Significant Enhancement of the Visible Light Photocatalytic Properties in 3D BiFeO3/Graphene Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of BFO and 3D BFO/Graphene Composites
2.2. Photocatalytic Experiments
2.3. Characterization
3. Results and Discussion
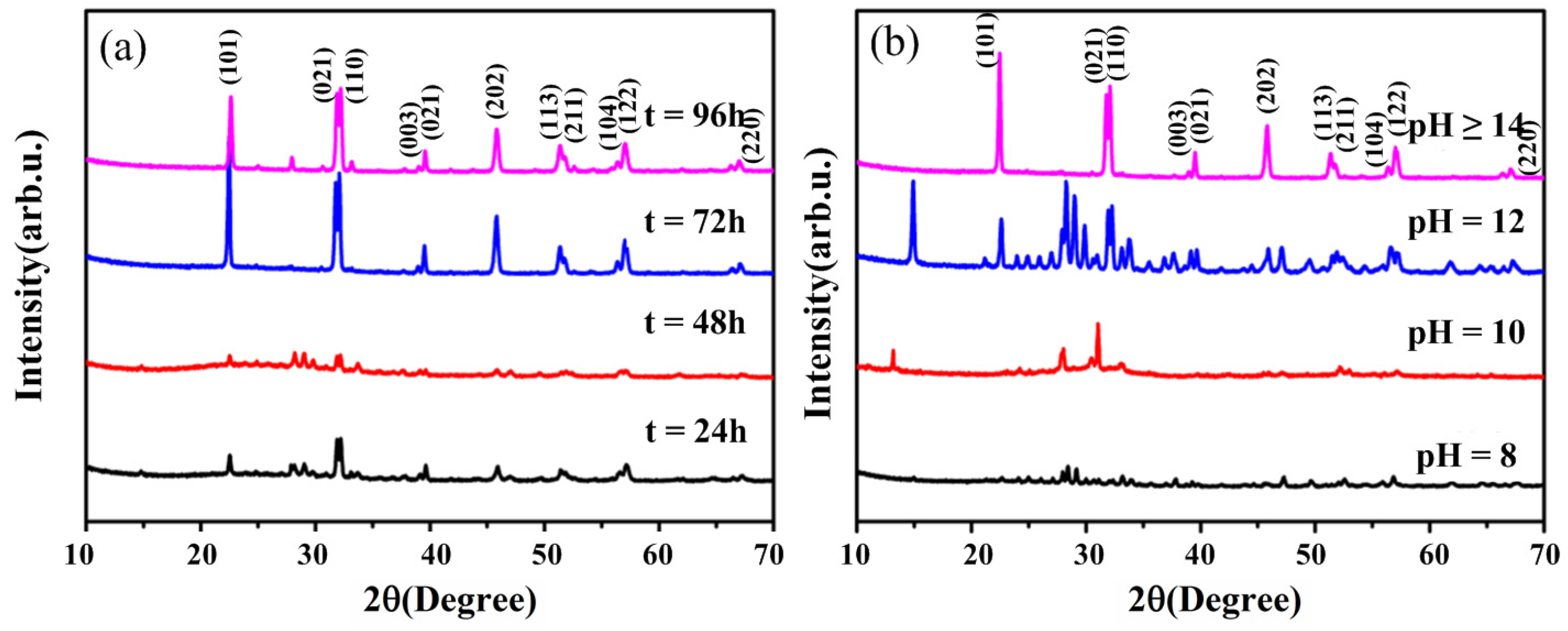
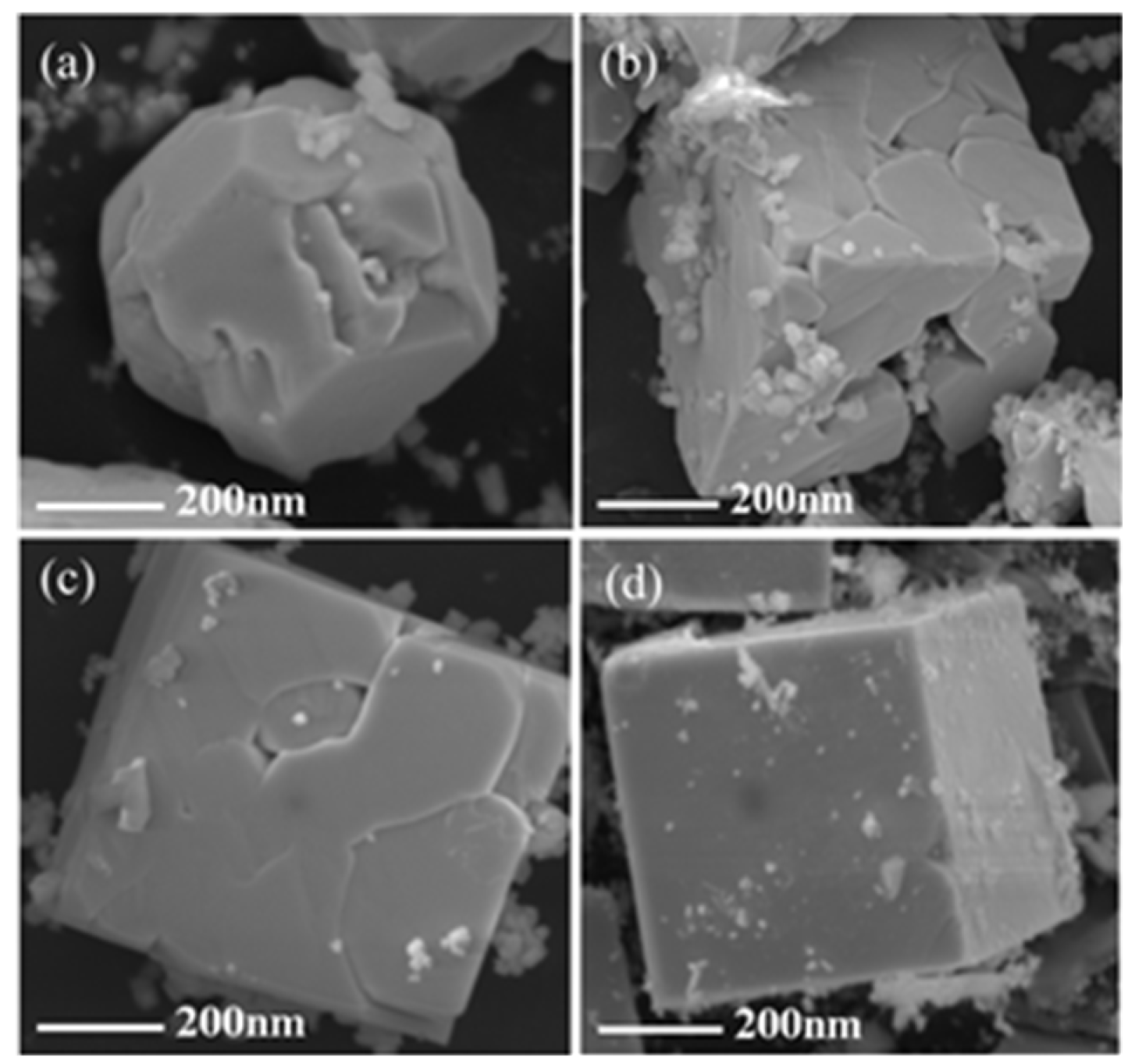
3.1. Effects of Hydrothermal Time and pH of Precursor on the Crystallization of BFO
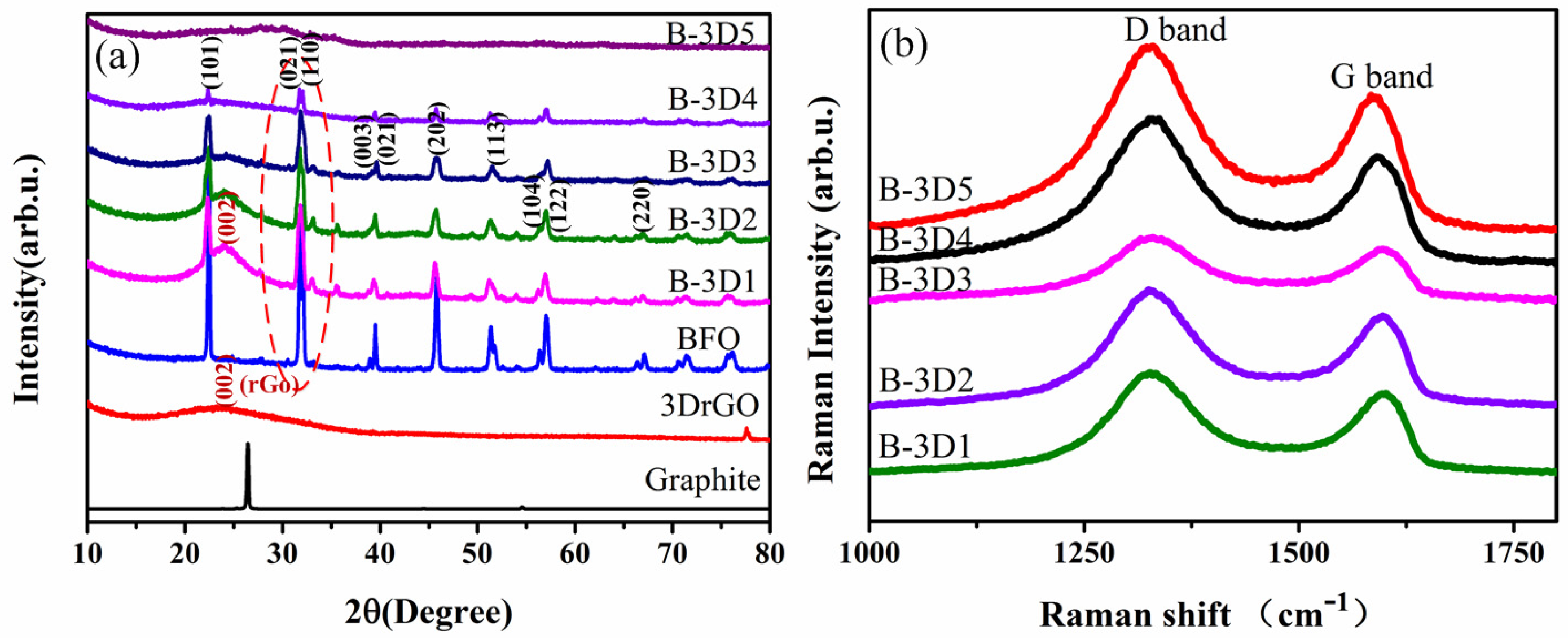
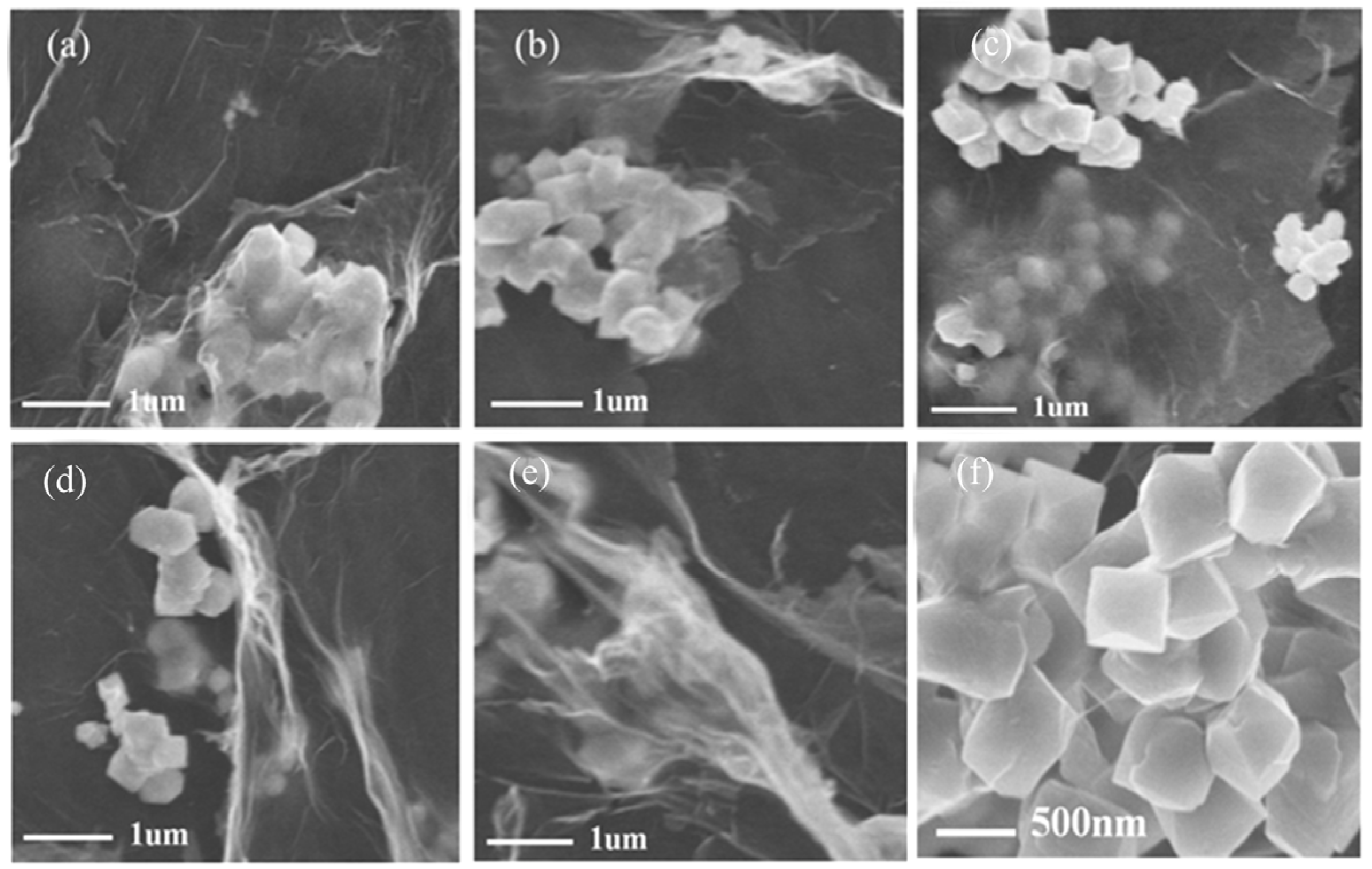
3.2. Effects of Graphene Oxide Concentration on the Crystallization of BFO
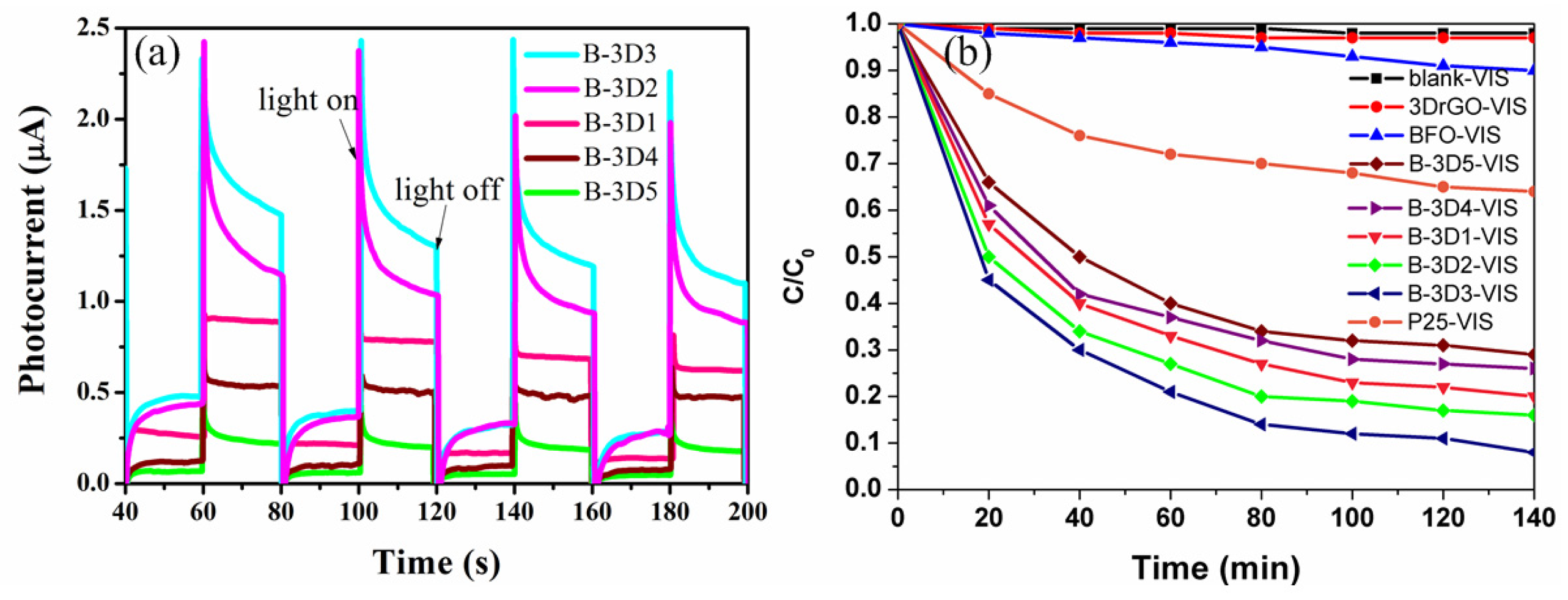
3.3. Photocatalytic Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mills, A.; Davies, R.H.; Worsley, D. Water-Purification by Semiconductor Photocatalysis. Chem. Soc. Rev. 1993, 22, 417–425. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.Y.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Bai, L.C.; Wang, X.; Tang, S.B.; Kang, Y.H.; Wang, J.H.; Yu, Y.; Zhou, Z.K.; Ma, C.; Zhang, X.; Jiang, J.; et al. Black Phosphorus/Platinum Heterostructure: A Highly Efficient Photocatalyst for Solar-Driven Chemical Reactions. Adv. Mater. 2018, 30. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Li, C. Roles of Phase Junction in Photocatalysis and Photoelectrocatalysis. J. Phys. Chem. C 2018, 122, 21083–21096. [Google Scholar] [CrossRef]
- Wang, W.Y.; Chen, J.; Li, C.; Tian, W.M. Achieving solar overall water splitting with hybrid photosystems of photosystem II and artificial photocatalysts. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Waiskopf, N.; Ben-Shahar, Y.; Banin, U. Photocatalytic Hybrid Semiconductor-Metal Nanoparticles; from Synergistic Properties to Emerging Applications. Adv. Mater. 2018, 30. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.P.; Ling, H.; Zhang, Z.; Li, Y.B.; Liang, L.H.; Chai, G.Z. Research progress on photocatalytic activity of ferroelectric materials. Acta Phys. Sin. 2017, 66. [Google Scholar] [CrossRef]
- Zhao, X.M.; Zhou, S.W.; Jiang, L.P.; Hou, W.H.; Shen, Q.M.; Zhu, J.J. Graphene-CdS Nanocomposites: Facile One-Step Synthesis and Enhanced Photoelectrochemical Cytosensing. Chem. Eur. J. 2012, 18, 4974–4981. [Google Scholar] [CrossRef]
