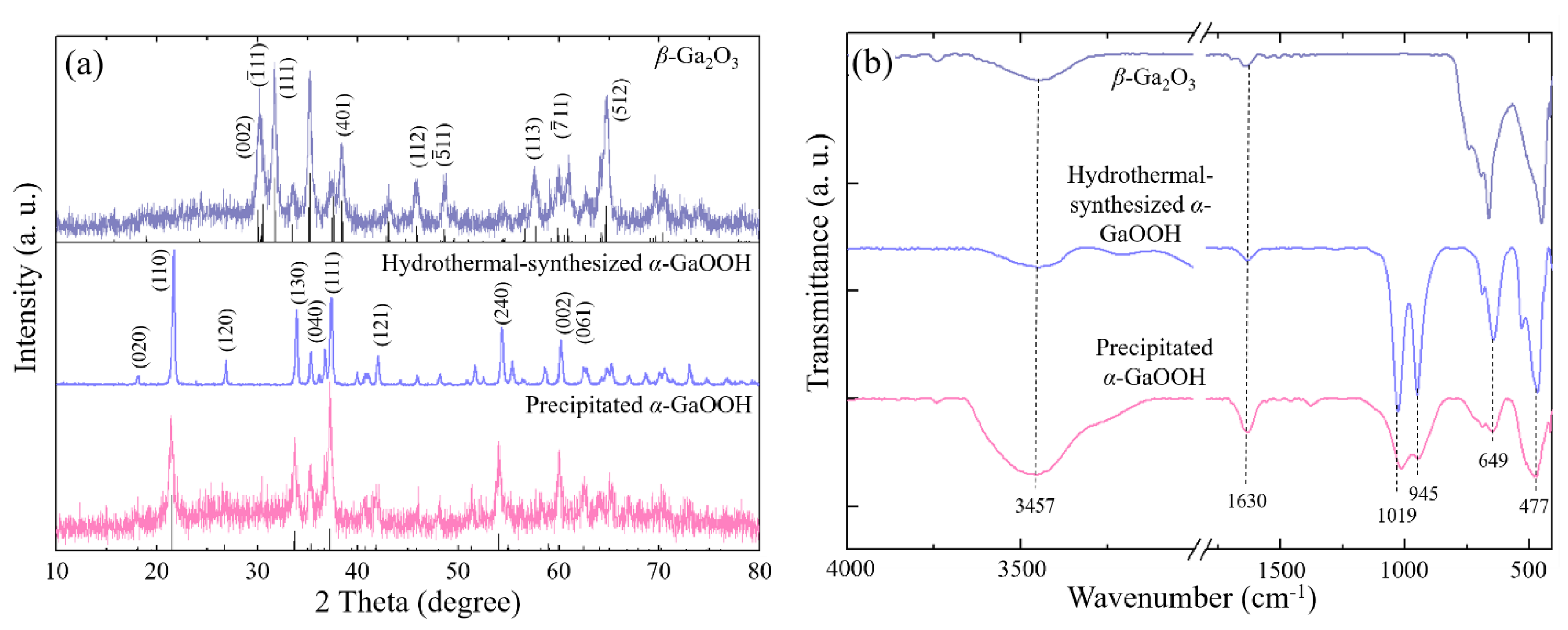
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Higashiwaki, M.; Sasaki, K.; Kuramata, A.; Masui, T.; Yamakoshi, S. Depletion-mode Ga2O3 metal-oxide-semiconductor field-effect transistors on β-Ga2O3 (010) substrates and temperature dependence of their device characteristics. Appl. Phys. Lett. 2012, 100, 013504. [Google Scholar] [CrossRef]
- Sasaki, K.; Kuramata, A.; Masui, T.; Víllora, E.G.; Shimamura, K.; Yamakoshi, S. Device-Quality β-Ga2O3 Epitaxial Films Fabricated by Ozone Molecular Beam Epitaxy. Appl. Phys. Exp. 2012, 5, 035502. [Google Scholar] [CrossRef]
- Zhou, H.; Maize, K.; Noh, J.; Shakouri, A.; Ye, P.D. Thermodynamic Studies of β-Ga2O3 Nanomembrane Field-Effect Transistors on a Sapphire Substrate. ACS Omega 2017, 2, 7723–7729. [Google Scholar] [CrossRef]
- Higashiwaki, M.; Sasaki, K.; Murakami, H.; Kumagai, Y.; Koukitu, A.; Kuramata, A.; Masui, T.; Yamakoshi, S. Recent progress in Ga2O3 power devices. Semicond. Sci. Technol. 2016, 31, 034001. [Google Scholar] [CrossRef]
- Li, X.; Zhen, X.; Meng, S.; Xian, J.; Shao, Y.; Fu, X.; Li, D. Structuring β-Ga2O3 Photonic Crystal Photocatalyst for Efficient Degradation of Organic Pollutants. Environ. Sci. Technol. 2013, 47, 9911–9917. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yamazaki, T.; Shen, Y.; Kikuta, T.; Nakatani, N.; Li, Y. O2 and CO sensing of Ga2O3 multiple nanowire gas sensors. Sens. Actuator B Chem. 2008, 129, 666–670. [Google Scholar] [CrossRef]
- Kim, J.; Mastro, M.A.; Tadjer, M.J.; Kim, J. Quasi-Two-Dimensional h-BN/β-Ga2O3 Heterostructure Metal-Insulator-Semiconductor Field-Effect Transistor. ACS Appl. Mater. Interfaces 2017, 9, 21322–21327. [Google Scholar] [CrossRef] [PubMed]
- Kalygina, V.M.; Zarubin, A.N.; Nayden, Y.P.; Novikov, V.A.; Petrova, Y.S.; Tolbanov, O.P.; Tyazhev, A.V.; Yaskevich, T.M. Ga2O3 Films Formed by Electrochemical Oxidation. Semiconductors 2011, 45, 1097–1102. [Google Scholar] [CrossRef]
- Jangir, R.; Ganguli, T.; Tiwari, P.; Porwal, S.; Srivastava, H.; Rai, S.K.; Khattak, B.Q.; Oak, S.M. Synthesis and characterization of β-Ga2O3 nanostructures grown on GaAs substrates. Appl. Surf. Sci. 2011, 257, 9323–9328. [Google Scholar] [CrossRef]
- Wang, F.; Han, Z.; Tong, L. Fabrication and characterization of β-Ga2O3 optical nanowires. Physica E 2005, 30, 150–154. [Google Scholar] [CrossRef]
