Water Dispersible Few-Layer Graphene Stabilized by a Novel Pyrene Derivative at Micromolar Concentration
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization of the Exfoliated Graphite Suspension
3. Results and Discussion
3.1. Synthesis of the Pyrene Derivative
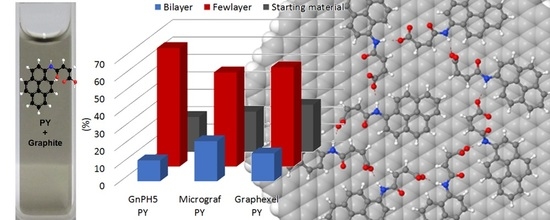
3.2. Exfoliation of Graphite
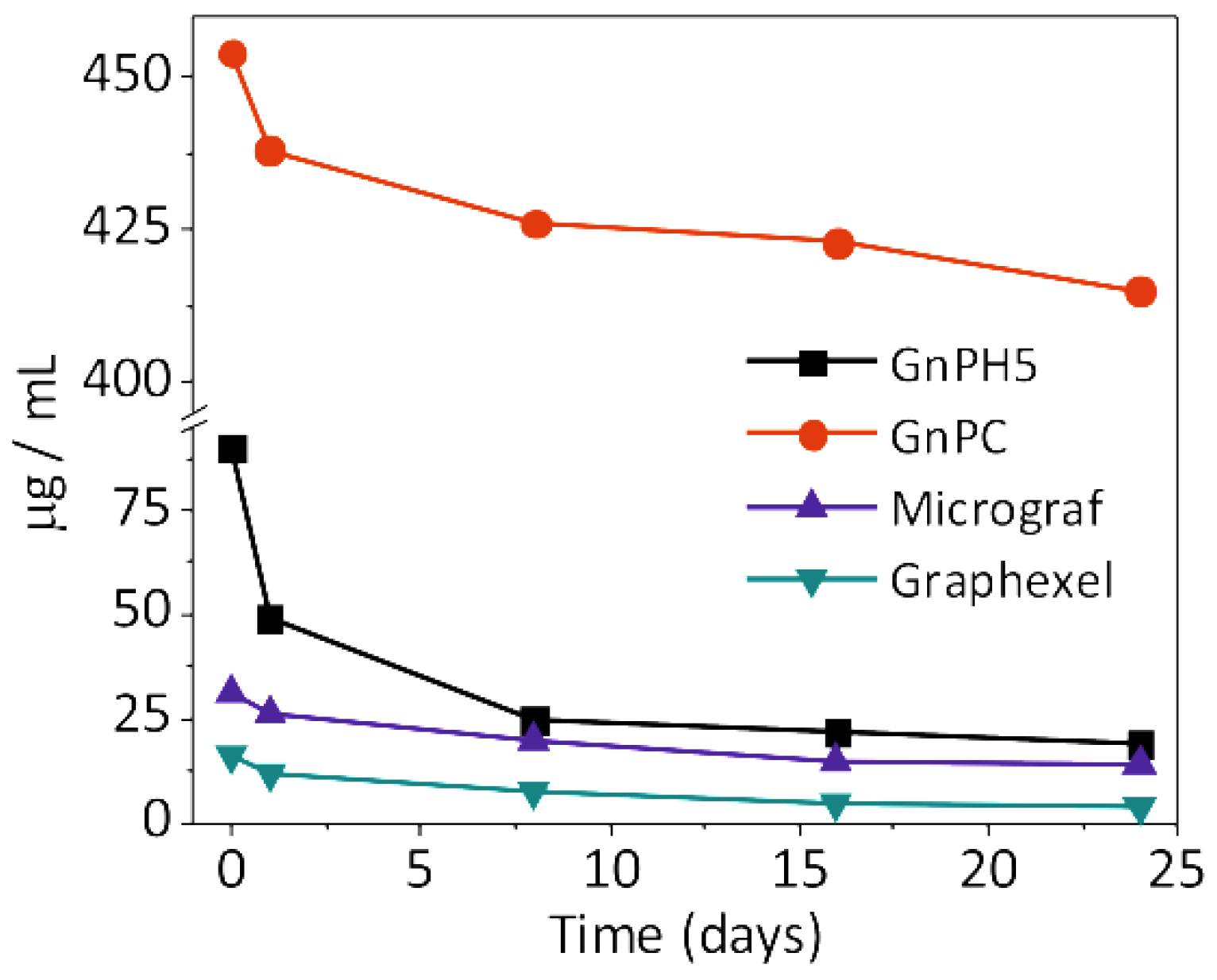
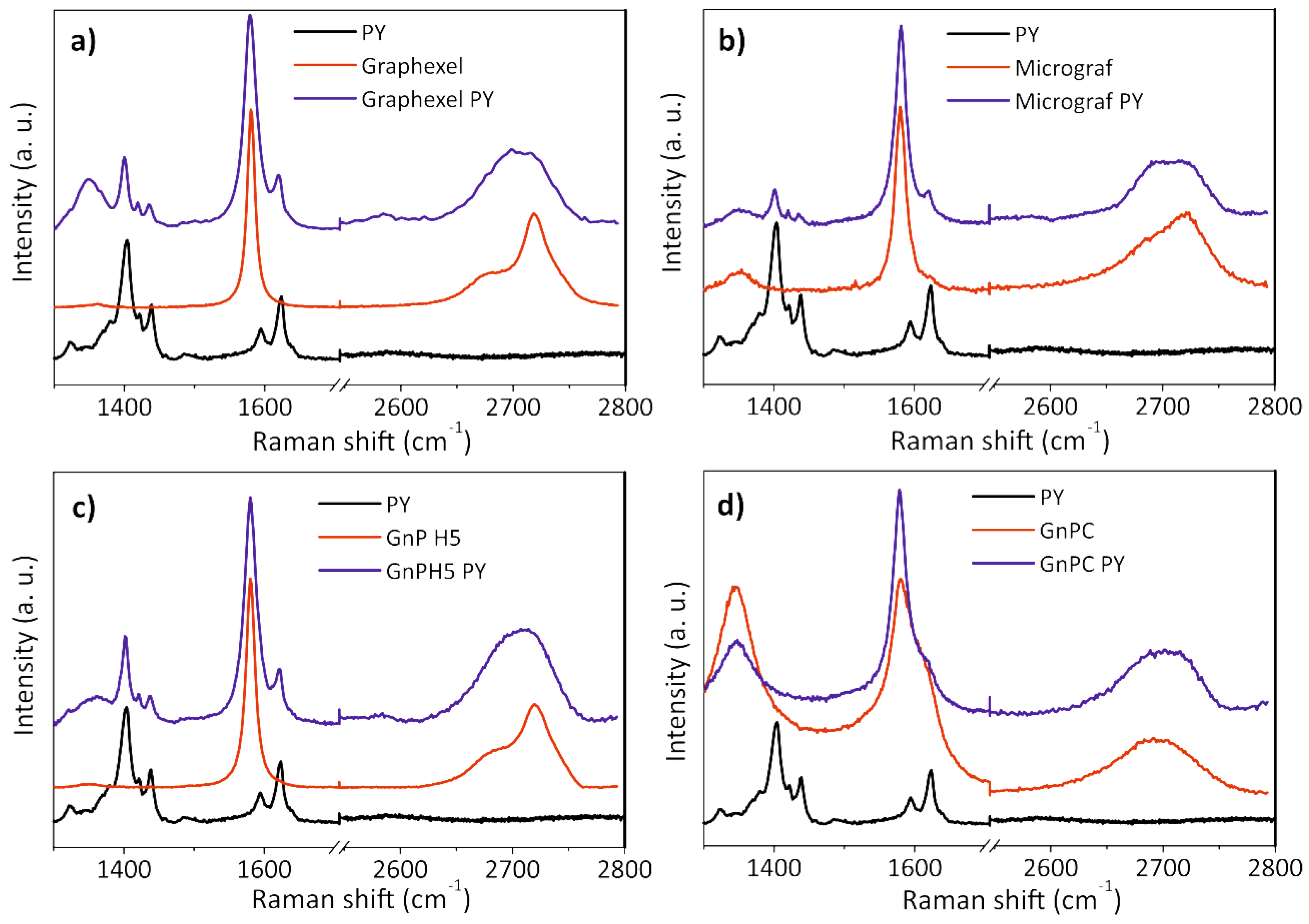
3.3. Characterization of the Exfoliated Materials
3.4. Study of the Pyrene Derivative/Exfoliated Graphite Interactions
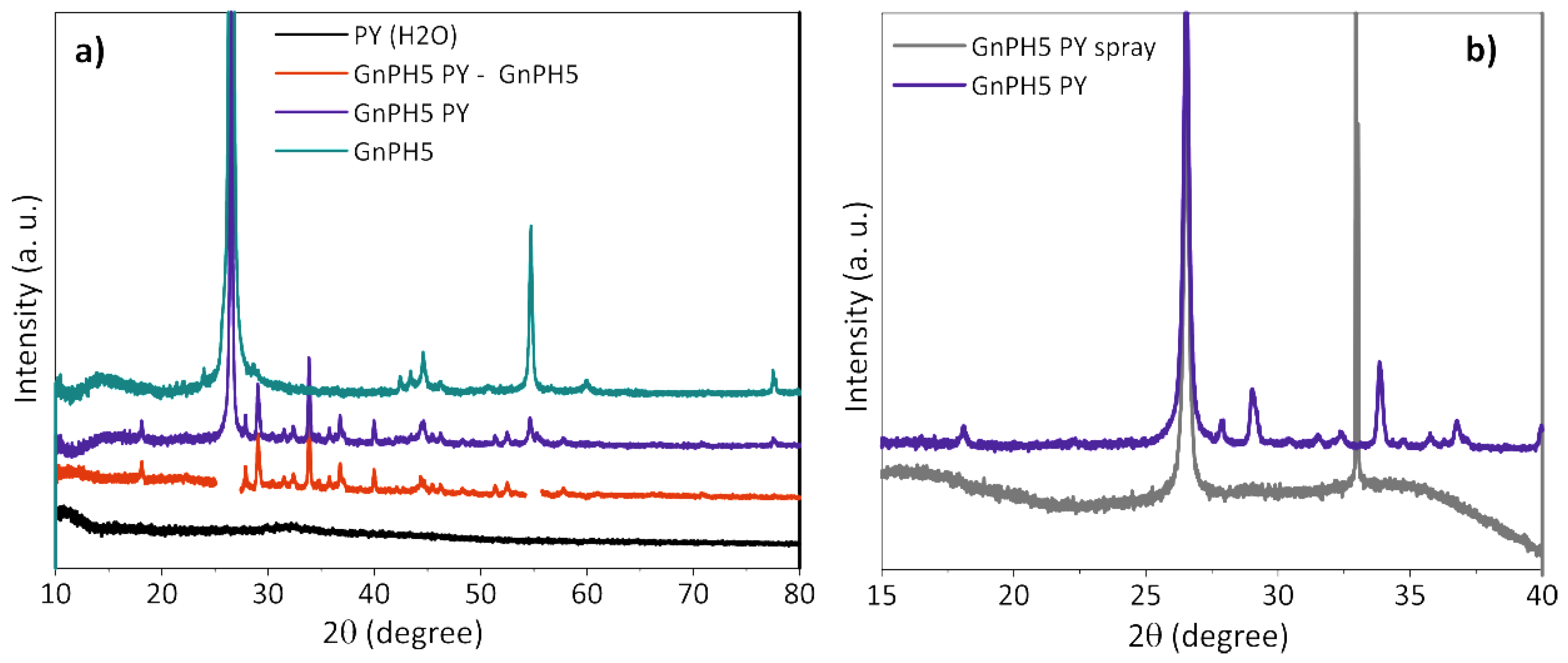
3.4.1. X-ray Diffraction
3.4.2. Molecular Modeling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Singh, V.; Joung, D.; Zhai, L.; Das, S.; Khondaker, S.I.; Seal, S. Graphene based materials: Past, present and future. Prog. Mater. Sci. 2011, 56, 1178–1271. [Google Scholar] [CrossRef]
- Huang, X.; Yin, Z.; Wu, S.; Qi, X.; He, Q.; Zhang, Q.; Yan, Q.; Boey, F.; Zhang, H. Graphene-based materials: Synthesis, characterization, properties, and applications. Small 2011, 7, 1876–1902. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Fal’ko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef] [PubMed]
- MacLeod, J.M.; Rosei, F. Molecular self-assembly on graphene. Small 2014, 10, 1038–1049. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.T.; Holland, B.; Byrne, M.; Gun’Ko, Y.K.; et al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol. 2008, 3, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Ciesielski, A.; Samori, P. Graphene via sonication assisted liquid-phase exfoliation. Chem. Soc. Rev. 2014, 43, 381–398. [Google Scholar] [CrossRef] [PubMed]
- Ciesielski, A.; Samori, P. Supramolecular approaches to graphene: From self-assembly to molecule-assisted liquid-phase exfoliation. Adv. Mater. 2016, 28, 6030–6051. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Bai, H.; Lu, G.; Li, C.; Shi, G. Flexible graphene films via the filtration of water-soluble noncovalent functionalized graphene sheets. J. Am. Chem. Soc. 2008, 130, 5856–5857. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Pang, S.P.; Alijani, V.; Li, C.; Feng, X.L.; Mullen, K. Composites of graphene with large aromatic molecules. Adv. Mater. 2009, 21, 3191–3195. [Google Scholar] [CrossRef]