- Shi, R.; Ye, H.F.; Liang, F.; Wang, Z.; Li, K.; Weng, Y.X.; Lin, Z.S.; Fu, W.F.; Che, C.M.; Chen, Y. Interstitial P-Doped CdS with Long-Lived Photogenerated Electrons for Photocatalytic Water Splitting without Sacrificial Agents. Adv. Mater. 2018, 30. [Google Scholar] [CrossRef]
- Chen, J.; Li, C.; Eda, G.; Zhang, Y.; Lei, W.; Chhowalla, M.; Milne, W.I.; Deng, W.Q. Incorporation of graphene in quantum dot sensitized solar cells based on ZnO nanorods. Chem. Commun. 2011, 47, 6084–6086. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Tang, Z.R.; Fu, X.Z.; Xu, Y.J. TiO2-Graphene Nanocomposites for Gas-Phase Photocatalytic Degradation of Volatile Aromatic Pollutant: Is TiO2-Graphene Truly Different from Other TiO2-Carbon Composite Materials? Acs Nano 2010, 4, 7303–7314. [Google Scholar] [CrossRef] [PubMed]
- Stengl, V.; Popelkova, D.; Vlacil, P. TiO2-Graphene Nanocomposite as High Performace Photocatalysts. J. Phys. Chem. C 2011, 115, 25209–25218. [Google Scholar] [CrossRef]
- Ma, C.; Dong, W.; Fang, L.; Zheng, F.G.; Shen, M.R.; Wang, Z.L. Synthesis of TiO2/Pt/TiO2 multilayer films via radio frequency magnetron sputtering and their enhanced photocatalytic activity. Thin Solid Films 2012, 520, 5727–5732. [Google Scholar] [CrossRef]
- Dong, B.B.; Liu, T.F.; Li, C.; Zhang, F.X. Species, engineering and characterizations of defects in TiO2-based photocatalyst. Chin. Chem. Lett. 2018, 29, 671–680. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Altomare, M.; Yoo, J.; Schmuki, P. Efficient Photocatalytic H-2 Evolution: Controlled Dewetting-Dealloying to Fabricate Site-Selective High-Activity Nanoporous Au Particles on Highly Ordered TiO2 Nanotube Arrays. Adv. Mater. 2015, 27, 3208–3215. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jin, Q.; Mao, D.; Qi, J.; Wei, Y.Z.; Yu, R.B.; Li, A.R.; Li, S.Z.; Zhao, H.J.; Ma, Y.W.; et al. Dually Ordered Porous TiO2-rGO Composites with Controllable Light Absorption Properties for Efficient Solar Energy Conversion. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef]
- Zhao, D.; Chen, C.C.; Yu, C.L.; Ma, W.H.; Zhao, J.C. Photoinduced Electron Storage in WO3/TiO2 Nanohybrid Material in the Presence of Oxygen and Postirradiated Reduction of Heavy Metal Ions. J. Phys. Chem. C 2009, 113, 13160–13165. [Google Scholar] [CrossRef]
