Solid-State Reactions for the Storage of Thermal Energy
Abstract
:1. Introduction
- Reactants and reaction products in the solid state can make it possible to conceive a TES system with the characteristic of simplicity the sensible storage system (only one reactor).
- Possible direct contact of the reacting material with the heat transfer fluid (allowing minimizing the use of expensive heat exchanger).
- Potential high energy density materials, allowing compact and low-cost systems.
2. Materials and Methods
2.1. Materials Selection
2.2. Nanocrystalline Materials Production
- (1)
- Two-step synthesis: ball milling of separated Mn and Ni using 2 balls of 8 mm diameter. A milling time of 4 h was applied to the pure materials with a ball-to-powder mass ratio (BPR) of 1.6. To obtain a good intermixing between Mn and Ni, the materials were placed in the ball milling reactor in the right molar ratio (Mn75-Ni25 and Mn52-Ni48) and subjected to mechanical treatment under mild conditions (for 15 min using 3 balls of 3 mm diameter).
- (2)
- One-step synthesis: ball milling of Mn and Ni directly in the right molar ratio (75/25, 52/48) with 2 balls of 8 mm diameter. A milling time of 4 h was applied with a BPR of 1.6.
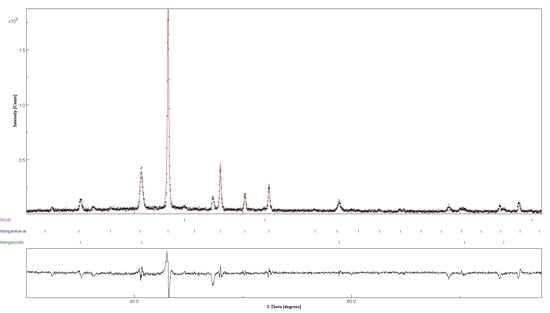
2.3. Structural Analysis
2.4. Reactivity and Thermodynamic Characterization
3. Results and Discussion
3.1. Materials Selection Results
3.2. Synthesis of Mn75-Ni25 and Mn52-Ni48 Nanocrystalline Materials
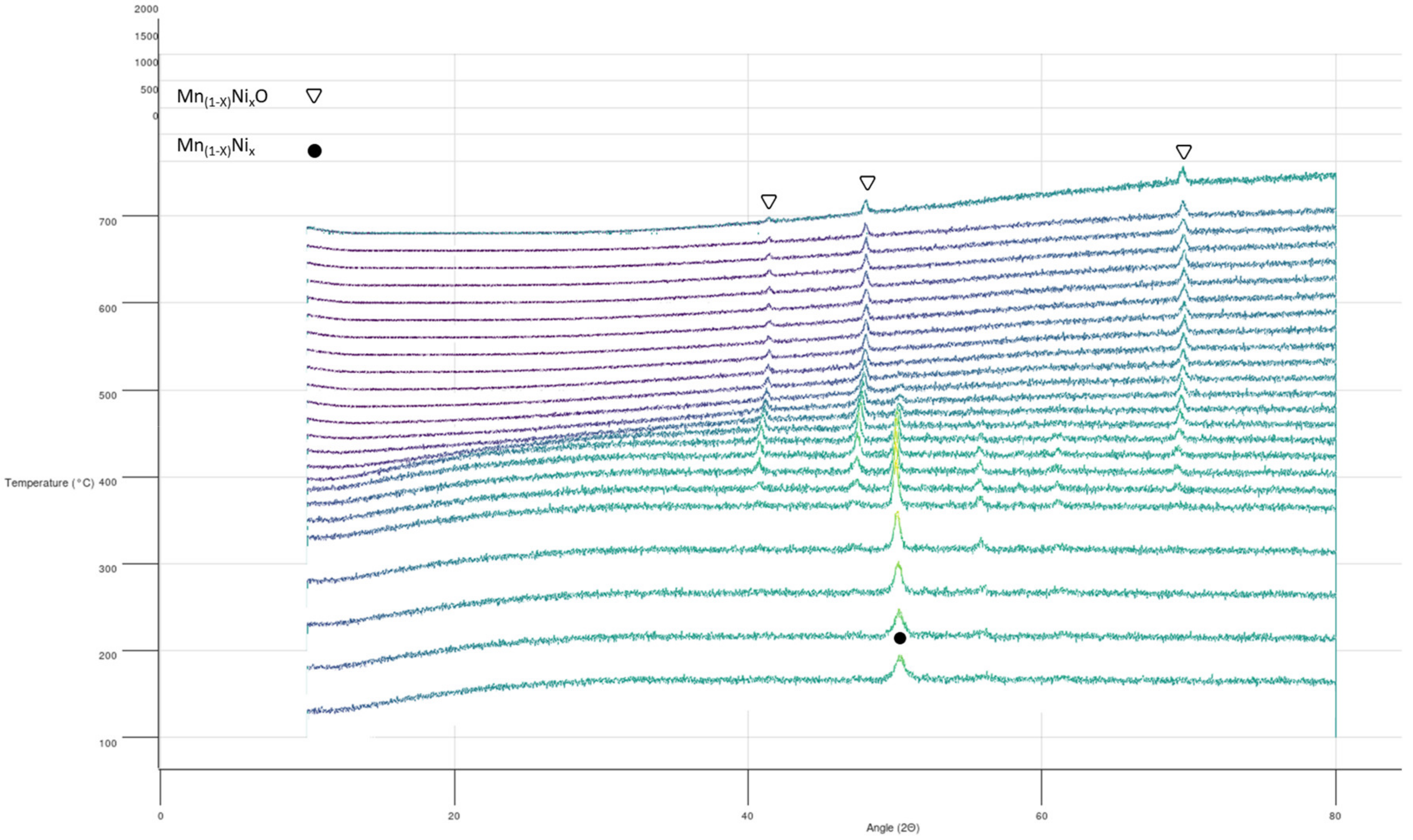
3.4. Reactivity upon Heating
4. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Materials Roadmap Enabling Low Carbon Energy Technologies. Available online: http://ec.europa.eu/research/industrial_technologies/pdf/materials-roadmap-elcet 13122011_en.pdf (accessed on 19 December 2018).
