Hydrothermal Synthesis of Ultra-Light Coal-Based Graphene Oxide Aerogel for Efficient Removal of Dyes from Aqueous Solutions
Abstract
:1. Introduction
2. Materials and Methods
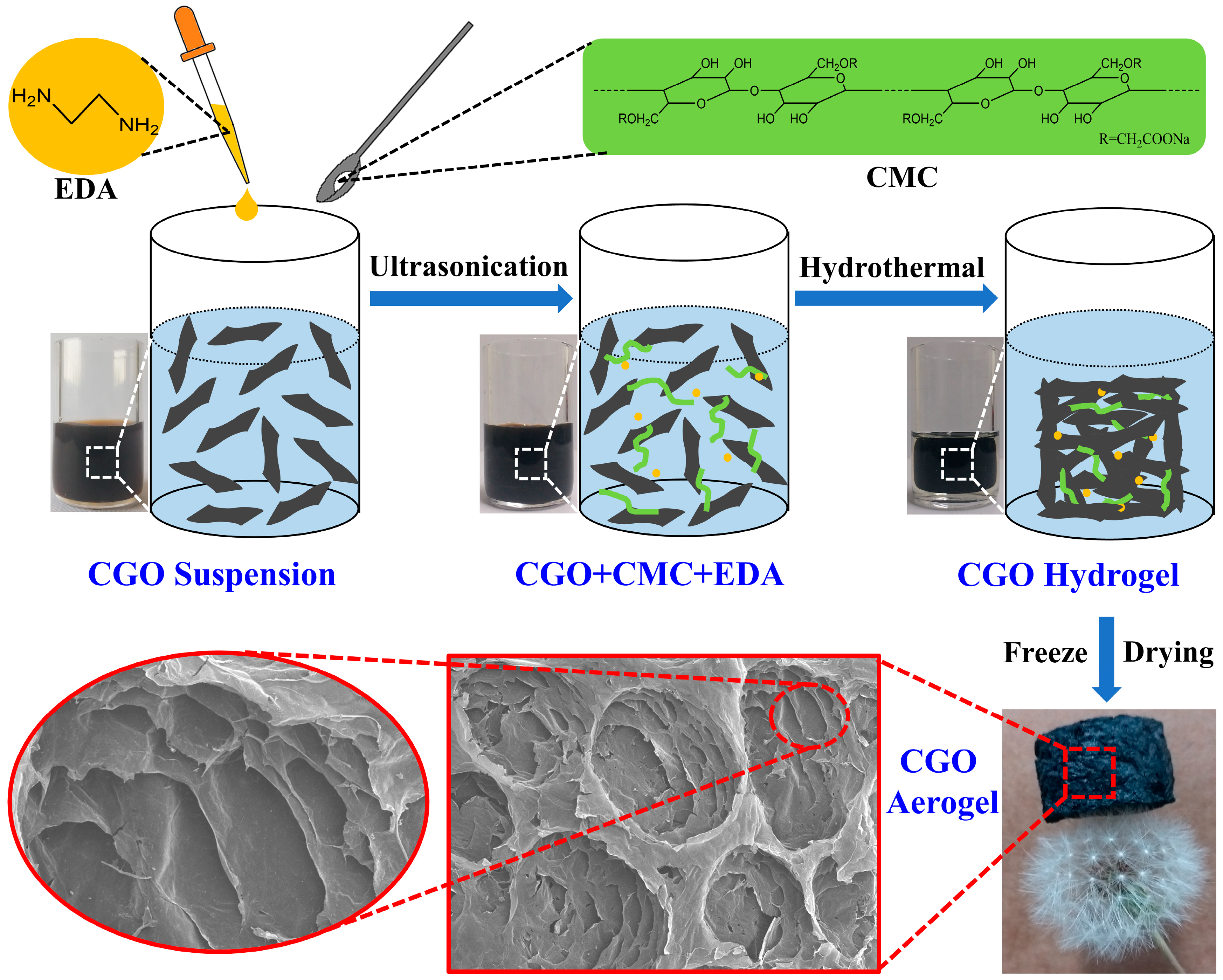
2.1. Preparation of Coal-Based Graphene Oxide (CGO) and CGO Aerogel
2.2. Characterization
2.3. Adsorption Experiments
3. Results and Discussion
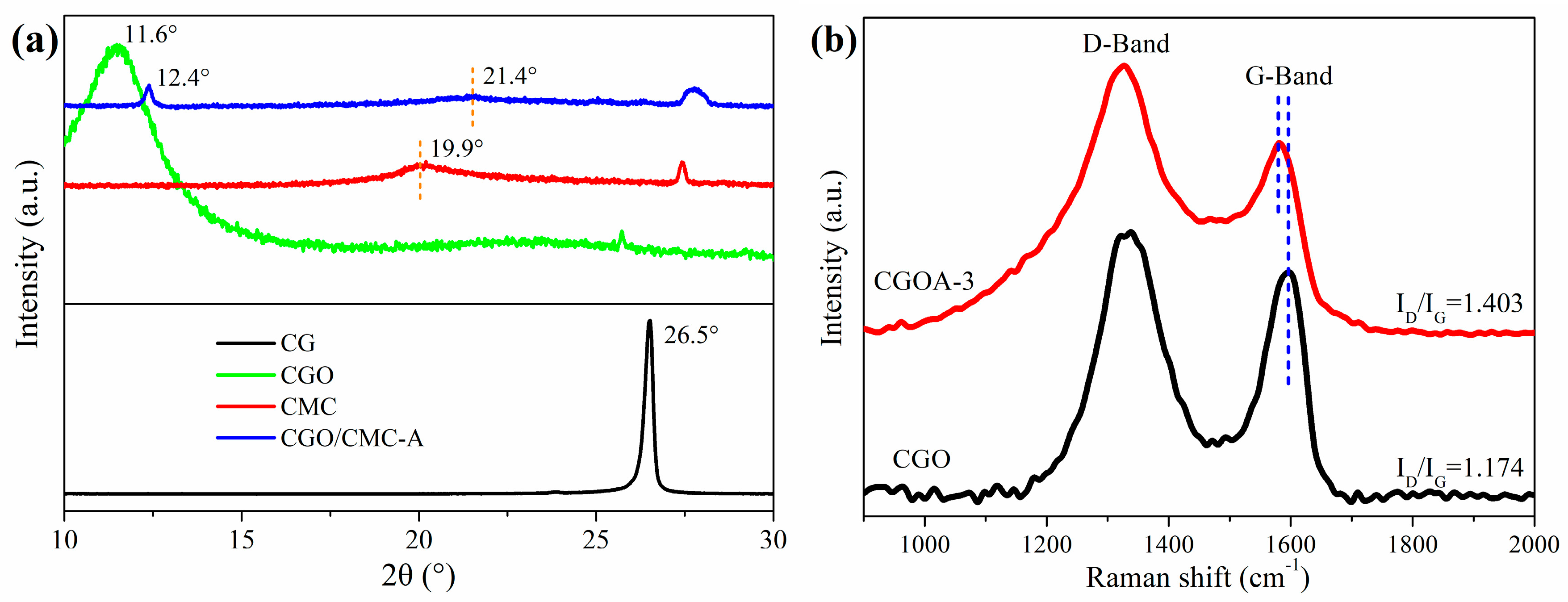
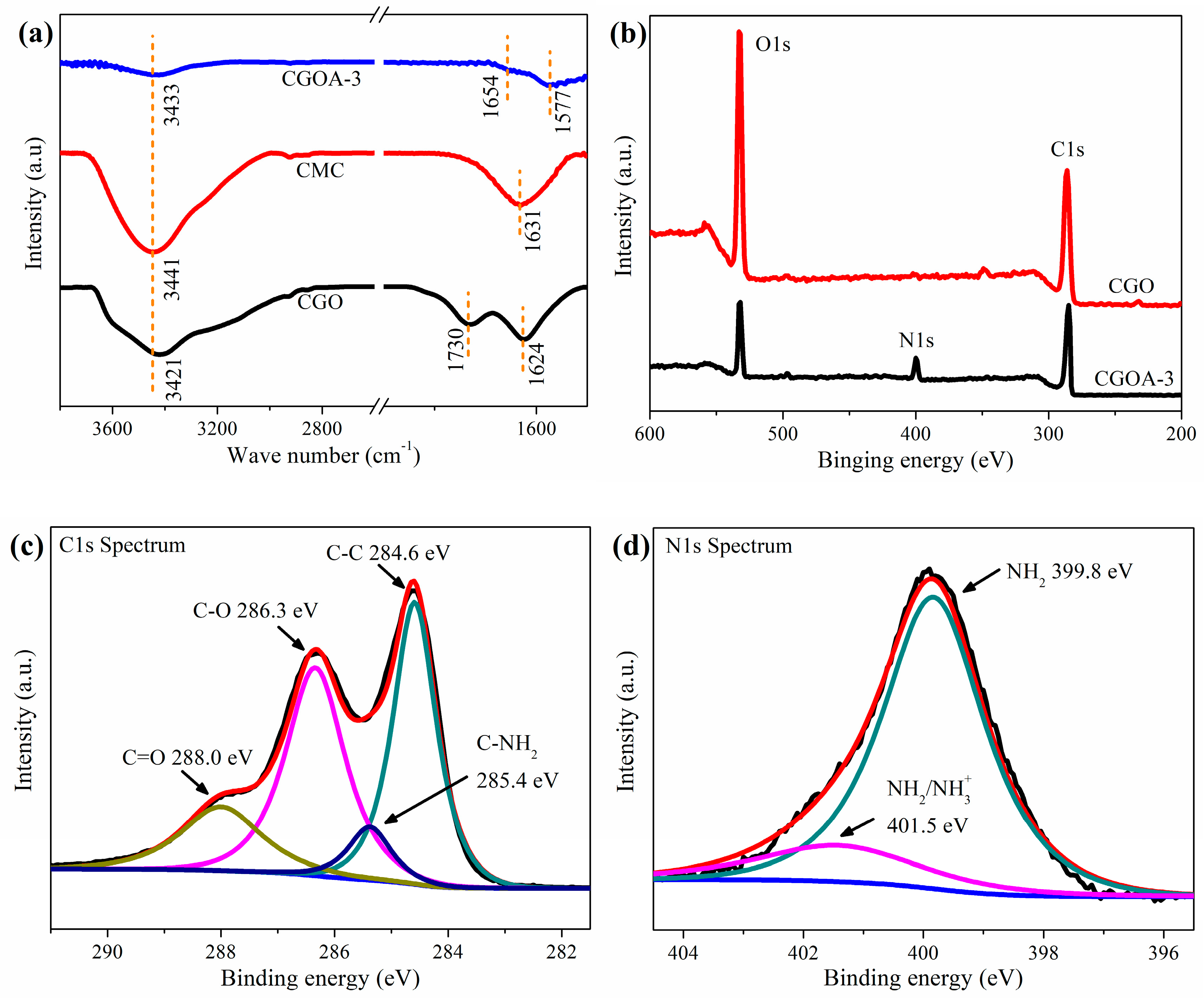
3.1. Structural Characterization
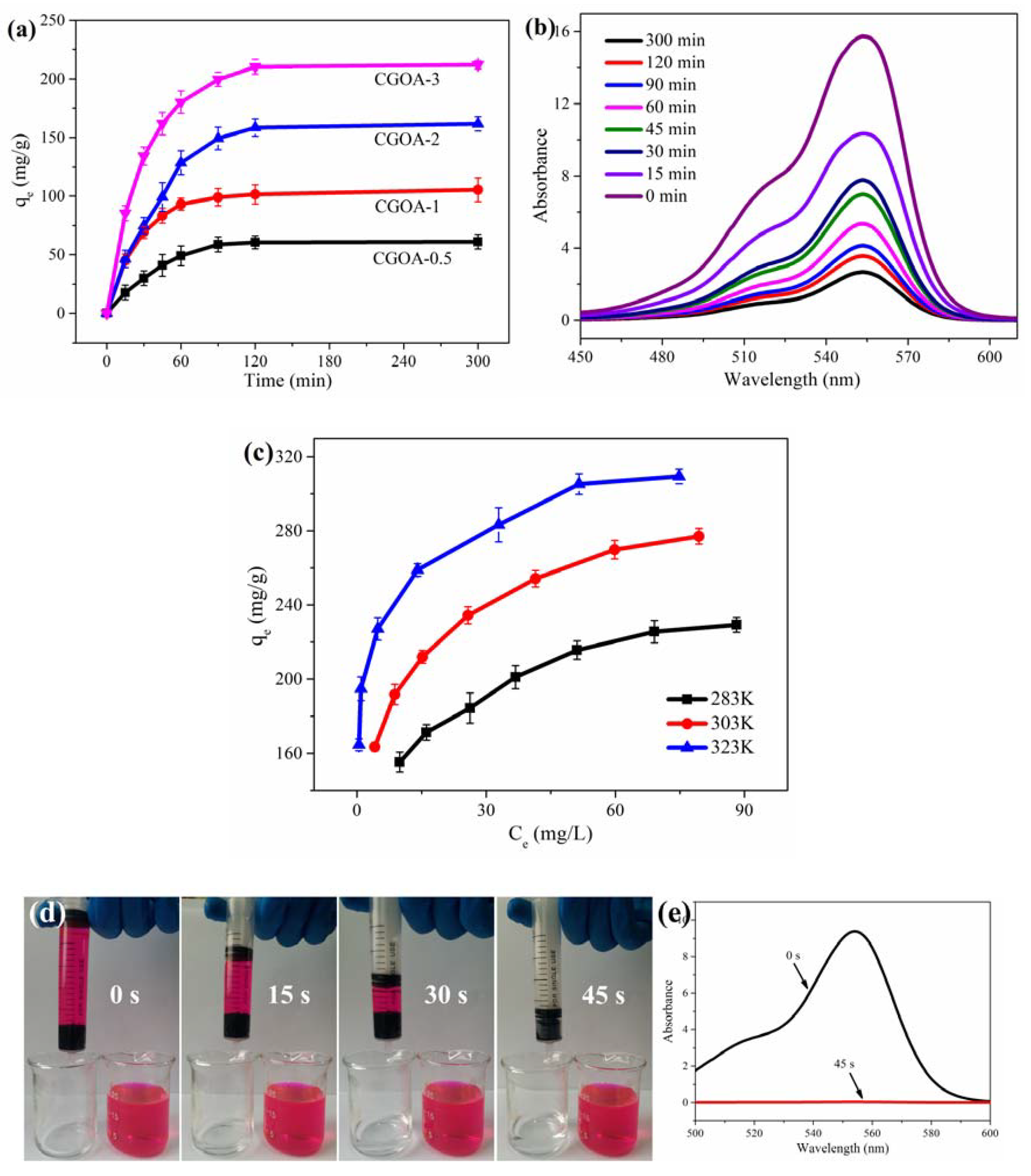
3.2. Adsorption Performance
3.2.1. Adsorption Kinetics
3.2.2. Adsorption Isotherm
3.2.3. Adsorption Thermodynamic
3.2.4. Effect of pH, Cycles and Different Dyes
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xiang, C.; Guo, R.; Lan, J.; Jiang, S.; Wang, C.; Du, Z.; Cheng, C. Self-assembling porous 3D titanium dioxide-reduced graphene oxide aerogel for the tunable absorption of oleic acid and rhodamineb dye. J. Alloy. Compd. 2017, 735, 246–252. [Google Scholar] [CrossRef]
- Gupta, V.K.; Kumar, R.; Nayak, A.; Saleh, T.A.; Barakat, M.A. Adsorptive removal of dyes from aqueous solution onto carbon nanotubes: A review. Adv. Colloid Interface Sci. 2013, 24, 193–194. [Google Scholar] [CrossRef] [PubMed]
- Xing, B.; Shi, C.; Zhang, C.; Yi, G.; Chen, L.; Guo, H.; Huang, G.; Cao, J. Preparation of TiO2/activated carbon composites for photocatalytic degradation of RhB under UV light irradiation. J. Nanomater. 2016, 2016, 3. [Google Scholar] [CrossRef]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of methylene blue on low-cost adsorbents: A review. J. Hazard. Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Rachna, K.; Aggarwal, A.; Singh, N.B. Preparation and characterization of zinc ferrite—polyaniline nanocomposite for removal of rhodamine b dye from aqueous solution. Environ. Nanotechnol. Monit. Manag. 2018, 9, 154–163. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, C.; Hou, B.; Wang, Y.; Hao, C.; Wu, J. Carbon composite lignin-based adsorbents for the adsorption of dyes. Chemosphere 2018, 206, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.; Yousef, S.; Abdelnaby, M.A.; Osman, T.A.; Hamawandi, B.; Toprak, M.S.; Muhammed, M.; Uheida, A. Photocatalytic degradation of organic dyes and enhanced mechanical properties of pan/cnts composite nanofibers. Sep. Purif. Technol. 2017, 182, 219–223. [Google Scholar] [CrossRef]
- Wang, J.; Qin, L.; Lin, J.; Zhu, J.; Zhang, Y.; Liu, J.; Bruggen, B.V.D. Enzymatic construction of antibacterial ultrathin membranes for dyes removal. Chem. Eng. J. 2017, 323, 56–63. [Google Scholar] [CrossRef]
- Oubagha, N.; Lemlikchi, W.; Sharrock, P.; Fiallo, M.; Mecherri, M.O. Hydroxyapatite precipitation with hydron blue dye. J. Environ. Manag. 2016, 203, 807–810. [Google Scholar] [CrossRef] [PubMed]
- Baban, A.; Yediler, A.; Avaz, G.; Hostede, S.S. Biological and oxidative treatment of cotton textile dye-bath effluents by fixed and fluidized bed reactors. Bioresour. Technol. 2010, 101, 1147–1152. [Google Scholar] [CrossRef] [PubMed]
- Ptaszkowska-Koniarz, M.; Goscianska, J.; Pietrzak, R. Removal of rhodamine b from water by modified carbon xerogels. Coll. Surf. A Physicochem. Eng. Asp. 2018, 543, 109–117. [Google Scholar] [CrossRef]
- Shu, D.; Feng, F.; Han, H.; Ma, Z. Prominent adsorption performance of amino-functionalized ultra-light graphene aerogel for methyl orange and amaranth. Chem. Eng. J. 2017, 324, 1–9. [Google Scholar] [CrossRef]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Ferralis, N. Probing mechanical properties of graphene with raman spectroscopy. J. Mater. Sci. 2010, 45, 5135–5149. [Google Scholar] [CrossRef]
- Wang, H.; Hu, X.; Guo, Y.; Qiu, C.; Long, S.; Hao, D.; Cai, X.; Xu, W.; Wang, Y.; Liu, Y. Removal of copper ions by few-layered graphene oxide nanosheets from aqueous solutions: External influences and adsorption mechanisms. J. Chem. Technol. Biotechnol. 2018, 93, 2447–2455. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, J.; Liu, P.; Song, W.; He, B. Study of water adsorption on graphene edges. RSC Adv. 2018, 8, 11216–11221. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, H.; Chu, Y.; Cui, Y.; Hayasaka, T.; Dasaka, V.; Nguyen, L.; Lin, L. Defect-induced gas adsorption on graphene transistors. Adv. Mater. Interfaces 2018, 5, 1701640. [Google Scholar] [CrossRef]