- Qiu, Y.; Wu, H.P.; Wang, J.; Lou, J.; Zhang, Z.; Liu, A.P.; Chai, G.Z. The enhanced piezoelectricity in compositionally graded ferroelectric thin films under electric field: A role of flexoelectric effect. J. Appl. Phys. 2018, 123. [Google Scholar] [CrossRef]
- Qiu, Y.; Wu, H.P.; Wang, J.; Lou, J.; Zhang, Z.; Liu, A.P.; Kitamura, T.; Chai, G.Z. Giant electrocaloric effect in ferroelectric ultrathin films at room temperature mediated by flexoelectric effect and work function. J. Appl. Phys. 2017, 122. [Google Scholar] [CrossRef]
- Wu, H.P.; Ma, X.F.; Zhang, Z.; Zhu, J.; Wang, J.; Chai, G.Z. Dielectric tunability of vertically aligned ferroelectric-metal oxide nanocomposite films controlled by out-of-plane misfit strain. J. Appl. Phys. 2016, 119. [Google Scholar] [CrossRef]
- Zheng, L.M.; Jing, Y.J.; Lu, X.Y.; Li, S.Y.; Yang, L.Y.; Lu, W.M.; Cao, W.W. Temperature dependent piezoelectric anisotropy in tetragonal 0.63Pb(Mg1/3Nb2/3)-0.37PbTiO3 single crystal. Appl. Phys. Lett. 2018, 113. [Google Scholar] [CrossRef]
- Li, Z.; Yang, Z.C.; Wu, J.G.; Zhou, B.Z.; Bao, Q.W.; Zhang, K.L.; Zhao, J.S.; Wei, J. Low leakage current resistive memory based on Bi1.10 (Fe0.95Mn0.05) O3 films. Semicond. Sci. Technol. 2018, 33. [Google Scholar] [CrossRef]
- Guo, R.Q.; Fang, L.; Dong, W.; Zheng, F.G.; Shen, M.R. Enhanced Photocatalytic Activity and Ferromagnetism in Gd Doped BiFeO3 Nanoparticles. J. Phys. Chem. C 2010, 114, 21390–21396. [Google Scholar] [CrossRef]
- Gao, T.; Chen, Z.; Niu, F.; Zhou, D.T.; Huang, Q.L.; Zhu, Y.X.; Qin, L.S.; Sun, X.G.; Huang, Y.X. Shape-controlled preparation of bismuth ferrite by hydrothermal method and their visible-light degradation properties. J. Alloys Compd. 2015, 648, 564–570. [Google Scholar] [CrossRef]
- Xu, Q.Y.; Zheng, X.H.; Wen, Z.; Yang, Y.; Wu, D.; Xu, M.X. Enhanced room temperature ferromagnetism in porous BiFeO3 prepared using. Solid State Commun. 2011, 151, 624–627. [Google Scholar] [CrossRef]
- Xu, X.; Lin, Y.H.; Li, P.; Shu, L.; Nan, C.W. Synthesis and Photocatalytic Behaviors of High Surface Area BiFeO3 Thin Films. J. Am. Ceram. Soc. 2011, 94, 2296–2299. [Google Scholar] [CrossRef]