- Hwang, W.S.; Verma, A.; Peelaers, H.; Protasenko, V.; Rouvimov, S.; Xing, H.; Seabaugh, A.; Haensch, W.; Walle, C.V.; Galazka, Z.; et al. High-voltage field effect transistors with wide-bandgap β-Ga2O3 nanomembranes. Appl. Phys. Lett. 2014, 104, 203111. [Google Scholar] [CrossRef]
- Kwon, Y.B.; Lee, G.Y.; Oh, S.Y.; Kim, J.H.; Pearton, S.J.; Ren, F. Tuning the thickness of exfoliated quasi-two-dimensional β-Ga2O3 flakes by plasma etching. Appl. Phys. Lett. 2017, 110, 131901. [Google Scholar] [CrossRef]
- Feng, W.; Wang, X.; Zhang, J.; Wang, L.; Zheng, W.; Hu, P.; Cao, W.; Yang, B. Synthesis of two-dimensional β-Ga2O3 nanosheets for high-performance solar blind photodetectors. J. Mater. Chem. C 2014, 2, 3254. [Google Scholar] [CrossRef]
- Yoon, Y.B.; Han, K.I.; Kim, B.H.; Lee, I.G.; Kim, Y.H.; Kim, J.P.; Hwang, W.S. Formation of β-Ga2O3 nanofibers of sub-50 nm diameter synthesized by electrospinning method. Thin Solid Films 2018, 1, 358–362. [Google Scholar] [CrossRef]
- Hailin, M.; Yan, L. One-Step Preparation of β-Ga2O3 Nanomaterial and Research the Electrical Transport Properties at High Temperature. Rare Metal. Mater. Eng. 2013, 42, 2245–2247. [Google Scholar] [CrossRef]
- Khan, A.; Jadwisienczak, W.M.; Kordesch, M.E. One-step preparation of ultra-wide β-Ga2O3 microbelts and their photoluminescence study. Physica E 2006, 35, 207–211. [Google Scholar] [CrossRef]
- Oh, S.Y.; Kim, J.H.; Ren, F.; Peartonc, S.J.; Kim, J.H. Quasi-two-dimensional β-gallium oxide solar-blind photodetectors with ultrahigh responsivity. J. Mater. Chem. C 2016, 4, 9245–9250. [Google Scholar] [CrossRef]
- Reddy, L.S.; Ko, Y.H.; Yu, J.S. Hydrothermal Synthesis and Photocatalytic Property of β-Ga2O3 Nanorods. Nanoscale Res. Lett. 2015, 10, 364. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Sunkara, M.K. Direct Synthesis of Gallium Oxide Tubes, Nanowires, and Nanopaintbrushes. J. Am. Chem. Soc. 2002, 124, 12288–12293. [Google Scholar] [CrossRef] [PubMed]
- Kokubun, Y.; Miura, K.; Endo, F.; Nakagomi, S. Sol-gel prepared β-Ga2O3 thin films for ultraviolet photodetectors. Appl. Phys. Lett. 2007, 90, 031912. [Google Scholar] [CrossRef]
- Kang, B.K.; Mang, S.R.; Song, K.M.; Lee, K.S.; Yoon, D.H. Hydrothermal synthesis and characterization of uniform β-Ga2O3 hollow nanostructures by carbon nanospheres. J. Ceram. Process. Res. 2014, 15, 200–203. [Google Scholar]
- Zhang, J.; Liu, Z.; Lin, C.; Lin, J. A simple method to synthesize β-Ga2O3 nanorods and their photoluminescence properties. J. Cryst. Growth 2005, 280, 99–106. [Google Scholar] [CrossRef]
