Carboxylic Acid Functionalization at the Meso-Position of the Bodipy Core and Its Influence on Photovoltaic Performance
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of Bodipy Dye 1 and 2
2.2.1. Bodipy Dye 1
2.2.2. Bodipy Dye 2
2.3. Preparation of Photoelectrochemical Cells (PECs)
2.4. Dye Sensitization
3. Characterisation
4. Results and Discussion
4.1. Synthesis
4.2. Optical Properties
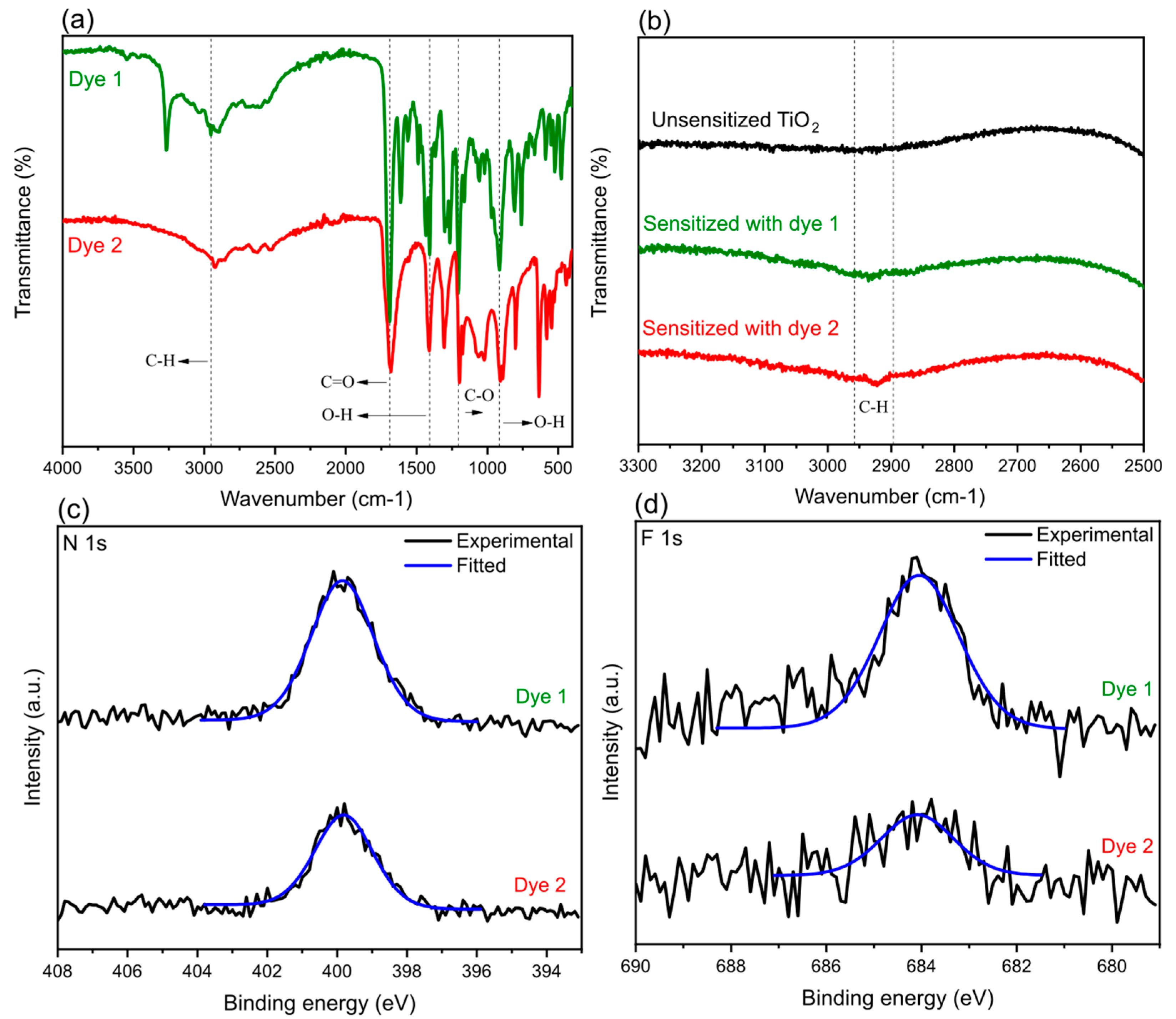
4.3. Spectroscopy Characterization
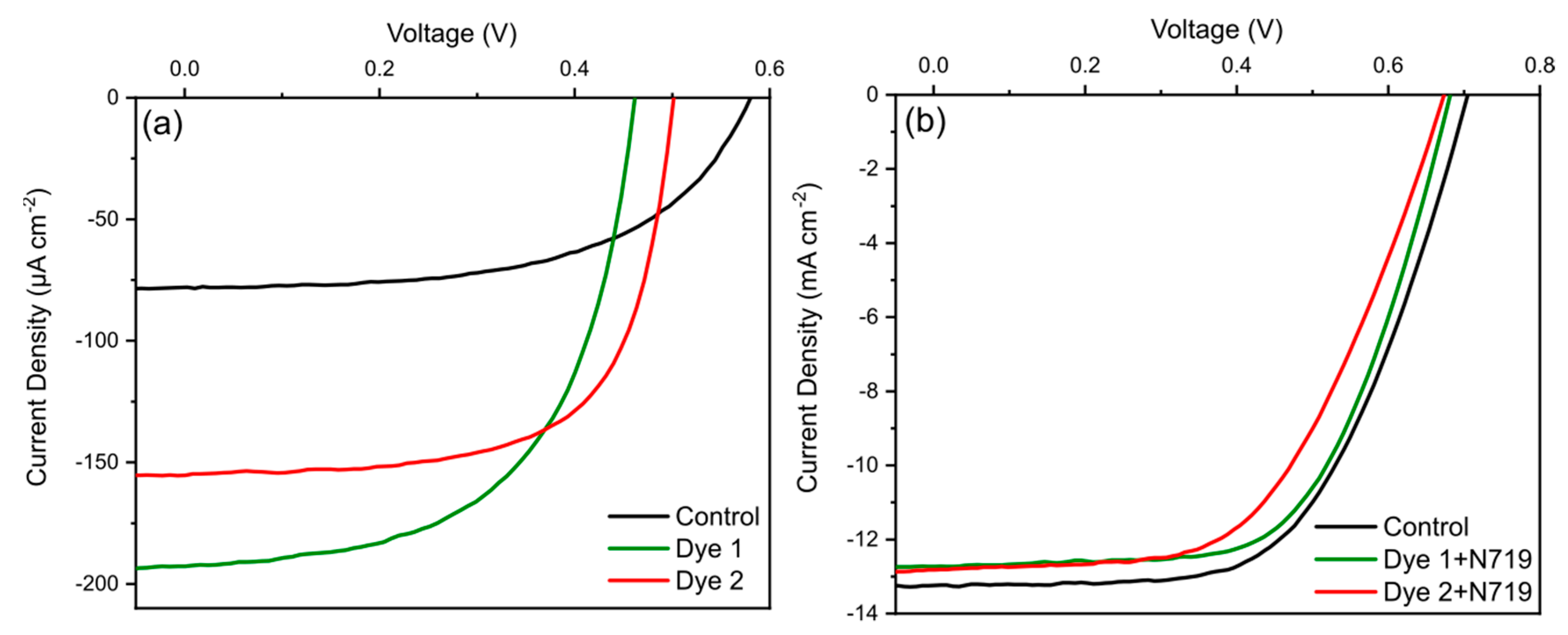
4.4. Photoelectrochemical Properties
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nayak, P.K.; Mahesh, S.; Snaith, H.J.; Cahen, D. Photovoltaic solar cell technologies: Analysing the state of the art. Nat. Rev. Mater. 2019, 4, 269. [Google Scholar] [CrossRef]
- Khatri, I.; Shudo, K.; Matsuura, J.; Sugiyama, M.; Nakada, T. Impact of heat-light soaking on potassium fluoride treated CIGS solar cells with CdS buffer layer. Prog. Photovolt. Res. Appl. 2018, 26, 171–178. [Google Scholar] [CrossRef]
- Munkhbayar, B.; Nine Md, J.; Jeoun, J.; Ji, M.; Jeong, H.; Chung, H. Synthesis of a graphene–tungsten composite with improved dispersibility of graphene in an ethanol solution and its use as a counter electrode for dye-sensitised solar cells. J. Power Sources 2013, 230, 207–217. [Google Scholar] [CrossRef]
- Batmunkh, M.; Shrestha, A.; Bat-Erdene, M.; Nine, M.J.; Shearer, C.J.; Gibson, C.T.; Slattery, A.D.; Tawfik, S.A.; Ford, M.J.; Dai, S.; et al. Electrocatalytic activity of a 2D phosphorene-based heteroelectrocatalyst for photoelectrochemical cells. Angew. Chem. Int. Ed. 2018, 57, 2644–2647. [Google Scholar] [CrossRef] [PubMed]