- Lam, S.M.; Jaffari, Z.H.; Sin, J.C.; Mohamed, A.R. Spindly BiFeO3 Nanoparticles for Photodegradation of Organic Pollutants Under a Compact Fluorescent Lamp. IOP Conf. Ser. Earth Environ. Sci. 2018, 151. [Google Scholar] [CrossRef]
- Liu, Y.C.; Lan, J.L.; Zhan, B.; Ding, J.X.; Liu, Y.; Lin, Y.H.; Zhang, B.P.; Nan, C.W. Thermoelectric Properties of Pb-Doped BiCuSeO Ceramics. J. Am. Ceram. Soc. 2013, 96, 2710–2713. [Google Scholar] [CrossRef]
- Li, S.; Lin, Y.H.; Zhang, B.P.; Wang, Y.; Nan, C.W. Controlled Fabrication of BiFeO3 Uniform Microcrystals and Their Magnetic and Photocatalytic Behaviors. J. Phys. Chem. C 2010, 114, 2903–2908. [Google Scholar] [CrossRef]
- Gao, F.; Chen, X.Y.; Yin, K.B.; Dong, S.; Ren, Z.F.; Yuan, F.; Yu, T.; Zou, Z.; Liu, J.M. Visible-light photocatalytic properties of weak magnetic BiFeO3 nanoparticles. Adv. Mater. 2007, 19, 2889–2892. [Google Scholar] [CrossRef]
- Soltani, T.; Lee, B.K. Sono-synthesis of nanocrystallized BiFeO3/reduced graphene oxide composites for visible photocatalytic degradation improvement of bisphenol A. Chem. Eng. J. 2016, 306, 204–213. [Google Scholar] [CrossRef]
- Kong, J.J.; Rui, Z.B.; Wang, X.Y.; Ji, H.B.; Tong, Y.X. Visible-light decomposition of gaseous toluene over BiFeO3-(BiNe)2O3 heterojunctions with enhanced performance. Chem. Eng. J. 2016, 302, 552–559. [Google Scholar] [CrossRef]
- Gao, F.; Yuan, Y.; Wang, K.F.; Chen, X.Y.; Chen, F.; Liu, J.M. Preparation and photoabsorption characterization of BiFeO3 nanowires. Appl. Phys. Lett. 2006, 89. [Google Scholar] [CrossRef]
- Hu, C.Z.; Vogler, H.; Aellen, M.; Shamsudhin, N.; Jang, B.; Burri, J.T.; Laubli, N.; Grossniklaus, U.; Pane, S.; Nelson, B.J. High precision, localized proton gradients and fluxes generated by a microelectrode device induce differential growth behaviors of pollen tubes. Lab Chip 2017, 17, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.F.; Mao, W.W.; Zhang, Q.X.; Wang, Q.; Zhu, Y.Y.; Zhang, J.; Yang, T.; Yang, J.P.; Li, X.A.; Huang, W. PVP assisted hydrothermal fabrication and morphology-controllable fabrication of BiFeO3 uniform nanostructures with enhanced photocatalytic activities. J. Alloys Compd. 2016, 677, 288–293. [Google Scholar] [CrossRef]