- Stobiecka, M.; Chalupa, A. Modulation of plasmon-enhanced resonance energy transfer to gold nanoparticles by protein survivin channeled-shell gating. J. Phys. Chem. B 2015, 119, 13227–13235. [Google Scholar] [CrossRef] [PubMed]
- Stobiecka, M. Novel plasmonic field-enhanced nanoassay for trace detection of proteins. Biosens. Bioelectron. 2014, 55, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, K.; Krazinski, B.; Kowalczyk, A.; Dworakowska, B.; Jakiela, S.; Stobiecka, M. Optical biosensing system for the detection of survivin mrna in colorectal cancer cells using a graphene oxide carrier-bound oligonucleotide molecular beacon. Nanomaterials 2018, 8, 510. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, K.; Stobiecka, M. Ternary interactions and energy transfer between fluorescein isothiocyanate, adenosine triphosphate, and graphene oxide nanocarriers. J. Phys. Chem. B 2017, 121, 6822–6830. [Google Scholar] [CrossRef] [PubMed]
- Stobiecka, M.; Dworakowska, B.; Jakiela, S.; Lukasiak, A.; Chalupa, A.; Zembrzycki, K. Sensing of survivin mrna in malignant astrocytes using graphene oxide nanocarrier-supported oligonucleotide molecular beacons. Sens. Actuators B Chem. 2016, 235, 136–145. [Google Scholar] [CrossRef]
- Lerf, A.; He, H.; Forster, M.; Klinowski, J. Structure of graphite oxide revisited‖. J. Phys. Chem. B 1998, 102, 4477–4482. [Google Scholar] [CrossRef]
- Cruzsilva, R.; Endo, M.; Terrones, M. Graphene oxide films, fibers, and membranes. Nanotechnol. Rev. 2016, 5. [Google Scholar] [CrossRef]
- Seabra, A.B.; Paula, A.J.; De, R.L.; Alves, O.L.; Durán, N. Nanotoxicity of graphene and graphene oxide. Chem. Res. Toxicol. 2014, 27, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Golkaram, M.; Van Duin, A.C.T. Revealing graphene oxide toxicity mechanisms: A reactive molecular dynamics study. Mater. Discov. 2015, 1, 54–62. [Google Scholar] [CrossRef]