- Liu, J.; Tao, L.; Yang, W.; Li, D.; Boyer, C.; Wuhrer, R.; Braet, F.; Davis, T.P. Synthesis, characterization, and multilayer assembly of pH sensitive graphene-polymer nanocomposites. Langmuir 2010, 26, 10068–10075. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, J.; Cui, L.; Wang, R.; Luo, X.; Barrow, C.; Yang, W. Preparation of graphene/polymer composites by direct exfoliation of graphite in functionalzed block copolymer matrix. Carbon 2013, 51, 148–155. [Google Scholar] [CrossRef]
- Zheng, X.L.; Xu, Q.; Li, J.B.; Li, L.H.; Wei, J.Y. High-throughput, direct exfoliation of graphite to graphene via a cooperation of supercritical CO2 and pyrene-polymers. RSC Adv. 2012, 2, 10632–10638. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, X.; Boulos, R.; Yasin, F.; Lu, H.; Raston, C.; Zhang, H. Pyrene-conjugated hyaluronan facilitated exfoliation and stabilisation of low dimentional nanomaterials in water. Chem. Commun. 2013, 49, 4845–4847. [Google Scholar] [CrossRef] [PubMed]
- Ihiawakrim, D.; Ersen, O.; Melin, F.; Hellwig, P.; Janowska, I.; Begin, D.; Baaziz, W.; Bedin-Colin, S.; Pham-Huu, C.; Batti, R. A single-stage functionalization and exfoliation method for the production of graphene in water: Stepwise construction of 2D-nanostructured composites with iron oxide nanoparticles. Nanoscale 2013, 5, 9073–9080. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Choi, J.Y.; Seo, T.S. DNA mediated water-dispersible graphene fabrication and gold nanoparticle-graphene hybrid. Chem. Commun. 2010, 46, 2844–2846. [Google Scholar] [CrossRef] [PubMed]