- Yilmaz, P.; Yeo, D.; Chang, H.; Loh, L.; Dunn, S. Perovskite BiFeO3 thin film photocathode performance with visible light activity. Nanotechnology 2016, 27. [Google Scholar] [CrossRef]
- Mukherjee, A.; Chakrabarty, S.; Kumari, N.; Su, W.N.; Basu, S. Visible-Light-Mediated Electrocatalytic Activity in Reduced Graphene Oxide-Supported Bismuth Ferrite. Acs Omega 2018, 3, 5946–5957. [Google Scholar] [CrossRef]
- Lu, H.B.; Huang, W.M. Synergistic effect of self-assembled carboxylic acid-functionalized carbon nanotubes and carbon fiber for improved electro-activated polymeric shape-memory nanocomposite. Appl. Phys. Lett. 2013, 102. [Google Scholar] [CrossRef]
- Lu, H.B.; Liang, F.; Yao, Y.T.; Gou, J.H.; Hui, D. Self-assembled multi-layered carbon nanofiber nanopaper for significantly improving electrical actuation of shape memory polymer nanocomposite. Compos. Part B Eng. 2014, 59, 191–195. [Google Scholar] [CrossRef]
- Zhang, C.; Dang, F.; Chen, Y.L.; Yan, Y.; Liu, Y.L.; Chen, X. Vibration-to-electric energy conversion with porous graphene oxide-nickel electrode. J. Power Sources 2017, 368, 73–77. [Google Scholar] [CrossRef]
- Qin, H.S.; Sun, Y.; Liu, J.Z.; Liu, Y.L. Mechanical properties of wrinkled graphene generated by topological defects. Carbon 2016, 108, 204–214. [Google Scholar] [CrossRef]
- Song, X.S.; Li, X.F.; Bai, Z.M.; Yan, B.; Xiong, D.B.; Lin, L.X.; Zhao, H.; Li, D.J.; Shao, Y.Y. Rationally-designed configuration of directly-coated Ni3S2/Ni electrode by RGO providing superior sodium storage. Carbon 2018, 133, 14–22. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Tang, Z.R.; Fu, X.; Xu, Y.J. Engineering the Unique 2D Mat of Graphene to Achieve Graphene-TiO2 Nanocomposite for Photocatalytic Selective Transformation: What Advantage does Graphene Have over Its Forebear Carbon Nanotube? Acs Nano 2011, 5, 7426–7435. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Yang, M.Q.; Tang, Z.R.; Xu, Y.J. Toward Improving the Graphene-Semiconductor Composite Photoactivity via the Addition of Metal Ions as Generic Interfacial Mediator. Acs Nano 2014, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Lin, R.; Jiang, L.Y.; Wan, J.W.; Chen, C. Fabrication and photocatalysis of ZnO nanotubes on transparent conductive graphene-based flexible substrates. Sci. China Mater. 