- Stroyuk, O.; Raevskaya, A.; Gaponik, N. Solar light harvesting with multinary metal chalcogenide nanocrystals. Chem. Soc. Rev. 2018, 47, 5354–5422. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.; Batmunkh, M.; Tricoli, A.; Qiao, S.Z.; Dai, S. Near-infrared active lead chalcogenide quantum dots: Preparation, post-synthesis ligand exchange, and applications in solar cells. Angew. Chem. Int. Ed. 2019, 58, 5202–5224. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Xiong, Y.; Chen, Z.; Zhang, Q.; Fei, Z.; Henry, R.; Heeney, M.; O’Connor, B.T.; You, W.; Ade, H. Sequential deposition of organic films with eco-compatible solvents improves performance and enables over 12%-efficiency nonfullerene solar cells. Adv. Mater. 2019, 31, 1808153. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tan, H.S.; Guo, X.; Facchetti, A.; Yan, H. Material insights and challenges for non-fullerene organic solar cells based on small molecular acceptors. Nat. Energy 2018, 3, 720–731. [Google Scholar] [CrossRef]
- Ye, L.; Hu, H.; Ghasemi, M.; Wang, T.; Collins, B.A.; Kim, J.-H.; Jiang, K.; Carpenter, J.H.; Li, H.; Li, Z.; et al. Quantitative relations between interaction parameter, miscibility and function in organic solar cells. Nat. Mater. 2018, 17, 253–260. [Google Scholar] [CrossRef]
- Jeon, N.J.; Na, H.; Jung, E.H.; Yang, T.-Y.; Lee, Y.G.; Kim, G.; Shin, H.-W.; Seok, S.I.; Lee, J.; Seo, J. A fluorene-terminated hole-transporting material for highly efficient and stable perovskite solar cells. Nat. Energy 2018, 3, 682. [Google Scholar] [CrossRef]
- Macdonald, T.J.; Batmunkh, M.; Lin, C.-T.; Kim, J.; Tune, D.D.; Ambroz, F.; Li, X.; Xu, S.; Sol, C.; Papakonstantinou, I.; et al. Origin of performance enhancement in TiO2-carbon nanotube composite perovskite solar cells. Small Methods 2019, 1900164. [Google Scholar] [CrossRef]