- Energy Roadmap 2050. Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=COM:2011:0885:FIN:EN:PDF (accessed on 19 December 2018).
- EASE/EERA Energy Storage Technology Development Roadmap towards 2030. Available online: http://ease-storage.eu/easeeera-energy-storage-technology-development-roadmap-towards-2030 (accessed on 19 December 2018).
- BCS Incorporated (March 2008). Available online: http://www1.eere.energy.gov/manufacturing/intensiveprocesses/pdfs/waste_heat_recovery.pdf (accessed on 19 December 2018).
- Siegel, N.P. Thermal energy storage for solar power production. Energy Environ. 2012, 1, 119–131. [Google Scholar] [CrossRef]
- Lane, G.A.; Shamsundar, N. Solar heat storage: Latent heat materials, Vol. I: Background and scientific principles.; American Society of Mechanical Engineers: New York, NY, USA, 1983; p. 467. [Google Scholar] [CrossRef]
- Zalba, B.; Marın, J.M.; Cabeza, L.F.; Mehling, H. Review on thermal energy storage with phase change: materials, heat transfer analysis and applications. Appl. Therm. Eng. 2003, 23, 251–283. [Google Scholar] [CrossRef]
- Felderhoff, M.; Urbanczyk, R.; Peil, S. Thermochemical heat storage for high temperature applications—A review. Green 2013, 3, 113–123. [Google Scholar] [CrossRef]
- Steinfeld, A.; Palumbo, R. Solar thermochemical process technology. Encycl. Phys. Sci. Technol. 2001, 15, 237–56. [Google Scholar] [CrossRef]
- Cahn, R.W.; Haasen, P. Physical metallurgy: fourth, revised and enhanced edition; Elsevier Science BV: Amsterdam, The Netherlands, 1996. [Google Scholar]
- Massalski, T.B. Binary alloy phase diagrams; ASM international: Materials Park, OH, USA, 1992. [Google Scholar]