2018, 61, 1007–1011. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.H.; Liu, A.P.; Xing, Y.; Duan, H.W.; Xu, W.Z.; Zhou, Q.; Wu, H.P.; Chen, C.; Chen, B.Y. Three-dimensional graphene biointerface with extremely high sensitivity to single cancer cell monitoring. Biosens. Bioelectron. 2018, 105, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Hong, C.C.; Lin, L.X.; Wu, H.P.; Su, Y.W.; Zhang, X.B.; Liu, A.P. Controllable nanoscale engineering of vertically aligned MoS2 ultrathin nanosheets by nitrogen doping of 3D graphene hydrogel for improved electrocatalytic hydrogen evolution. Carbon 2017, 116, 223–231. [Google Scholar] [CrossRef]
- Liu, A.P.; Zhao, L.; Zhang, J.M.; Lin, L.X.; Wu, H.P. Solvent-Assisted Oxygen Incorporation of Vertically Aligned MoS2 Ultrathin Nanosheets Decorated on Reduced Graphene Oxide for Improved Electrocatalytic Hydrogen Evolution. Acs Appl. Mater. Interfaces 2016, 8, 25210–25218. [Google Scholar] [CrossRef]
- Yuan, M.; Liu, A.P.; Zhao, M.; Dong, W.J.; Zhao, T.Y.; Wang, J.J.; Tang, W.H. Bimetallic PdCu nanoparticle decorated three-dimensional graphene hydrogel for non-enzymatic amperometric glucose sensor. Sens. Actuators B Chem. 2014, 190, 707–714. [Google Scholar] [CrossRef]
- Zheng, X.L.; Xiong, X.; Yang, J.W.; Chen, D.L.; Jian, R.K.; Lin, L.X. A strong and compressible three dimensional graphene/polyurushiol composite for efficient water cleanup. Chem. Eng. J. 2018, 333, 153–161. [Google Scholar] [CrossRef]
- Qiu, B.C.; Xing, M.Y.; Zhang, J.L. Recent advances in three-dimensional graphene based materials for catalysis applications. Chem. Soc. Rev. 2018, 47, 2165–2216. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.T.; Liang, Y.N.; Zhao, J.; Zhang, Y.D.; Yang, E.H.; Chen, J.M.; Lim, T.T. Ultra-effective integrated technologies for water disinfection with a novel OD-2D-3D nanostructured rGO-AgNP/Bi2Fe4O9 composite. Appl. Catal. B Environ. 2018, 227, 548–556. [Google Scholar] [CrossRef]
- Zhang, J.J.; Fang, S.S.; Mei, J.Y.; Zheng, G.P.; Zheng, X.C.; Guan, X.X. High-efficiency removal of rhodamine B dye in water using g-C3N4 and TiO2 co-hybridized 3D graphene aerogel composites. Sep. Purif. Technol. 2018, 194, 96–103. [Google Scholar] [CrossRef]