- Parviz, D.; Das, S.; Ahmed, H.S.; Irin, F.; Bhattacharia, S.; Green, M.J. Dispersions of non-covalently functionalized graphene with minimal stabilizer. ACS Nano 2012, 6, 8857–8867. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Simmons, T.; Shah, R.; Wolfe, C.; Lewis, K.M.; Washington, M.; Nayak, S.; Talapatra, S.; Kar, S. Stable aqueous dispersions of noncovalently functionalized graphene from graphite and their multifunctional high-performance applications. Nano Lett. 2010, 10, 4295–4301. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; An, X.H.; Shah, R.; Rawat, D.; Dave, B.; Kar, S.; Talapatra, S. Effect of 1-pyrene carboxylic-acid functionalization of graphene on its capacitive energy storage. J. Phys. Chem. C 2012, 116, 20688–20693. [Google Scholar] [CrossRef]
- Yang, H.; Hernandez, Y.; Schlierf, A.; Felten, A.; Eckmann, A.; Johal, S.; Louette, P.; Pireaux, J.-J; Feng, X.; Mullen, K.; et al. A simple method for graphene production based on exfoliation of graphite in water using 1-pyrenesulfonic acid sodium salt. Carbon 2013, 53, 357–365. [Google Scholar] [CrossRef]
- Viinikanoja, A.; Kauppila, J.; Damlin, P.; Makila, E.; Leiro, J.; Aaritalo, T.; Lukkari, J. Interactions between graphene sheets and ionic molecules used for the shear-assisted exfoliation of natural graphite. Carbon 2014, 68, 195–209. [Google Scholar] [CrossRef]
- Zhang, M.; Parajuli, R.R.; Mastrogiovanni, D.; Dai, B.; Lo, P.; Cheung, W.; Brukh, R.; Chiu, P.L.; Zhou, T.; Liu, Z.; et al. Production of graphene sheets by direct dispersion with aromatic healing agents. Small 2010, 6, 1100–1107. [Google Scholar] [CrossRef] [PubMed]
- Schlierf, A.; Yang, H.F.; Gebremedhn, E.; Treossi, E.; Ortolani, L.; Chen, L.P.; Minoia, A.; Morandi, V.; Samorì, P.; Casiraghi, C.; et al. Nanoscale insight into the exfoliation mechanism of graphene with organic dyes: Effect of charge, dipole and molecular structure. Nanoscale 2013, 5, 4205–4216. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Shi, Y.; Zhao, Y.; Chen, D.; Ye, J.; Yao, Y.; Gao, F.; Ni, Z.; Yu, T.; Shen, Z.; et al. Symmetry breaking of graphene monolayers by molecular decoration. Phys. Rev. Lett. 2009, 102, 135501. [Google Scholar] [CrossRef] [PubMed]