- Rong, Y.; Hu, Y.; Mei, A.; Tan, H.; Saidaminov, M.I.; Seok, S.I.; McGehee, M.D.; Sargent, E.H.; Han, H. Challenges for commercializing perovskite solar cells. Science 2018, 361, eaat8235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, Y.; Sun, D.; Cao, Y.; Tsao, H.N.; Yuan, Y.; Zakeeruddin, S.M.; Wang, P.; Grätzel, M. A stable blue photosensitizer for color palette of dye-sensitized solar cells reaching 12.6% efficiency. J. Am. Chem. Soc. 2018, 140, 2405–2408. [Google Scholar] [CrossRef] [PubMed]
- Ambroz, F.; Sathasivam, S.; Lee, R.; Gadipelli, S.; Lin, C.-T.; Xu, S.; Poduval, R.K.; Mclachlan, M.A.; Papakonstantinou, I.; Parkin, I.P.; et al. Influence of lithium and lanthanum treatment on TiO2 nanofibers and their application in n-i-p solar cells. ChemElectroChem 2019, 6, 3590–3598. [Google Scholar] [CrossRef]
- Macdonald, T.J.; Ambroz, F.; Batmunkh, M.; Li, Y.; Kim, D.; Contini, C.; Poduval, R.; Liu, H.; Shapter, J.G.; Papakonstantinou, I.; et al. TiO2 nanofiber photoelectrochemical cells loaded with sub-12 nm AuNPs: Size dependent performance evaluation. Mater. Today Energy 2018, 9, 254–263. [Google Scholar] [CrossRef]
- Nazeeruddin, M.K.; Klein, C.; Liska, P.; Grätzel, M. Synthesis of novel ruthenium sensitizers and their application in dye-sensitized solar cells. Coord. Chem. Rev. 2005, 249, 1460–1467. [Google Scholar] [CrossRef]
- Nazeeruddin, M.K.; Kay, A.; Rodicio, I.; Humphry-Baker, R.; Mueller, E.; Liska, P.; Vlachopoulos, N.; Graetzel, M. Conversion of light to electricity by cis-X2bis(2,2′-bipyridyl-4,4′-dicarboxylate)ruthenium(II) charge-transfer sensitizers (X = Cl-, Br-, I-, CN-, and SCN-) on nanocrystalline titanium dioxide electrodes. J. Am. Chem. Soc. 1993, 115, 6382–6390. [Google Scholar] [CrossRef]