- EL-Sayed, E.I.; Al-Ghamdi, A.A.; Al-Heniti, S.; Al-Marzouki, F.; El-Tantawy, F. Synthesis of ultrafine β-Ga2O3 nanopowder via hydrothermal approach: A strong UV “excimer-like” emission. Mater. Lett. 2011, 65, 317–321. [Google Scholar] [CrossRef]
- Quan, Y.; Fang, D.; Zhang, X.; Liu, S.; Huang, K. Synthesis and characterization of gallium oxide nanowires via a hydrothermal method. Mater. Chem. Phys. 2010, 121, 142–146. [Google Scholar] [CrossRef]
- Sun, M.; Li, D.; Zhang, W.; Fu, X.; Shao, Y.; Li, W.; Xiao, G.; He, Y. Rapid microwave hydrothermal synthesis of GaOOH nanorods with photocatalytic activity toward aromatic compounds. Nanotechnology 2010, 21, 355601. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Frost, R.L.; Yang, J.; Martens, W.N. Size and Morphology Control of Gallium Oxide Hydroxide GaO(OH), Nano- to Micro-Sized Particles by Soft-Chemistry Route without Surfactant. J. Phys. Chem. C 2008, 112, 3568–3579. [Google Scholar] [CrossRef]
- Kumar, S.; Tessarek, C.; Sarau, G.; Christiansen, S.; Singh, R. Self-Catalytic Growth of β-Ga2O3 Nanostructures by Chemical Vapor Deposition. Adv. Eng. Mater. 2015, 17, 709–715. [Google Scholar] [CrossRef]
- Hu, J.Q.; Li, Q.; Meng, X.M.; Lee, C.S.; Lee, S.T. Synthesis of β-Ga2O3 Nanowires by Laser Ablation. J. Phys. Chem. B 2002, 106, 9536–9539. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, Y.; Hao, R.; Liu, F.; Wang, Y.; Tan, M.; Tang, J.; Ren, D.; Zhao, D. Synthesis of mesoporous β-Ga2O3 nanorods using PEG as template: Preparation, characterization and photocatalytic properties. J. Hazard. Mater. 2011, 192, 1548–1554. [Google Scholar] [CrossRef] [PubMed]
- Girija, K.; Thirumalairajan, S.; Mangalaraj, D. Morphology controllable synthesis of parallely arranged single-crystalline β-Ga2O3 nanorods for photocatalytic and antimicrobial activities. Chem. Eng. J. 2014, 236, 181–190. [Google Scholar] [CrossRef]
- Huang, C.C.; Yeh, C.S. GaOOH, and β- and γ-Ga2O3 nanowires: Preparation and photoluminescence. New J. Chem. 2010, 34, 103–107. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, J.; Shi, L.; Tang, S.; Li, Y.; Jiang, L.; Cui, Q. Template-free synthesis of α-GaOOH hyperbranched nanoarchitectures via crystal splitting and their optical properties. RSC Adv. 2014, 4, 8209–8215. [Google Scholar] [CrossRef]
