Protective Properties of a Microstructure Composed of Barrier Nanostructured Organics and SiOx Layers Deposited on a Polymer Matrix
Abstract
:1. Introduction
2. Materials and Methods
2.1. Source Materials, Substrates and Polymeric Matrices
2.2. Apparatuses for the Samples Preparation
2.3. Measuring Instruments
3. Results and Discussion
3.1. Protective Properties
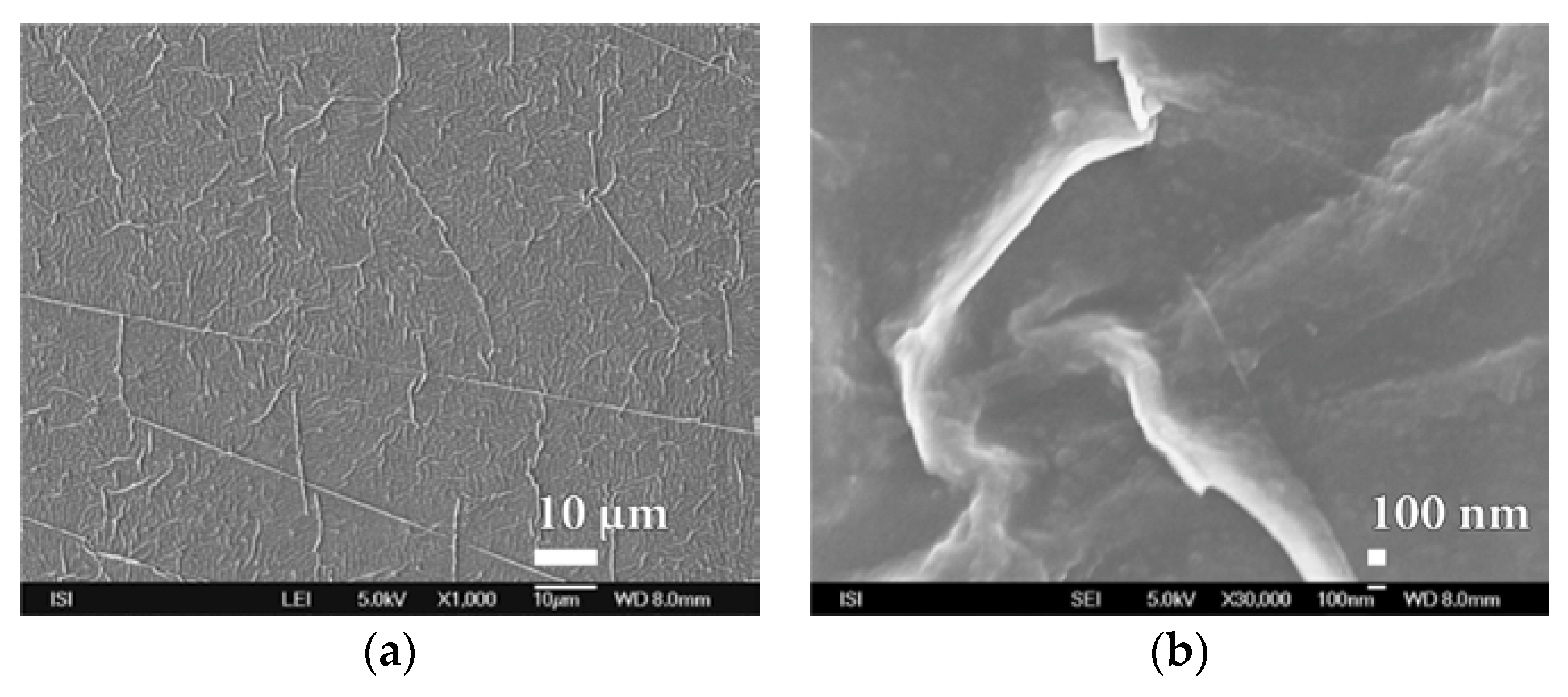
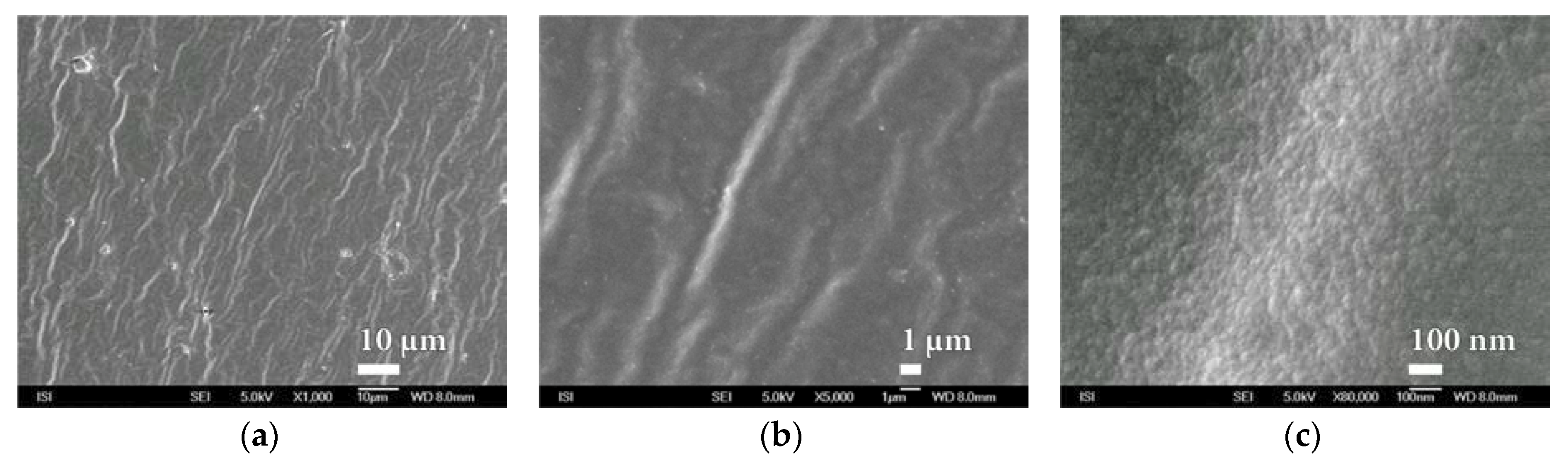
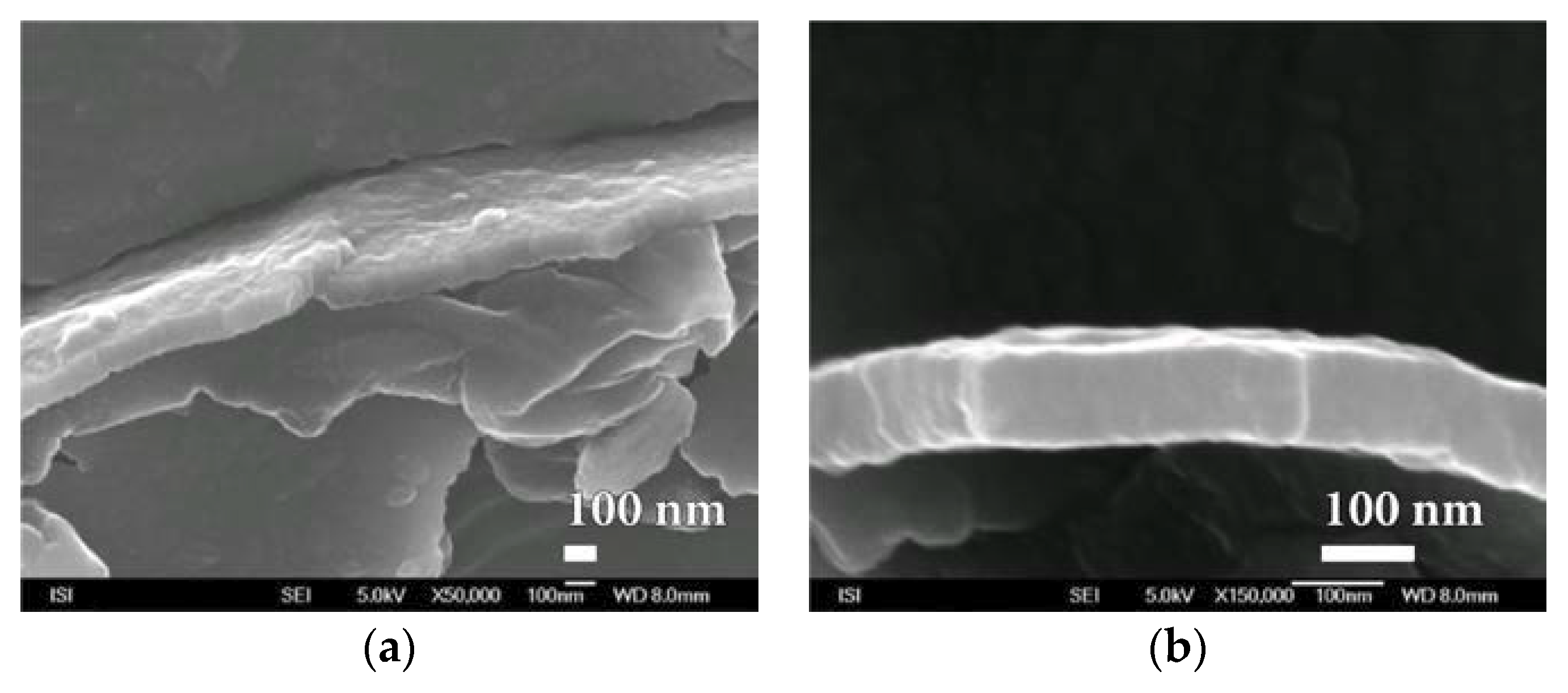
3.2. Microstructure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kogelschatz, U.; Eliasson, B.; Egli, W. Dielectric-barrier discharges principle and applications. J. Phys. Arch. 1997, 7, C4-47–C4-66. [Google Scholar] [CrossRef]
- Otrisal, P.; Florus, S.; Svorc, L.; Barsan, G.; Mosteanu, D. A new colorimetric assay for determination of selected toxic vapours and liquids permeation through barrier materials using the minitest device. Rev. Plast. 2017, 54, 748–751. [Google Scholar]