- Saunders, N.; Miodownik, A.P. CALPHAD (calculation of phase diagrams): a comprehensive guide; Elsevier: New York, NY, USA, 1998. [Google Scholar]
- Chang, Y.A.; Chen, S.; Zhang, F.; Yan, X.; Xie, F.; Schmid-Fetzer, R.; Oates, W.A. Phase diagram calculation: past, present and future. Prog. Mater Sci. 2004, 49, 313–345. [Google Scholar] [CrossRef]
- Cacciamani, G. An Introduction to the CALPHAD Method and the COMPOUND Energy Formalism (CEF). Tecnol. Metal. Mater. Mineração 2016, 13, 16. [Google Scholar] [CrossRef]
- Bale, C.W.; Bélisle, E.; Chartrand, P.; Decterov, S.A.; Eriksson, G.; Gheribi, A.E.; Hack, K.; Jung, I.H.; Kang, Y.B.; Melançon, J.; et al. FactSage Thermochemical Software and Databases-2010-2016. Calphad 2016, 54, 35–53. [Google Scholar] [CrossRef]
- Lutterotti, L.; Gialanella, S. X-ray diffraction characterization of heavily deformed metallic specimens. Acta Mater. 1998, 46, 101–110. [Google Scholar] [CrossRef]
- Rietveld, H. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef] [Green Version]
- Suryanarayana, C. Structure and properties of nanocrystalline materials. Bull. Mater. Sci. 1994, 17, 307–346. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Igathinathane, C.; Pordesimo, L.O.; Columbus, E.P.; Batchelor, W.D.; Methuku, S.R. Shape identification and particles size distribution from basic shape parameters using ImageJ. Comput. Electron. Agric. 2008, 63, 168–182. [Google Scholar] [CrossRef]
- Bergman, B.; ÅGREN, J. Thermodynamic Assessment of the System MnO-NiO. J. Am. Ceram. Soc. 1985, 68, 444–450. [Google Scholar] [CrossRef]

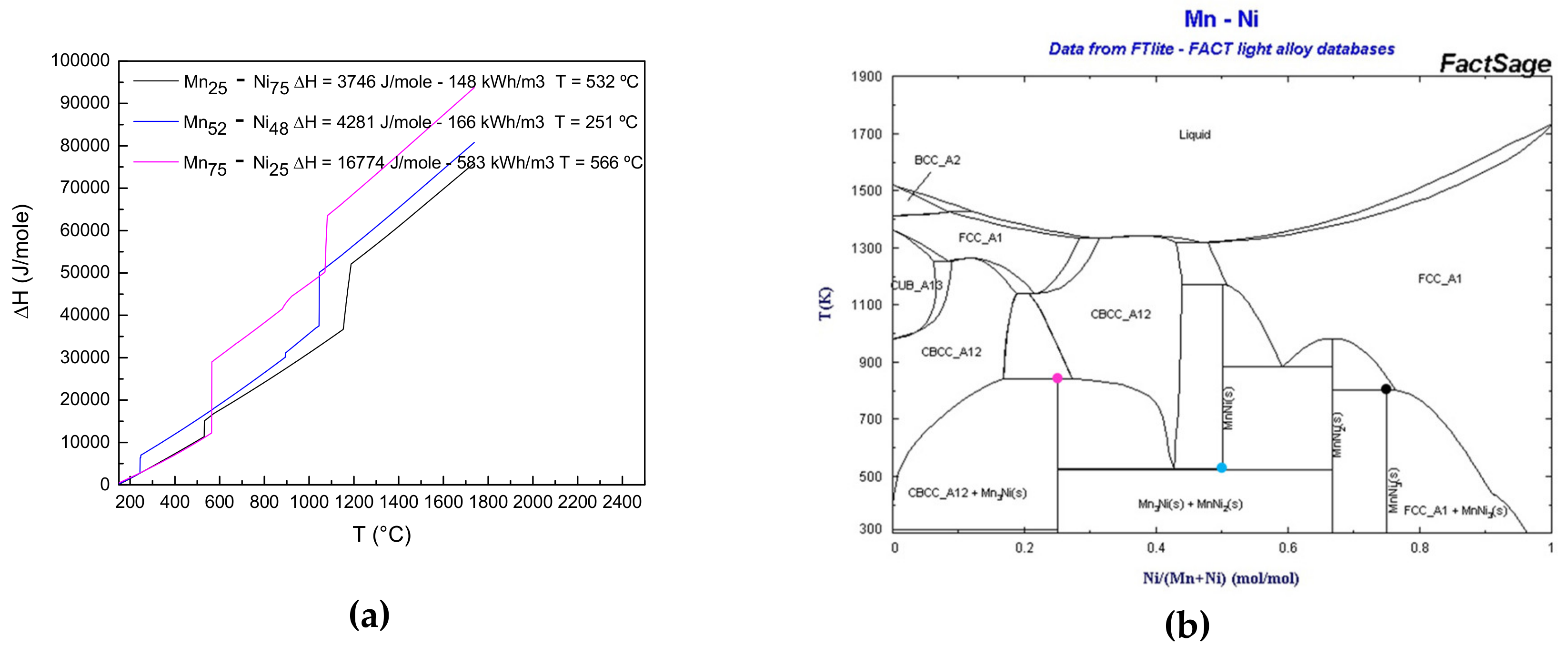





| System | Temperature °C | ΔHr J/mol | Volumetric Energy Density [kWh/m3] | Volumetric Energy Density (ΔT = 100 K)[kWh/m3] |
|---|---|---|---|---|
| Mn/Ni (52/48) | 251 | 4281 | 166 | 281 |