- Kolemen, S.; Bozdemir, O.A.; Cakmak, Y.; Barin, G.; Erten-Ela, S.; Marszalek, M.; Yum, J.-H.; Zakeeruddin, S.M.; Nazeeruddin, M.K.; Grätzel, M.; et al. Optimization of distyryl-Bodipy chromophores for efficient panchromatic sensitization in dye sensitized solar cells. Chem. Sci. 2011, 2, 949–954. [Google Scholar] [CrossRef] [Green Version]
- Ji, J.-M.; Zhou, H.; Kim, H.K. Rational design criteria for D–π–A structured organic and porphyrin sensitizers for highly efficient dye-sensitized solar cells. J. Mater. Chem. A 2018, 6, 14518–14545. [Google Scholar] [CrossRef]
- Song, H.; Liu, Q.; Xie, Y. Porphyrin-sensitized solar cells: systematic molecular optimization, coadsorption and cosensitization. Chem. Commun. 2018, 54, 1811–1824. [Google Scholar] [CrossRef]
- Kuang, D.; Walter, P.; Nüesch, F.; Kim, S.; Ko, J.; Comte, P.; Zakeeruddin, S.M.; Nazeeruddin, M.K.; Grätzel, M. Co-sensitization of organic dyes for efficient ionic liquid electrolyte-based dye-sensitized solar cells. Langmuir 2007, 23, 10906–10909. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, L.; Wu, J.; Wang, G.; Huang, W.; Melkonyan, F.S.; Lu, Z.; Huang, Y.; Marks, T.J.; Facchetti, A. Performance, morphology, and charge recombination correlations in ternary squaraine solar cells. Chem. Mater. 2018, 30, 6810–6820. [Google Scholar] [CrossRef]
- Erten-Ela, S.; Ueno, Y.; Asaba, T.; Kubo, Y. Synthesis of a dibenzo-Bodipy-incorporating phenothiazine dye as a panchromatic sensitizer for dye-sensitized solar cells. New J. Chem. 2017, 41, 10367–10375. [Google Scholar] [CrossRef]
- Singh, S.P.; Gayathri, T. Evolution of BODIPY dyes as potential sensitizers for dye-sensitized solar cells. Eur. J. Org. Chem. 2014, 2014, 4689–4707. [Google Scholar] [CrossRef]