- Kmochova, A.; Tichy, A.; Zarybnicka, L.; Sinkorova, Z.; Vavrova, J.; Rehacek, V.; Durisova, K.; Kubelkova, K.; Pejchal, J.; Kuca, K. Modulation of ionizing radiation-induced effects by NU7441, KU55933 and VE821 in peripheral blood lymphocytes. J. Appl. Biomed. 2016, 14, 19–24. [Google Scholar] [CrossRef]
- Krumpolec, R.; Zahoranova, A.; Cernak, M.; Kovacik, D. Chemical and physical evaluation of hydrophobic pp-HMDSO layers deposited by plasma polymerization at atmospheric pressure. Chem. Listy 2012, 106, 1450–1454. [Google Scholar]
- Radovan, K.M.; Negovan, I.D.; Dalibor, J.B.; Smiljana, M.M.; Dejan, I.R.; Marija, M.D.; Branko, K.V. Dynamic adsorption characteristics of thin layered activated charcoal materials used in chemical protective overgarments. Indian J. Fibre Text. Res. 2016, 41, 402–410. [Google Scholar]
- Mosteanu, D.; Barsan, G.; Otrisal, P.; Giurgiu, L.; Oancea, R. Obtaining the volatile oils from wormwood and tarragon plants by a new microwave hydrodistillation method. Rev. Chim. 2017, 68, 2499–2502. [Google Scholar]
- Sun, Y.J.; Fu, Y.B.; Chen, Q.; Zhang, C.M.; Sang, L.J.; Zhang, Y.F. Silicon dioxide coating deposited by PDPs on PET films and influence on oxygen transmission rate. Chin. Phys. Lett. 2008, 25, 1753–1756. [Google Scholar]
- Felts, J.T. Transparent barrier coatings update: Flexible substrates. J. Plast. Film. Sheeting 1993, 9, 139–158. [Google Scholar] [CrossRef]
- Macák, J.M.; Hromádko, L.; Bulánek, R.; Koudelková, E.; Buk, J.; Tejkl, M. Process for Preparing Submicron Fibers of Amorphous Silica and Submicron Fibers of Amorphous Silica Prepared in Such a Manner. Available online: https://1url.cz/qtyDc (accessed on 12 June 2018).