- Li, J.Q.; Xu, J.; Xie, Z.Q.; Gao, X.; Zhou, J.Y.; Xiong, Y.; Chen, C.G.; Zhang, J.; Liu, Z.F. Diatomite-Templated Synthesis of Freestanding 3D Graphdiyne for Energy Storage and Catalysis Application. Adv. Mater. 2018, 30. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.H.; Li, S.S.; Chen, Q.T.; Zheng, X.J.; Liu, P.R.; Fang, S.M. 3D graphene aerogels-supported Ag and Ag@Ag3PO4 heterostructure for the efficient adsorption-photocatalysis capture of different dye pollutants in water. Mater. Res. Bull. 2018, 105, 334–341. [Google Scholar] [CrossRef]
- Si, Y.H.; Xia, Y.; Shang, S.K.; Xiong, X.B.; Zeng, X.R.; Zhou, J.; Li, Y.Y. Enhanced Visible Light Driven Photocatalytic Behavior of BiFeO3/Reduced Graphene Oxide Composites. Nanomaterials 2018, 8, 526. [Google Scholar] [CrossRef]
- Xu, Y.; Gao, Y.F.; Xing, H.J.; Zhang, J.J. Room temperature spontaneous exchange bias in BiFeO3 micro/nano powders synthesized by hydrothermal method. Ceram. Int. 2018, 44, 17459–17463. [Google Scholar] [CrossRef]
- Chen, X.Y.; Yu, T.; Gao, F.; Zhang, H.T.; Liu, L.F.; Wang, Y.M.; Li, Z.S.; Zou, Z.G.; Liu, J.M. Application of weak ferromagnetic BiFeO3 films as the photoelectrode material under visible-light irradiation. Appl. Phys. Lett. 2007, 91. [Google Scholar] [CrossRef]
- Wu, G.S.; Shen, Q.P.; Yu, H.L.; Zhao, T.Y.; Lu, C.D.; Liu, A.P. Reduced graphene oxide encapsulated Cu2O with controlled crystallographic facets for enhanced visible-light photocatalytic degradation. Funct. Mater. Lett. 2017, 10. [Google Scholar] [CrossRef]
- Li, Y.; Cui, W.Q.; Liu, L.; Zong, R.L.; Yao, W.Q.; Liang, Y.H.; Zhu, Y.F. Removal of Cr(VI) by 3D TiO2-graphene hydrogel via adsorption enriched with photocatalytic reduction. Appl. Catal. B Environ. 2016, 199, 412–423. [Google Scholar] [CrossRef]
- Hu, Z.T.; Liu, J.C.; Yan, X.L.; Oh, W.D.; Lim, T.T. Low-temperature synthesis of graphene/Bi2Fe4O9 composite for synergistic adsorption-photocatalytic degradation of hydrophobic pollutant under solar irradiation. Chem. Eng. J. 2015, 262, 1022–1032. [Google Scholar] [CrossRef]