- He, H.; Ji, S.; He, Y.; Zhu, A.; Zou, Y.; Deng, Y.; Ke, H.; Yang, H.; Zhao, Y.; Guo, Z.; et al. Photoconversion-tunable fluorophore vesicles for wavelength-dependent photoinduced cancer therapy. Adv. Mater. 2017, 29, 1606690. [Google Scholar] [CrossRef]
- Turksoy, A.; Yildiz, D.; Akkaya, E.U. Photosensitization and controlled photosensitization with bodipy dyes. Coord. Chem. Rev. 2019, 379, 47–64. [Google Scholar] [CrossRef]
- Lee, C.Y.; Hupp, J.T. Dye sensitized solar cells: TiO2 sensitization with a bodipy-porphyrin antenna system. Langmuir 2010, 26, 3760–3765. [Google Scholar] [CrossRef]
- Urbani, M.; Grätzel, M.; Nazeeruddin, M.K.; Torres, T. Meso-substituted porphyrins for dye-sensitized solar cells. Chem. Rev. 2014, 114, 12330–12396. [Google Scholar] [CrossRef]
- Klfout, H.; Stewart, A.; Elkhalifa, M.; He, H. BODIPYs for dye-sensitized solar cells. ACS Appl. Mater. Interfaces 2017, 9, 39873–39889. [Google Scholar] [CrossRef]
- Islam, A.; Chowdhury, T.H.; Qin, C.; Han, L.; Lee, J.-J.; Bedja, I.M.; Akhtaruzzaman, M.; Sopian, K.; Mirloup, A.; Leclerc, N. Panchromatic absorption of dye sensitized solar cells by co-Sensitization of triple organic dyes. Sustain. Energy Fuels 2017, 2, 209–214. [Google Scholar] [CrossRef]
- Cheema, H.; Younts, R.; Gautam, B.; Gundogdu, K.; El-Shafei, A. Design and synthesis of BODIPY sensitizers with long alkyl chains tethered to N-carbazole and their application for dye sensitized solar cells. Mater. Chem. Phys. 2016, 184, 57–63. [Google Scholar] [CrossRef]
- Qin, C.; Mirloup, A.; Leclerc, N.; Islam, A.; El-Shafei, A.; Han, L.; Ziessel, R. Molecular engineering of new thienyl-bodipy dyes for highly efficient panchromatic sensitized solar cells. Adv. Energy Mater. 2014, 4, 1400085. [Google Scholar] [CrossRef]