- Varenik, M.; Green, M.J.; Regev, O. Distinguishing self-assembled pyrene structures from exfoliated graphene. Langmuir 2016, 32, 10699–10704. [Google Scholar] [CrossRef] [PubMed]
- Becherer, M.S.; Schade, B.; Bottcher, C.; Hirsch, A. Supramolecular assembly of self-labeled amphicalixarenes. Chem. Eur. J. 2009, 15, 1637–1648. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Shen, Z.G. A review on mechanical exfoliation for the scalable production of graphene. J. Mater. Chem. A 2015, 3, 11700–11715. [Google Scholar] [CrossRef]
- Halbig, C.E.; Nacken, T.J.; Walter, J.; Damm, C.; Eigler, S.; Peukert, W. Quantitative investigation of the fragmentation process and defect density evolution of oxo-functionalized graphene due to ultrasonication and milling. Carbon 2016, 96, 897–903. [Google Scholar] [CrossRef]
- Bianco, A.; Cheng, H.M.; Enoki, T.; Gogotsi, Y.; Hurt, R.H.; Koratkar, N.; Kyotani, T.; Monthioux, M.; Park, C.R.; Tascon, J.M.D.; et al. All in the graphene family - A recommended nomenclature for two-dimensional carbon materials. Carbon 2013, 65, 1–6. [Google Scholar] [CrossRef]
- Kung, Y.; Hsiao, S. Fluorescent and electrochromic polyamideswith pyrenylamine chromophore. J. Mater. Chem. 2010, 20, 5481–5492. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D. Spectrometric Identification of Organic Compounds, 7th ed.; Wiley: New York, NY, USA, 2005; Chapter 3; ISBN 978-0-471-39362-7. [Google Scholar]
- Li, D.; Muller, M.B.; Gilje, S.; Kaner, R.B.; Wallace, G.G. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2008, 3, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Ehli, C.; Rahman, G.M.; Jux, N.; Balbinot, D.; Guldi, D.M.; Paolucci, F.; Marcaccio, M.; Paolucci, D.; Melle-Franco, M.; Zerbetto, F.; et al. Interactions in single wall carbon nanotubes/pyrene/porphyrin nanohybrids. J. Am. Chem. Soc. 2006, 128, 11222–11231. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yao, B.W.; Li, C.; Shi, G.Q. An improved Hummers method for eco-friendly synthesis of graphene oxide. Carbon 2013, 64, 225–229. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Ma, H.L.; Zhang, Q.L.; Peng, J.; Li, J.Q.; Zhai, M.L.; Yu, Z.Z. Facile synthesis of well-dispersed graphene by gamma-ray induced reduction of graphene oxide. J. Mater. Chem. 2012, 22, 13064–13069. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [PubMed]