- Maleki, H. Photocatalytic activity and magnetic enhancements by addition of lanthanum into the BiFeO3 structure and the effect of synthesis method. J. Mater. Sci. Mater. Electron. 2018, 29, 11862–11869. [Google Scholar] [CrossRef]
- Chen, F.Y.; An, W.J.; Liu, L.; Liang, Y.H.; Cui, W.Q. Highly efficient removal of bisphenol A by a three-dimensional graphene hydrogel-AgBr@rGO exhibiting adsorption/photocatalysis synergy. Appl. Catal. B Environ. 2017, 217, 65–80. [Google Scholar] [CrossRef]
- Sha, J.W.; Li, Y.L.; Salvatierra, R.V.; Wang, T.; Dong, P.; Ji, Y.S.; Lee, S.K.; Zhang, C.H.; Zhang, J.B.; Smith, R.H.; et al. Three-Dimensional Printed Graphene Foams. Acs Nano 2017, 11, 6860–6867. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.B.; Chen, Z.W.; Zhong, C.F.; Lu, Z.Y. Facile synthesis of BiFeO3 nanosheets with enhanced visible-light photocatalytic activity. J. Mater. Sci. Mater. Electron. 2018, 29, 4817–4829. [Google Scholar] [CrossRef]
- Hao, C.X.; Wen, F.S.; Xiang, J.Y.; Hou, H.; Lv, W.M.; Lv, Y.F.; Hu, W.T.; Liu, Z.Y. Photocatalytic performances of BiFeO3 particles with the average size in nanometer, submicrometer, and micrometer. Mater. Res. Bull. 2014, 50, 369–373. [Google Scholar] [CrossRef]
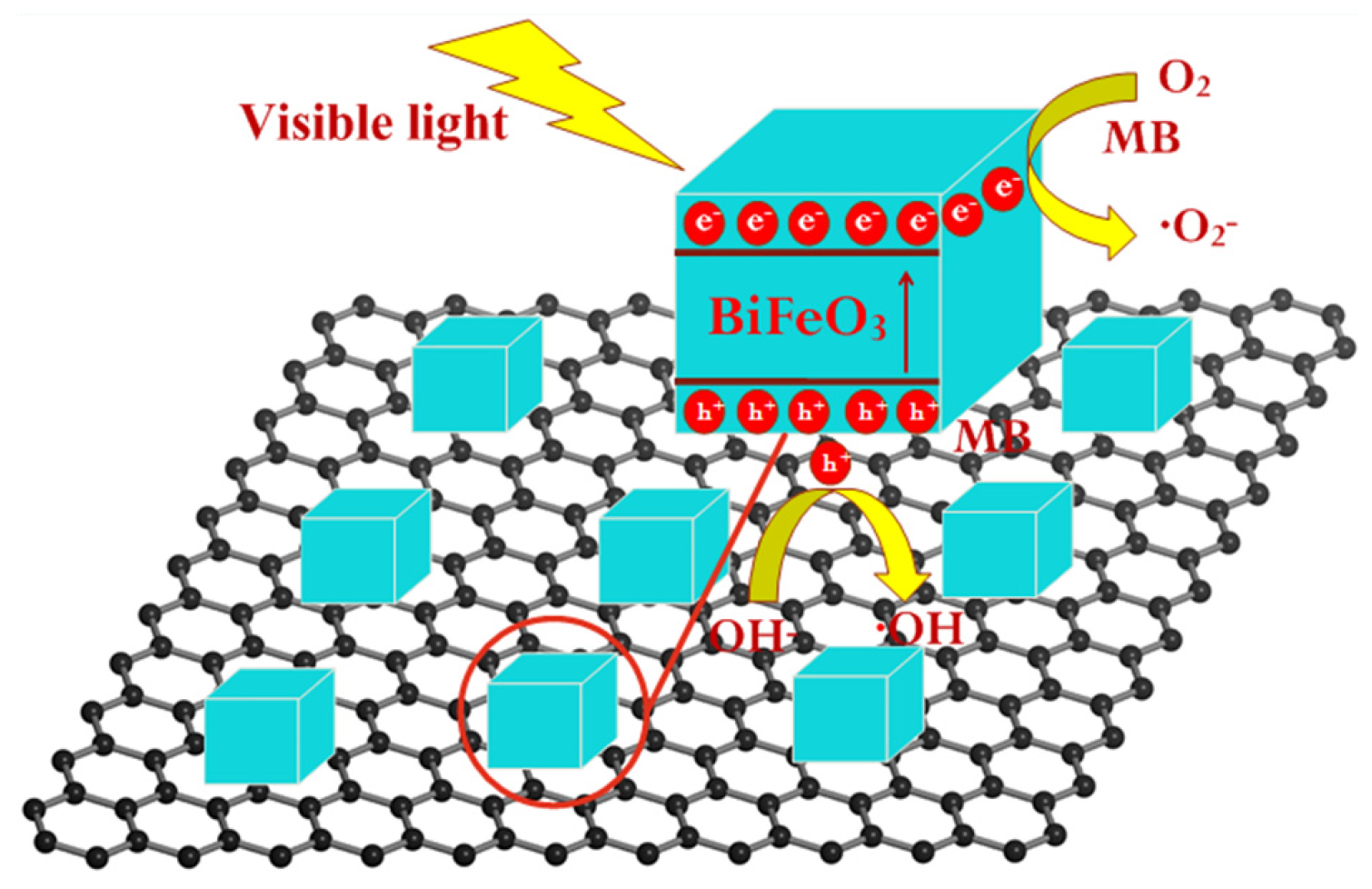


| Sample | BFO | B-3D1 | B-3D2 | B-3D3 | B-3D4 | B-3D5 |
|---|---|---|---|---|---|---|
| Eg (eV) | 2.90 | 2.60 | 2.56 | 2.40 | 2.68 | 2.75 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Wang, Y.; Ling, H.; Qiu, Y.; Lou, J.; Hou, X.; Bag, S.P.; Wang, J.; Wu, H.; Chai, G. Significant Enhancement of the Visible Light Photocatalytic Properties in 3D BiFeO3/Graphene Composites. Nanomaterials 2019, 9, 65. https://doi.org/10.3390/nano9010065
Li J, Wang Y, Ling H, Qiu Y, Lou J, Hou X, Bag SP, Wang J, Wu H, Chai G. Significant Enhancement of the Visible Light Photocatalytic Properties in 3D BiFeO3/Graphene Composites. Nanomaterials. 2019; 9(1):65. https://doi.org/10.3390/nano9010065
Chicago/Turabian StyleLi, Jiquan, Youyan Wang, Huan Ling, Ye Qiu, Jia Lou, Xu Hou, Sankar Parsad Bag, Jie Wang, Huaping Wu, and Guozhong Chai. 2019. "Significant Enhancement of the Visible Light Photocatalytic Properties in 3D BiFeO3/Graphene Composites" Nanomaterials 9, no. 1: 65. https://doi.org/10.3390/nano9010065