- Li, Z.; Mintzer, E.; Bittman, R. First synthesis of free cholesterol−BODIPY conjugates. J. Org. Chem. 2006, 71, 1718–1721. [Google Scholar] [CrossRef] [PubMed]
- Pakhomov, A.A.; Kononevich, Y.N.; Stukalova, M.V.; Svidchenko, E.A.; Surin, N.M.; Cherkaev, G.V.; Shchegolikhina, O.I.; Martynov, V.I.; Muzafarov, A.M. Synthesis and photophysical properties of a new BODIPY-based siloxane dye. Tetrahedron Lett. 2016, 57, 979–982. [Google Scholar] [CrossRef]
- Wanwong, S.; Sangkhun, W.; Wootthikanokkhan, J. The effect of co-sensitization methods between N719 and boron dipyrromethene triads on dye-sensitized solar cell performance. RSC Adv. 2018, 8, 9202–9210. [Google Scholar] [CrossRef] [Green Version]
- Krauss, T.N.; Barrena, E.; Zhang, X.N.; de Oteyza, D.G.; Major, J.; Dehm, V.; Würthner, F.; Cavalcanti, L.P.; Dosch, H. Three-dimensional molecular packing of thin organic films of PTCDI-C8 determined by surface X-Ray diffraction. Langmuir 2008, 24, 12742–12744. [Google Scholar] [CrossRef] [PubMed]
- Dharma, J.; Pisal, A. Simple Method of Measuring the Band Gap Energy Value of TiO2 in the Powder Form Using a UV/Vis/NIR Spectrometer; Perkin Elmer: Shelton, CT, USA, 2012. [Google Scholar]
- Jiang, X.; Li, S.; Xiang, G.; Li, Q.; Fan, L.; He, L.; Gu, K. Determination of the acid values of edible oils via FTIR spectroscopy based on the OH stretching band. Food Chem. 2016, 212, 585–589. [Google Scholar] [CrossRef]
- Mao, M.; Song, Q.-H. The structure-property relationships of D-π-A BODIPY dyes for dye-sensitized solar cells. Chem. Rec. 2016, 16, 719–733. [Google Scholar] [CrossRef]
- Mishra, A.; Fischer, M.K.R.; Bäuerle, P. Metal-free organic dyes for dye-sensitized solar cells: From structure: Property relationships to design rules. Angew. Chem. Int. Ed. 2009, 48, 2474–2499. [Google Scholar] [CrossRef]