- Qian, H.S.; Gunawan, P.; Zhang, Y.X.; Lin, G.F.; Zheng, J.W.; Xu, R. Template-Free Synthesis of Highly Uniform α-GaOOH Spindles and Conversion to α-Ga2O3 and β-Ga2O3. Cryst. Growth Des. 2008, 8, 1282–1287. [Google Scholar] [CrossRef]
- Sat, T.; Nakamura, T. Studies of the crystallisation of gallium hydroxide precipitated from hydrochloric acid solutions by various alkalis. J. Chem. Technol. Biotechnol. 1982, 32, 469–475. [Google Scholar] [CrossRef]
- Guo, D.; Xie, G.; Luo, J. Mechanical properties of nanoparticles: Basics and applications. J. Phys. D Appl. Phys. 2014, 47, 013001. [Google Scholar] [CrossRef]
- Eduardo, J.H.L.; Ribeiro, C.; Longo, E.; Leite, E.R. Oriented Attachment: An Effective Mechanism in the Formation of Anisotropic Nanocrystals. J. Phys. Chem. B 2005, 109, 20842–20846. [Google Scholar]
- Markov, I.V. Crystal Growth for Beginners, 2nd ed.; World Scientific Publishing: Singapore, 2003; p. 15. ISBN 978-981-4486-90-3. [Google Scholar]
- Yang, W.; Xu, Y.; Tang, Y.; Wang, C.; Hu, Y.; Huang, L.; Liu, J.; Luo, J.; Guo, H.; Chen, Y.; et al. Three-dimensional self-branching anatase TiO2 nanorods: Morphology control, growth mechanism and dye-sensitized solar cell application. J. Mater. Chem. A 2014, 2, 16030–16038. [Google Scholar] [CrossRef]
- Kang, B.K.; Lim, G.H.; Lim, B.K.; Yoon, D.H. Synthesis and Characterization of Monodispersed β-Ga2O3 Nanospheres via Morphology Controlled Ga4(OH)10SO4 Precursors. J. Alloys Compd. 2016, 675, 57–63. [Google Scholar] [CrossRef]
- Liu, X.; Qiu, G.; Zhao, Y.; Zhang, N.; Yi, R. Gallium oxide nanorods by the conversion of gallium oxide hydroxide nanorods. J. Alloys Compd. 2007, 439, 275–278. [Google Scholar] [CrossRef]
- Xu, X.; Huang, K.; Bi, K.; Liang, C.; Lin, S.; Wang, W.J.; Yang, T.Z.; Liu, J.; Fan, D.Y.; Yang, H.J.; et al. Controlled fabrication of α-GaOOH with a novel needle-like submicron tubular structure and its enhanced photocatalytic performance. J. Alloys Compd. 2015, 644, 485–490. [Google Scholar] [CrossRef]
- Li, G.; Peng, C.; Li, C.; Yang, P.; Hou, Z.; Fan, Y.; Cheng, Z.; Lin, J. Shape-Controllable Synthesis and Morphology-Dependent Luminescence Properties of GaOOH:Dy3+ and β-Ga2O3:Dy3+. Inorg. Chem. 2010, 49, 1449–1457. [Google Scholar] [CrossRef] [PubMed]
- Rambabu, U.; Munirathnam, N.R.; Prakash, T.L.; Vengalrao, B.; Buddhudu, S. Synthesis and characterization of morphologically different high purity gallium oxide nanopowders. J. Mater. Sci. 2007, 42, 9262–9266. [Google Scholar] [CrossRef]