- Young, R.J.; Kinloch, I.A.; Gong, L.; Novoselov, K.S. The mechanics of graphene nanocomposites: A review. Compos. Sci. Technol. 2012, 72, 1459–1476. [Google Scholar] [CrossRef]
- Malard, L.M.; Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S. Raman spectroscopy in graphene. Phys. Rep. 2009, 473, 51–87. [Google Scholar] [CrossRef]
- Rajagopalan, B.; Chung, J.S. Reduced chemically modified graphene oxide for supercapacitor electrode. Nanoscale Res. Lett. 2014, 9, 535. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.H.; Rangappa, D.; Kwon, Y.U.; Honma, I. Direct preparation of 1-PSA modified graphene nanosheets by supercritical fluidic exfoliation and its electrochemical properties. J. Mater. Chem. 2011, 21, 3462–3466. [Google Scholar] [CrossRef]
- Strutynski, K.; Gomes, J.A.; Melle-Franco, M. Accuracy of dispersion interactions in semiempirical and molecular mechanics models: The benzene dimer case. J. Phys. Chem. A 2014, 118, 9561–9567. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Bannwarth, C.; Shushkov, P. A robust and accurate tight-binding quantum chemical method for structures, vibrational frequencies, and noncovalent interactions of large molecular systems parametrized for all spd-block elements (Z = 1–86). J. Chem. Theory Comput. 2017, 13, 1989–2009. [Google Scholar] [CrossRef] [PubMed]







| Starting Material | Equivalent Diameter (µm) | Pyrene Derivative Concentration (mM) | Sonication Time (h) | Yield of Exfoliation (%) | Reference |
|---|---|---|---|---|---|
| Graphite + 1-pyrenecarboxylic acid | 45 | 1.3 | 24.0 a | 1.0 | [17] |
| Expanded graphite + Sodium 1-pyrenesulfonate | 15 | 3.0 | 1.0 b | 2.0 | [16] |
| Natural Graphite + Sulfonate substituted pyrene | 45 | 6.0 | 1.0 b | 2.4 | [20] |