- Guo, X.; Mei, N. Assessment of the toxic potential of graphene family nanomaterials. J. Food Drug Anal. 2014, 22, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Huang, T.; Tian, S.Q.; Xiao, Y.J.; Yang, J.H.; Zhang, N.; Wang, Y.; Zhou, Z.W. High structure stability and outstanding adsorption performance of graphene oxide aerogel supported by polyvinyl alcohol for waste water treatment. Mater. Des. 2016, 107, 187–197. [Google Scholar] [CrossRef]
- Huang, T.; Dai, J.; Yang, J.H.; Zhang, N.; Wang, Y.; Zhou, Z.W. Polydopamine coated graphene oxide aerogels and their ultrahigh adsorption ability. Diam. Relat. Mater. 2018, 86, 117–127. [Google Scholar] [CrossRef]
- Wei, X.; Huang, T.; Yang, J.H.; Zhang, N.; Wang, Y.; Zhou, Z.W. Green synthesis of hybrid graphene oxide/microcrystalline cellulose aerogels and their use as superabsorbents. J. Hazard. Mater. 2017, 335, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, Y.; Du, Q.; Sun, J.; Chen, L.; Hu, S.; Wang, Z.; Xia, Y.; Xia, L. Highly effective removal of basic fuchsin from aqueous solutions by anionic polyacrylamide/graphene oxide aerogels. J. Colloid Interface Sci. 2015, 453, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yao, F.; Oderinde, O.; Zhang, Z.; Fu, G. Green synthesis of oriented xanthan gum–graphene oxide hybrid aerogels for water purification. Carbohydr. Polym. 2017, 174, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Luo, H.; Geng, J.; Chen, J. Facile one-pot preparation of nitrogen-doped ultra-light graphene oxide aerogel and its prominent adsorption performance of cr(vi). Chem. Eng. J. 2017, 338, 62–71. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Chen, T.; Duan, T.; Yao, W.; Zheng, K.; Dai, L.; Zhu, W. Bioassembly of fungal hypha/graphene oxide aerogel as high performance adsorbents for U(VI) removal. Chem. Eng. J. 2018, 347, 407–414. [Google Scholar] [CrossRef]