| Cu/Sn (84/16) | 505 | 1505 | 48 | 98 |
| Mn/Zn (68/32) | 529 | 1407 | 48 | 149 |
| Mn/Ni (25/75) | 532 | 3746 | 171 | 331 |
| Mn/Ni (75/25) | 566 | 16774 | 583 | 707 |
| Li2SO4/Na2SO4 (50/50) | 515 | 40080 | 217 | 319 |
| Al/Ni (38/62) | 698 | 1504 | 52 | 176 |
| Cu/Sn (75/25) | 676 | 3124 | 84 | 465 (ΔT = 58 K) |
| Fe/Sn (60/40) | 612 | 4011 | 97.7 | 176 |
| Mn/Ni (41/59) | 607 | 2784 | 117 | 265 |
| Al/Mn (45/55) | 842 | 6708 | 218 | 342 |
| Fe/Si (30/70) | 960 | 4996 | 165 | 263 |
| Fe/Si (67/33) | 962 | 3824 | 121 | 226 |
| 4 h Ball Milling | a (nm) | Error (nm) | <d> (nm) | Error (nm) | ε strain | Error |
|---|---|---|---|---|---|---|
| Mn (4 h) | 0.89088 | ±4.1 × 10−5 | 19.3 | ±0.20 | 3.7 × 10−3 | ±9.2 × 10−5 |
| Mn (Mn75-Ni25 One-step synthesis) | 0.88802 | ±7.7 × 10−5 | 14.5 | ±0.27 | 1.8 × 10−3 | ±2.5 × 10−4 |
| Mn (Mn75-Ni25 Two-step synthesis) | 0.89107 | ±4.4 × 10−5 | 16.6 | ±0.16 | 2.6 × 10−3 | ±8.0 × 10−5 |
| Mn (Mn52-Ni48 One-step synthesis) | 0.88879 | ±9.7 × 10−5 | 14.4 | ±0.33 | 1.4 × 10−3 | ±4.4 × 10−4 |
| Mn (Mn52-Ni48 Two-step synthesis) | 0.89039 | ±9.7 × 10−6 | 17.2 | ±0.22 | 3.7 × 10−3 | ±6.6 × 10−5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doppiu, S.; Dauvergne, J.-L.; Palomo del Barrio, E. Solid-State Reactions for the Storage of Thermal Energy. Nanomaterials 2019, 9, 226. https://doi.org/10.3390/nano9020226
Doppiu S, Dauvergne J-L, Palomo del Barrio E. Solid-State Reactions for the Storage of Thermal Energy. Nanomaterials. 2019; 9(2):226. https://doi.org/10.3390/nano9020226
Chicago/Turabian StyleDoppiu, Stefania, Jean-Luc Dauvergne, and Elena Palomo del Barrio. 2019. "Solid-State Reactions for the Storage of Thermal Energy" Nanomaterials 9, no. 2: 226. https://doi.org/10.3390/nano9020226