| Graphite + 1-pyrenecarboxylic acid | 50 | 10.0 | 1.5 a | 2.5 | [19] |
| Graphite (Graphexel) | 180 | 0.05 | 1.0 b | 2.0 | Present work |
| Micronized graphite (Micrograf) | 10 | 0.05 | 1.0 b | 4.0 | |
| Expanded graphite (GnPH5) | 5 | 0.05 | 1.0 b | 5.0 | |
| Expanded graphite (GnPC) | 1–2 | 0.05 | 1.0 b | 85 |
| EBinding | ||
|---|---|---|
| # PY | PY (Carboxylate) in Water | PY (carboxylic) in Vacuum |
| 1 (1 adlayer) | −39 | −38 |
| 4 (1 adlayer) | −39 | −50 |
| 8 (1 adlayer) | −39 | −49 |
| 10 (1 adlayer) | −38 | −46 |
| 10 (>1 adlayer) | −35 | −48 |
| 12 (>1 adlayer) | −36 | −47 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cunha, E.; Proença, M.F.; Pereira, M.G.; Fernandes, M.J.; Young, R.J.; Strutyński, K.; Melle-Franco, M.; Gonzalez-Debs, M.; Lopes, P.E.; Paiva, M.D.C. Water Dispersible Few-Layer Graphene Stabilized by a Novel Pyrene Derivative at Micromolar Concentration. Nanomaterials 2018, 8, 675. https://doi.org/10.3390/nano8090675
Cunha E, Proença MF, Pereira MG, Fernandes MJ, Young RJ, Strutyński K, Melle-Franco M, Gonzalez-Debs M, Lopes PE, Paiva MDC. Water Dispersible Few-Layer Graphene Stabilized by a Novel Pyrene Derivative at Micromolar Concentration. Nanomaterials. 2018; 8(9):675. https://doi.org/10.3390/nano8090675
Chicago/Turabian StyleCunha, Eunice, Maria Fernanda Proença, Maria Goreti Pereira, Maria José Fernandes, Robert J. Young, Karol Strutyński, Manuel Melle-Franco, Mariam Gonzalez-Debs, Paulo E. Lopes, and Maria Da Conceição Paiva. 2018. "Water Dispersible Few-Layer Graphene Stabilized by a Novel Pyrene Derivative at Micromolar Concentration" Nanomaterials 8, no. 9: 675. https://doi.org/10.3390/nano8090675
APA StyleCunha, E., Proença, M. F., Pereira, M. G., Fernandes, M. J., Young, R. J., Strutyński, K., Melle-Franco, M., Gonzalez-Debs, M., Lopes, P. E., & Paiva, M. D. C. (2018). Water Dispersible Few-Layer Graphene Stabilized by a Novel Pyrene Derivative at Micromolar Concentration. Nanomaterials, 8(9), 675. https://doi.org/10.3390/nano8090675