- Mi, X.; Huang, G.; Xie, W.; Wang, W.; Liu, Y.; Gao, J. Preparation of graphene oxide aerogel and its adsorption for Cu2+ ions. Carbon 2012, 50, 4856–4864. [Google Scholar] [CrossRef]
- Wang, J.; Yao, Q.; Sheng, C.; Jin, C.; Sun, Q. One step preparation of graphene oxide/cellulose nanofibril hybrid aerogel for adsorptive removal of four kinds of antibiotics. J. Nanomater. 2017, 2017, 5150613. [Google Scholar] [CrossRef]
- Yao, Q.; Fan, B.; Ye, X.; Jin, C.; Sun, Q.; Sheng, C. 3D assembly based on 2D structure of cellulose nanofibril/graphene oxide hybrid aerogel for adsorptive removal of antibiotics in water. Sci. Rep. 2017, 7, 45914. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, K.; Tehrani, A.D.; Adeli, M. Bioconjugated graphene oxide hydrogel as an effective adsorbent for cationic dyes removal. Ecotoxicol. Environ. Saf. 2018, 147, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Li, J. Graphene oxide/cellulose aerogels nanocomposite: Preparation, pyrolysis, and application for electromagnetic interference shielding. Carbohydr. Polym. 2016, 150, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Rhee, K.; Jung, I.; Park, S. Eco-friendly synthesis, characterization and properties of a sodium carboxymethyl cellulose/graphene oxide nanocomposite film. Cellulose 2013, 20, 687–698. [Google Scholar] [CrossRef]
- Begum, H.A.; Mahbub, M.K.B. Effectiveness of carboxymethyl cellulose for the removal of methylene blue from aqueous solution. Dhaka Univ. J. Sci. 2013, 61, 2. [Google Scholar] [CrossRef]
- Layek, R.K.; Kundu, A.; Nandi, A.K. High-performance nanocomposites of sodium carboxymethylcellulose and graphene oxide. Macromol. Mater. Eng. 2013, 298, 1166–1175. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Wang, X.; Sun, Z.; Ma, J.; Wu, T.; Xing, F.; Gao, J. Porous graphene oxide/carboxymethyl cellulose monoliths, with high metal ion adsorption. Carbohydr. Polym. 2014, 101, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chu, H.; Wei, H.; Zhu, H.; Wang, G.; Zhu, J.; He, J. Facile fabrication of carboxymethyl cellulose sodium/graphene oxide hydrogel microparticles for water purification. RSC Adv. 2016, 6, 50061–50069. [Google Scholar] [CrossRef]