- Fan, K.; Yu, J.; Ho, W. Improving photoanodes to obtain highly efficient dye-sensitized solar cells: A brief review. Mater. Horiz. 2017, 4, 319–344. [Google Scholar] [CrossRef]

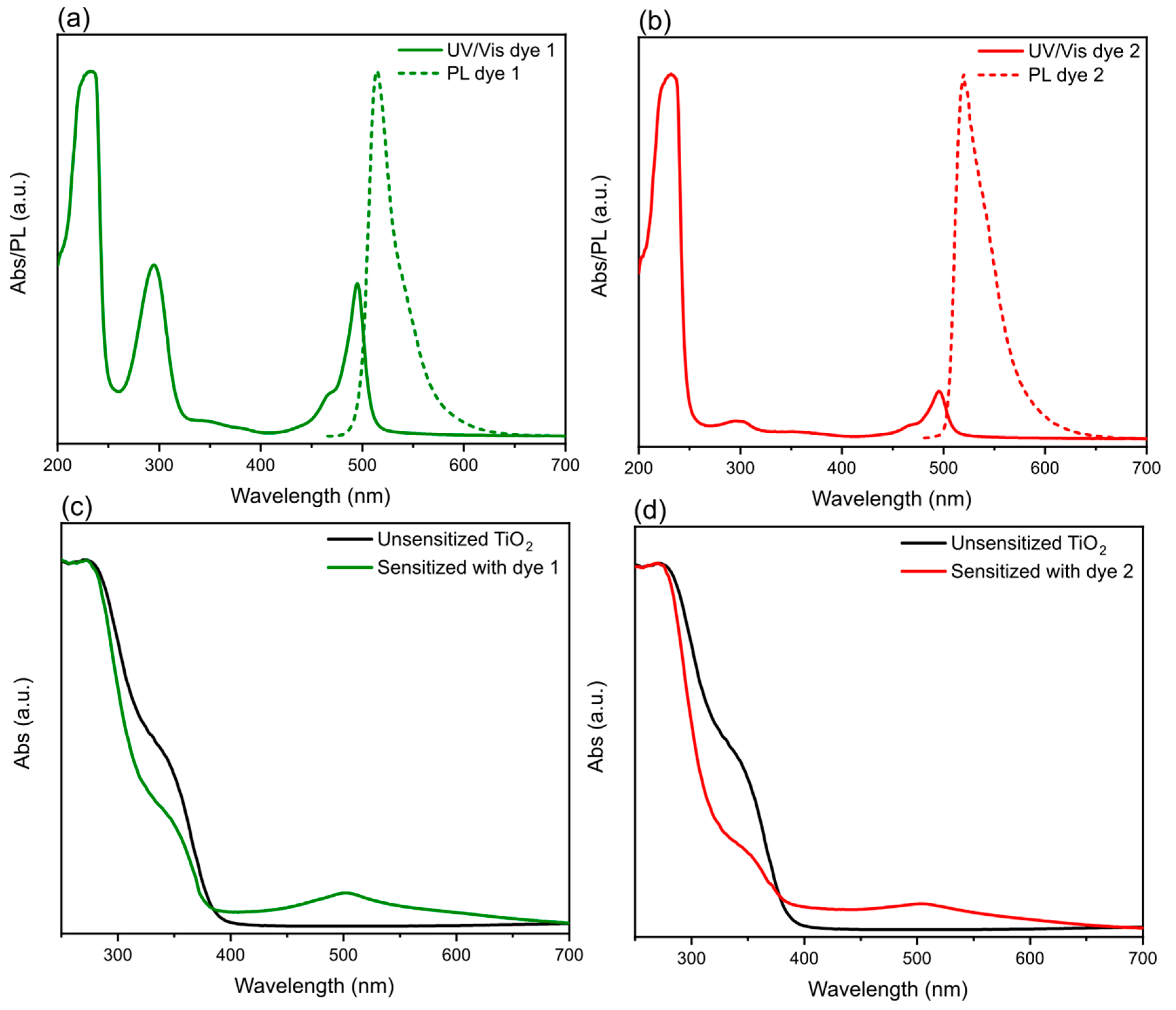
| Sensitizer | Solution Abs λmax (nm) | Solution PL λmax (nm) | Photoelectrode Abs λmax (nm) | Eg (eV) | ε (M−1 cm−1) |
|---|---|---|---|---|---|
| Dye 1 | 232, 295, 495 | 514 | 502 | 2.39 | 6124 (at 495 nm) |
| Dye 2 | 232, 295, 496 | 520 | 504 | 2.38 | 1350 (at 496 nm) |
| PEC | JSC (µA cm−2) | Voc (mV) | FF (%) | PCE (%) |
|---|---|---|---|---|
| Control (unsensitized) | 78.2 | 579.6 | 56.3 | 0.025 |
| Dye 1 | 192.6 | 461.3 | 58.0 | 0.051 |
| Dye 2 | 154.8 | 501.0 | 66.4 | 0.051 |
| Control (N719 sensitized) | 13.2 | 704.7 | 59.2 | 5.5 |
| Dye 1 + N719 | 12.7 | 681.3 | 61.6 | 5.3 |
| Dye 2 + N719 | 12.8 | 673.1 | 55.5 | 4.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ambroz, F.; Donnelly, J.L.; Wilden, J.D.; Macdonald, T.J.; Parkin, I.P. Carboxylic Acid Functionalization at the Meso-Position of the Bodipy Core and Its Influence on Photovoltaic Performance. Nanomaterials 2019, 9, 1346. https://doi.org/10.3390/nano9101346
Ambroz F, Donnelly JL, Wilden JD, Macdonald TJ, Parkin IP. Carboxylic Acid Functionalization at the Meso-Position of the Bodipy Core and Its Influence on Photovoltaic Performance. Nanomaterials. 2019; 9(10):1346. https://doi.org/10.3390/nano9101346
Chicago/Turabian StyleAmbroz, Filip, Joanna L. Donnelly, Jonathan D. Wilden, Thomas J. Macdonald, and Ivan P. Parkin. 2019. "Carboxylic Acid Functionalization at the Meso-Position of the Bodipy Core and Its Influence on Photovoltaic Performance" Nanomaterials 9, no. 10: 1346. https://doi.org/10.3390/nano9101346