- Girija, K.; Thirumalairajan, S.; Mastelaro, V.R.; Mangalaraj, D. Photocatalytic degradation of organic pollutants by shape selective synthesis of β-Ga2O3 microspheres constituted by nanospheres for environmental remediation. J. Mater. Chem. A 2015, 3, 2617–2627. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, Y.; Frost, R.L. Infrared and infrared emission spectroscopy of gallium oxide α-GaO(OH) nanostructures. Spectrochim. Acta A 2009, 74, 398–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tas, A.C.; Majewski, P.J.; Aldinger, F. Synthesis of Gallium Oxide Hydroxide Crystals in Aqueous Solutions with or without Urea and Their Calcination Behavior. J. Am. Ceram. Soc. 2002, 85, 1421–1429. [Google Scholar] [CrossRef] [Green Version]
- Krehula, S.; Ristic, M.; Kubuki, S.; Iida, Y.; Fabián, M.; Music, S. The formation and microstructural properties of uniform α-GaOOH particles and their calcination products. J. Alloys Compd. 2015, 620, 217–227. [Google Scholar] [CrossRef]
- Hill, R.J. Crystal structure refinement and electron density distribution in diaspore. Phys. Chem. Min. 1979, 5, 179–200. [Google Scholar] [CrossRef]
- Sasaki, K.; Higashiwaki, M.; Kuramata, A.; Masui, T.; Yamakoshi, S. Si-Ion Implantation Doping in β-Ga2O3 and Its Application to Fabrication of Low-Resistance Ohmic Contacts. Appl. Phys. Express 2013, 6, 086502. [Google Scholar] [CrossRef]
- Zhou, W.; Xia, C.; Sai, Q.; Zhang, H. Controlling n-type conductivity of β-Ga2O3 by Nb doping. Appl. Phys. Lett. 2017, 111, 242103. [Google Scholar] [CrossRef]
- Zeng, K.; Wallace, J.S.; Heimburger, C.; Sasaki, K.; Kiramata, A.; Masui, T.; Gardella, J.A., Jr.; Singisetti, U. Ga2O3 MOSFETs using spin-on-glass source/drain doping technology. IEEE Electron Device Lett. 2017, 38, 513. [Google Scholar] [CrossRef]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, H.J.; Yoo, T.H.; Yoon, Y.; Lee, I.G.; Kim, J.P.; Cho, B.J.; Hwang, W.S. High-Aspect Ratio β-Ga2O3 Nanorods via Hydrothermal Synthesis. Nanomaterials 2018, 8, 594. https://doi.org/10.3390/nano8080594
Bae HJ, Yoo TH, Yoon Y, Lee IG, Kim JP, Cho BJ, Hwang WS. High-Aspect Ratio β-Ga2O3 Nanorods via Hydrothermal Synthesis. Nanomaterials. 2018; 8(8):594. https://doi.org/10.3390/nano8080594
Chicago/Turabian StyleBae, Hyun Jeong, Tae Hee Yoo, Youngbin Yoon, In Gyu Lee, Jong Pil Kim, Byung Jin Cho, and Wan Sik Hwang. 2018. "High-Aspect Ratio β-Ga2O3 Nanorods via Hydrothermal Synthesis" Nanomaterials 8, no. 8: 594. https://doi.org/10.3390/nano8080594