- Varaprasad, K.; Jayaramudu, T.; Sadiku, E.R. Removal of dye by carboxymethyl cellulose, acrylamide and graphene oxide via a free radical polymerization process. Carbohydr. Polym. 2017, 164, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Xing, B.; Zhang, C.; Cao, Y.; Huang, G.; Liu, Q.; Zhang, C.; Chen, Z.; Yi, G.; Chen, L.; Yu, J. Preparation of synthetic graphite from bituminous coal as anode materials for high performance lithium-ion batteries. Fuel Process. Technol. 2018, 172, 162–171. [Google Scholar] [CrossRef]
- Hu, H.; Zhao, Z.; Wan, W.; Gogotsi, Y.; Qiu, J. Ultralight and highly compressible graphene aerogels. Adv. Mater. 2013, 25, 2219–2223. [Google Scholar] [CrossRef] [PubMed]
- Kota, M.; Yu, X.; Yeon, S.H.; Cheong, H.W.; Park, H.S. Ice-templated three dimensional nitrogen doped graphene for enhanced supercapacitor performance. J. Power Source 2016, 303, 372–378. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of raman spectra of disordered and amorphous carbon. Phys. Rev. B Cond. Matter 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Ge, X.; Shan, Y.; Wu, L.; Mu, X.; Peng, H.; Jiang, Y. High-strength and morphology-controlled aerogel based on carboxymethyl cellulose and graphene oxide. Carbohydr. Polym. 2018, 197, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Wang, D.W.; Yin, L.C.; Li, N.; Li, F.; Cheng, H.M. Oxygen bridges between nio nanosheets and graphene for improvement of lithium storage. ACS Nano 2012, 6, 3214–3223. [Google Scholar] [CrossRef] [PubMed]
- Quan, Z.; Zhao, Z.; Zhang, Y.; Bo, M.; Zhou, A.; Qiu, J. Graphene sheets from graphitized anthracite coal: Preparation, decoration, and application. Energy Fuels 2012, 26, 5186–5192. [Google Scholar]
- El Achaby, M.; El Miri, N.; Snik, A.; Zahouily, M.; Abdelouahdi, K.; Fihri, A.; Barakat, A.; Solhy, A. Mechanically strong nanocomposite films based on highly filled carboxymethyl cellulose with graphene oxide. J. Appl. Polym. Sci. 2015, 133. [Google Scholar] [CrossRef]