- Madocks, J.; Marcus, P.; Morse, P. Silicon dioxide coatings on plastic substrates by large area plasma enhanced chemical vapour deposition process. In Proceedings of the 49th Annual Technical Conference, Washington, DC, USA, 22–27 April 2006; pp. 569–573. [Google Scholar]
- Hoskova-Mayerova, S.; Maturo, A. Algebraic hyperstructures and social relations. Italian J. Pure. Appl. Math. 2018, 39, 701–709. [Google Scholar]
- Anonymous. CERAMIS® Barrier Films: Transparent SiOx Barrier Films for Ultimate Product Protection. Available online: https://1url.cz/Pt734 (accessed on 23 June 2018).
- Roberts, A.P.; Henry, B.M.; Sutton, A.P.; Grovenor, C.R.M.; Briggs, G.A.D.; Miyamoto, T.; Kano, M.; Tsukahara, Y.; Yanaka, M. Gas permeation in silicon-oxide/polymer (SiOx/PET) barrier films: Role of the oxide lattice, nano-defects and macro-defects. J. Membr. Sci. 2002, 208, 75–88. [Google Scholar] [CrossRef]
- Erlat, A.G.; Spontak, R.J.; Clarke, R.P.; Robinson, T.C.; Haaland, P.D.; Tropsha, Y.; Harvey, N.G.; Vogler, E.A. SiOx gas barrier coatings on polymer substrates: Morphology and gas transport considerations. J. Phys. Chem. B 1999, 103, 6047–6055. [Google Scholar] [CrossRef]
- Felts, J.; Finson, E.J. Transparent SiO2 barrier coatings: Conversion and production status. In Proceedings of the 37th Annual Technical Conference Proceedings, Boston, MA, USA, 8–13 May1994; pp. 139–143. [Google Scholar]
- Foest, R.; Schafer, J.; Quade, A.; Ohl, A.; Weltmann, K.D. Miniaturized atmospheric pressure plasma jet (APPJ) for deposition of SiOx films with different Silicon-organics compounds—A comparative study. In Proceedings of the 35th EPS Conference on Plasma Phys, Hersonissos, Greece, 9–13 June 2008. [Google Scholar]
- Gaur, S.; Vergason, G. Plasma polymerization: For industrial production. In Proceedings of the 44th Annual Technical Conference Proceedings, Philadelphia, PA, USA, 21–26 April 2001. [Google Scholar]
- Huang, X. Fabrication and Properties of Carbon Fibers. Materials 2009, 1, 938–947. [Google Scholar] [CrossRef]
- Horova, I.; Kolacek, J.; Vopatova, K. Full bandwidth matrix selectors for gradient kernel density estimate. Comput. Stat. Data Anal. 2013, 57, 364–376. [Google Scholar] [CrossRef]
- Raynaud, P.; Clergereaux, R.; Segui, Y.; Gherardi, N.; Despax, B. Plasma Nanolayering, Nanoclustering, Nanotexturing: Challenges for Future Processes for Mass Produstion. Available online: https://1url.cz/Yt73j (accessed on 23 June 2018).