- Rao, Z.; Ge, H.; Liu, L.; Zhu, C.; Min, L.; Liu, M.; Fan, L.; Li, D. Carboxymethyl cellulose modified graphene oxide as ph-sensitive drug delivery system. Int. J. Biol. Macromol. 2017, 107, 1184–1192. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Lin, B.; Huang, G.; Zhang, C.; Hou, W.; Yao, Y.; Xu, B.; Xing, B. Nitrogen and oxygen co-doped porous carbon for high performance supercapacitors. J. Mater. Sci. Mater. Electron. 2018, 29, 3340–3347. [Google Scholar] [CrossRef]
- Compton, O.C.; Dikin, D.A.; Putz, K.W.; Brinson, L.C.; Nguyen, S.T. Electrically conductive “alkylated” graphene paper via chemical reduction of amine-functionalized graphene oxide paper. Adv. Mater. 2010, 22, 892–896. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Li, W.; Zhu, Y. Decontamination of bisphenol a from aqueous solution by graphene adsorption. Langmuir 2012, 28, 8418–8425. [Google Scholar]
- Gerçel, Ö.; Gerçel, H.F. Adsorption of lead(ii) ions from aqueous solutions by activated carbon prepared from biomass plant material of euphorbia rigida. Chem. Eng. J. 2007, 132, 289–297. [Google Scholar] [CrossRef]
- Doğan, M.; Alkan, M.; Demirbaş, Ö.; Özdemir, Y.; Özmetin, C. Adsorption kinetics of maxilon blue grl onto sepiolite from aqueous solutions. Chem. Eng. J. 2006, 124, 89–101. [Google Scholar] [CrossRef]
- Ho, Y.S. Second-order kinetic model for the sorption of cadmium onto tree fern: A comparison of linear and non-linear methods. Water Res. 2006, 40, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Neghlani, P.K.; Rafizadeh, M.; Taromi, F.A. Preparation of aminated-polyacrylonitrile nanofiber membranes for the adsorption of metal ions: Comparison with microfibers. J. Hazard. Mater. 2011, 186, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, H.; Xu, A.; Tang, K.; Huang, Y.; Lu, C. In situ reduced and assembled three-dimensional graphene aerogel for efficient dye removal. J. Alloy. Compd. 2017, 714, 522–529. [Google Scholar] [CrossRef]

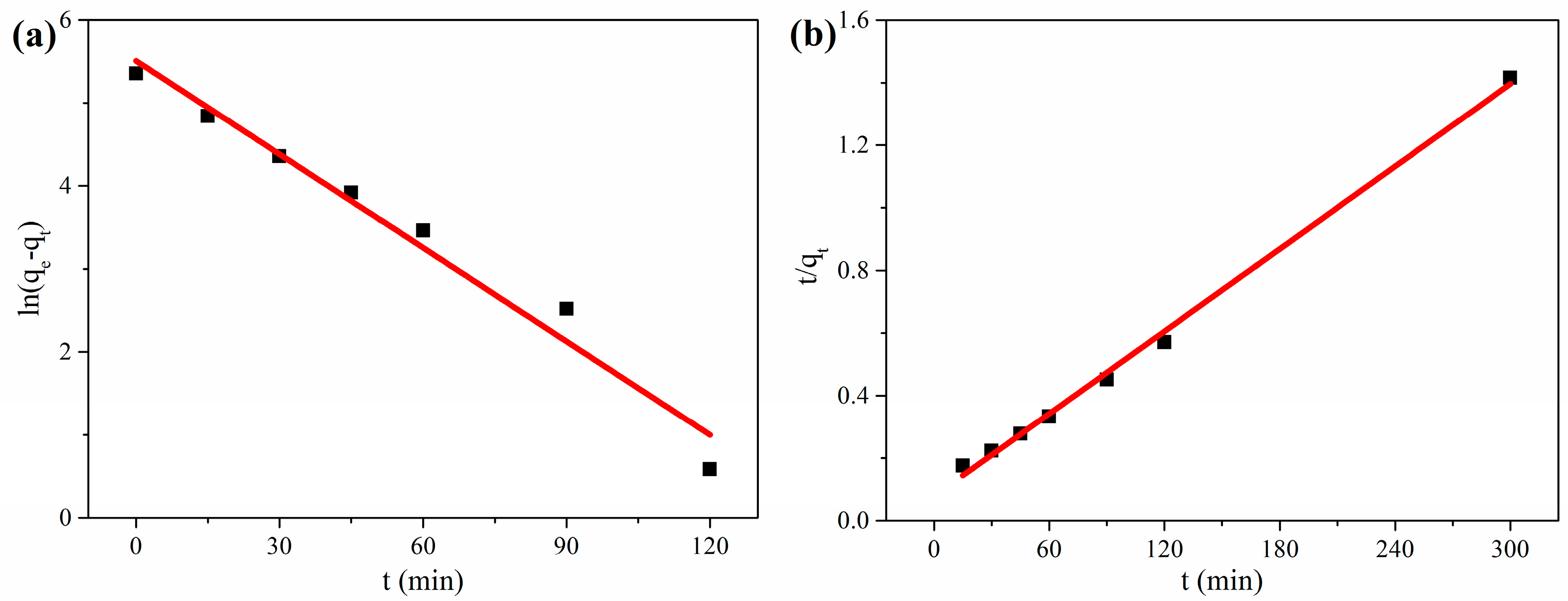
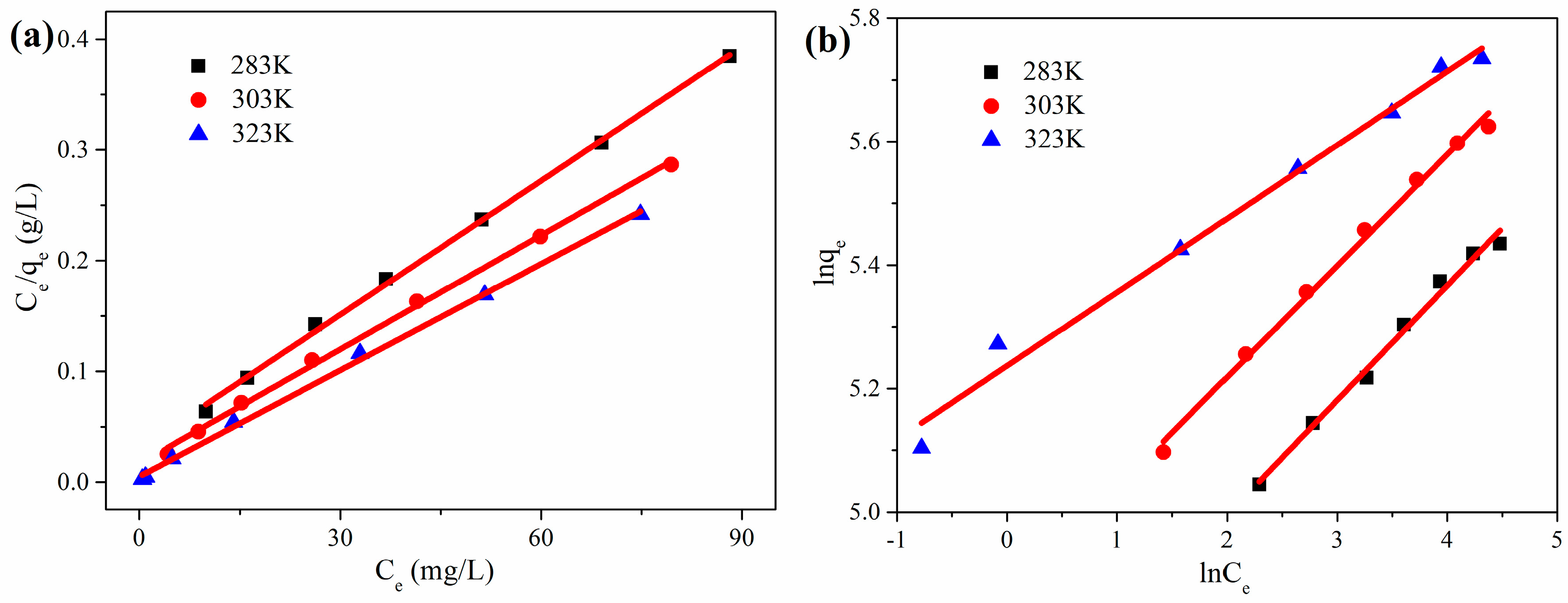
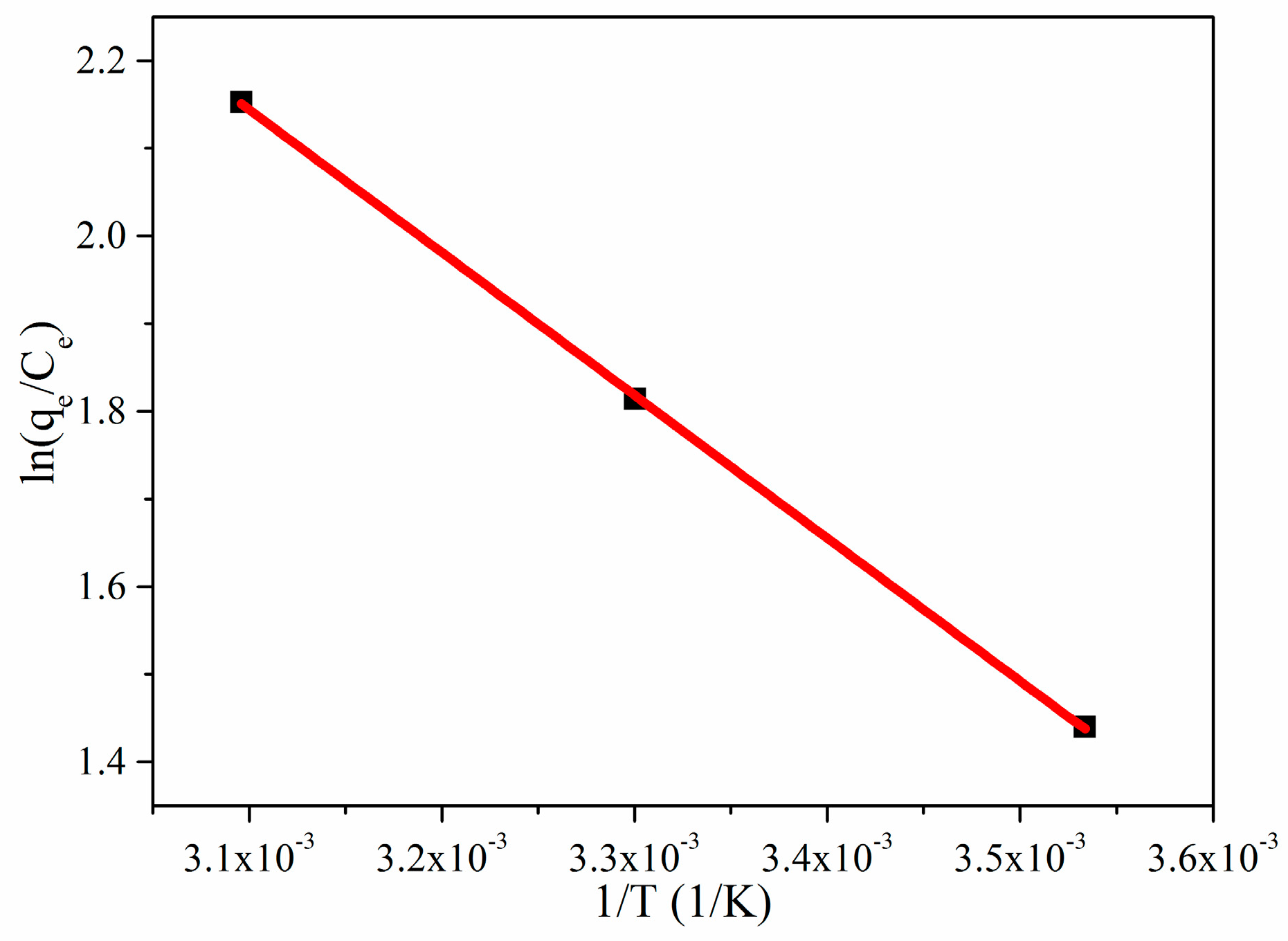
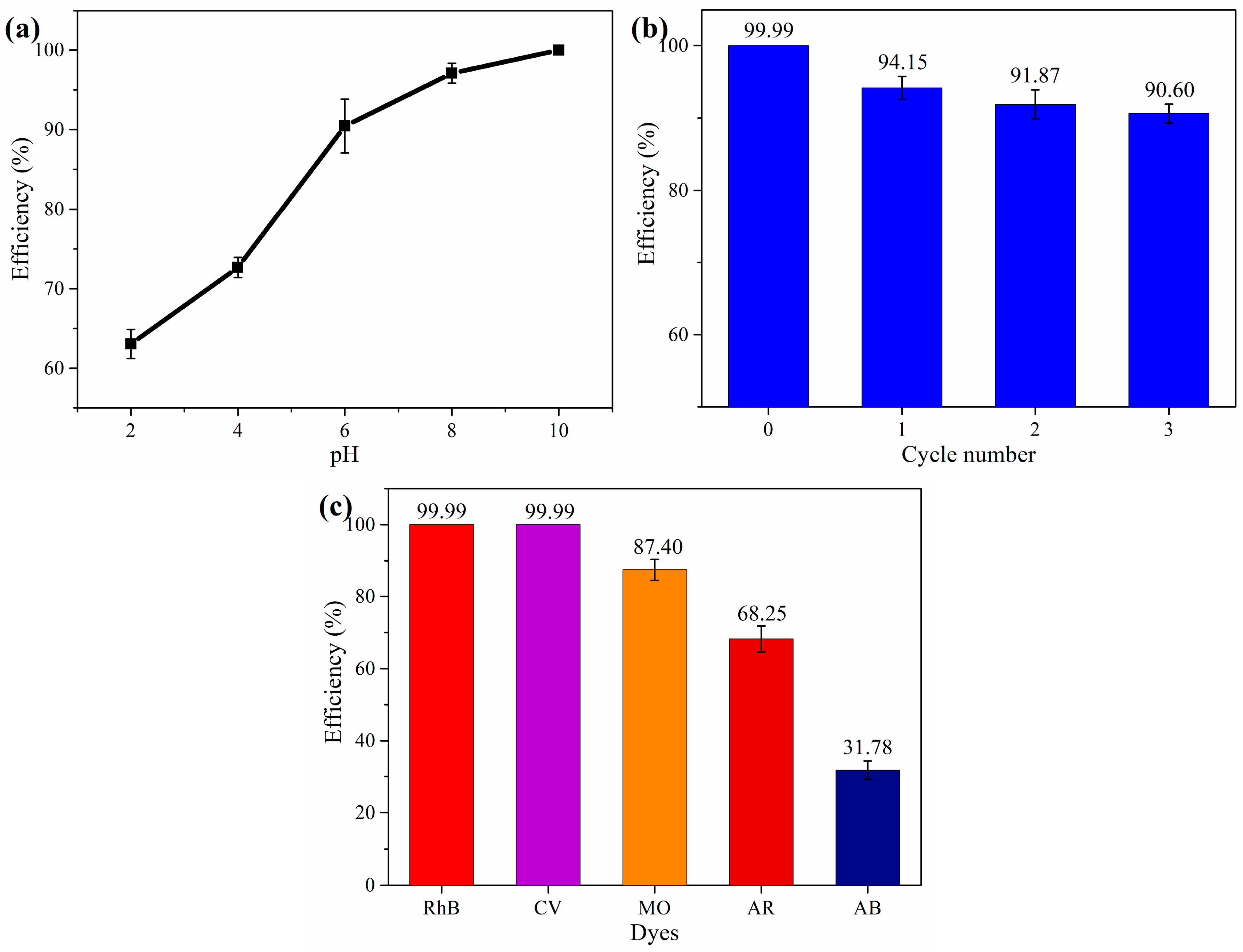
| Kinetic | Parameters | Values |
|---|---|---|
| Pseudo-first-order | qe (mg·g−1) | 246.6894 |
| k1 | 0.0376 | |
| R12 | 0.9682 | |
| Pseudo-second-order | qe (mg·g−1) | 227.7904 |
| k2 | 0.0002 | |
| R22 | 0.9964 |
| T(K) | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| qmax | KL | RL2 | KF | n | RF2 | |
| 283 | 248.14 | 0.1332 | 0.9984 | 101.82 | 5.3783 | 0.9889 |
| 303 | 291.55 | 0.2021 | 0.9981 | 128.73 | 5.5454 | 0.9938 |
| 323 | 312.50 | 0.6275 | 0.9980 | 188.04 | 8.3843 | 0.9851 |
| Adsorbent | Dyes | qmax (mg·g−1) | References |
|---|---|---|---|
| CMC/GO | Methylene blue | 59 | [44] |
| Eosin Y | 66 | ||
| CMC-AM-GO | Acid Blue-133 | 185.45 | [45] |
| CGOA | Rhodamine B | 312.50 | This work |
| T (K) | ∆G (kJ·mol−1) | ∆H (kJ·mol−1) | ∆S (J·mol−1·K−1) | R2 |
|---|---|---|---|---|
| 283 | −3.3833 | 13.540 | 59.780 | 0.9998 |
| 303 | −4.5793 | |||
| 323 | −5.7753 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, Y.; Xing, B.; Zheng, M.; Yi, G.; Huang, G.; Zhang, C.; Yuan, R.; Chen, Z.; Cao, Y. Hydrothermal Synthesis of Ultra-Light Coal-Based Graphene Oxide Aerogel for Efficient Removal of Dyes from Aqueous Solutions. Nanomaterials 2018, 8, 670. https://doi.org/10.3390/nano8090670
Lv Y, Xing B, Zheng M, Yi G, Huang G, Zhang C, Yuan R, Chen Z, Cao Y. Hydrothermal Synthesis of Ultra-Light Coal-Based Graphene Oxide Aerogel for Efficient Removal of Dyes from Aqueous Solutions. Nanomaterials. 2018; 8(9):670. https://doi.org/10.3390/nano8090670
Chicago/Turabian StyleLv, You, Baolin Xing, Mingkun Zheng, Guiyun Yi, Guangxu Huang, Chuanxiang Zhang, Ruifu Yuan, Zhengfei Chen, and Yijun Cao. 2018. "Hydrothermal Synthesis of Ultra-Light Coal-Based Graphene Oxide Aerogel for Efficient Removal of Dyes from Aqueous Solutions" Nanomaterials 8, no. 9: 670. https://doi.org/10.3390/nano8090670