- Starostin, S.A.; Aldea, E.; Vries, H.W.; Creatore, M.; van de Sanden, M.C.M. Application of atmospheric pressure glow discharge (APGD) for deposition of thin silica like films on polymeric webs. In Proceedings of the 28th International Conference on Phenomena in Ionized Gases (ICPIG), Prague, Czech Republic, 15–20 July 2007. [Google Scholar]
- Fortin, J.B.; Lu, T.-M. Chemical Vapor Deposition Polymerization: The Growth and Properties of Parylene Thin Films; Springer Science & Business Media: New York, NY, USA, 2003. [Google Scholar] [CrossRef]
- Gorham, W.F. A new, general synthetic method for the preparation of linear poly-p-xylylenes. J. Polym. Sci. Part A 1966, 4, 3027–3039. [Google Scholar] [CrossRef]
- Míša, M.; Obšel, V. System for Measuring Permeation of Gases and Vapors Through Barrier Membranes. Available online: https://1url.cz/Bty4h (accessed on 11 June 2018).
- Yasuda, H. Plasma Polymerization; 1st Issue; Academic Press: Orlando, FL, USA, 1985. [Google Scholar]
- Smith, A.W.; Copeland, N. Clear barrier coatings by plasma CVD for packaging applications. In Proceedings of the 43th Annual Technical Conference Proceedings, Washington, DC, USA, 22–27 April 2006; pp. 636–641. [Google Scholar]
- Izu, M.; Dotter, B.; Ovshinsky, S.R. Roll-to-roll microwave PECVD machine for high-barrier film coatings. J. Photopolym. Sci. Technol. 1995, 8, 195–204. [Google Scholar] [CrossRef]
- Hones, P.; Levy, F.; Randall, N.X. Influence of deposition parameters on mechanical properties of sputter-deposited Cr2O3 thin films. J. Mater. Res. 1999, 14, 3623–3629. [Google Scholar] [CrossRef]
- Jahromi, S.; Moosheimer, S.U. Oxygen barrier coatings based on supramolecular assembly of melamine. Macromolecules 2002, 33, 7582–7587. [Google Scholar] [CrossRef]
- Howells, D.G.; Henry, B.M.; Leterrier, Y.; Manson, J.A.E.; Madocks, J.; Assender, H.E. Mechanical properties of SiOx gas barrier coatings on polyester films. Surf. Coat. Technol. 2008, 202, 3529–3537. [Google Scholar] [CrossRef]
- Ozeki, K.; Nagashima, Y.; Ohgoe, Y.; Hirakuri, K.K.; Mukaibayashi, H.; Masuzawa, T. Gas barrier properties of diamond-like carbon films coated on PTFE. Appl. Surf. Sci. 2009, 25, 7286–7290. [Google Scholar] [CrossRef]
- Stodola, P. Using metaheuristics on the multi-depot vehicle routing problem with modified optimization criterion. Algorithms 2018, 11, 74. [Google Scholar] [CrossRef]















© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prikryl, R.; Otrisal, P.; Obsel, V.; Svorc, L.; Karkalic, R.; Buk, J. Protective Properties of a Microstructure Composed of Barrier Nanostructured Organics and SiOx Layers Deposited on a Polymer Matrix. Nanomaterials 2018, 8, 679. https://doi.org/10.3390/nano8090679
Prikryl R, Otrisal P, Obsel V, Svorc L, Karkalic R, Buk J. Protective Properties of a Microstructure Composed of Barrier Nanostructured Organics and SiOx Layers Deposited on a Polymer Matrix. Nanomaterials. 2018; 8(9):679. https://doi.org/10.3390/nano8090679
Chicago/Turabian StylePrikryl, Radek, Pavel Otrisal, Vladimir Obsel, Lubomír Svorc, Radovan Karkalic, and Jan Buk. 2018. "Protective Properties of a Microstructure Composed of Barrier Nanostructured Organics and SiOx Layers Deposited on a Polymer Matrix" Nanomaterials 8, no. 9: 679. https://doi.org/10.3390/nano8090679




