New Composite Water Sorbents CaCl2-PHTS for Low-Temperature Sorption Heat Storage: Determination of Structural Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
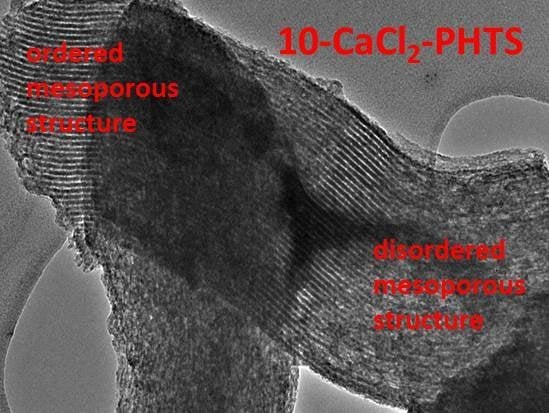
3.1. Structural Properties of As-Prepared Smples
3.2. Structural Properties of the Samples After Water Sorption and Cycling Test
3.3. Water Sorption and Heat Storage Capacity Calculation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Scapino, L.; Zondag, H.A.; Van Bael, J.; Diriken, J.; Rindt, C.C.M. Sorption heat storage for long-term low-temperature applications: A review on the advancements at material and prototype scale. Appl. Energy 2017, 190, 920–948. [Google Scholar] [CrossRef]
- Mastronardo, E.; Bonaccorsi, L.; Kato, Y.; Piperopoulos, E.; Milone, C. Efficiency improvement of heat storage materials for MgO/H2O/Mg(OH)2 chemical heat pumps. Appl. Energy 2016, 162, 31–39. [Google Scholar] [CrossRef]
- Ristić, A.; Fischer, F.; Hauer, A.; Zabukovec Logar, N. Improved performance of binder-free zeolite Y for low-temperature sorption heat storage. J. Mater. Chem. A 2018, 6, 11521–11530. [Google Scholar] [CrossRef]
- Grekova, A.; Gordeeva, L.; Aristov, Y. Composite sorbents “Li/Ca halogenides inside Multi-wall Carbon Nano-tubes” for Thermal Energy Storage. Sol. Energy Mater. Sol. Cells 2016, 155, 176–183. [Google Scholar] [CrossRef]
- Henninger, S.K.; Ernst, S.J.; Gordeeva, L.; Bendix, P.; Froechlich, D.; Grekova, A.D.; Bonaccorsi, L.; Aristov, Y.; Jaenchen, J. New materials for adsorption heat transformation and storage. Renew. Energy 2017, 110, 59–68. [Google Scholar] [CrossRef]
- Vasta, S.; Brancato, V.; La Rosa, D.; Palomba, V.; Restuccia, G.; Sapienza, A.; Frazzica, A. Adsorption Heat Storage: State-of-the-Art and Future Perspectives. Nanomaterials 2018, 8, 522. [Google Scholar] [CrossRef] [PubMed]
- Grekova, A.D.; Gordeeva, L.G.; Aristov, Y.I. Composite “LiCl/vermiculite” as advanced water sorbent for thermal energy storage. Appl. Therm. Eng. 2017, 124, 1401–1408. [Google Scholar] [CrossRef]
- Grekova, A.D.; Girnik, I.S.; Nikulin, V.V.; Tokarev, M.M.; Gordeeva, L.G.; Aristov, Y.I. New composite sorbents of water and methanol “salt in anodic alumina”: Evaluation for adsorption heat transformation. Energy 2016, 106, 231–239. [Google Scholar] [CrossRef]
- Gordeeva, L.G.; Aristov, Y.I. Composites “salt inside porous matrix” for adsorption heat transformation: A current state-of-the-art and new trends. Int. J. Low-Carbon Technol. 2012, 7, 288–302. [Google Scholar] [CrossRef]
- Gaeini, M.; Rouws, A.L.; Salari, J.W.O.; Zondag, H.A.; Rindt, C.C.M. Characterization of microencapsulated and impregnated porous host materials based on calcium chloride for thermochemical energy storage. Appl. Energy 2018, 212, 1165–1177. [Google Scholar] [CrossRef]
- Aristov, Y.I. New family of solid sorbents for adsorptive cooling: Material scientist approach. J. Eng. Thermophys. 2007, 16, 63–72. [Google Scholar] [CrossRef]
- Courbon, E.; D’Ans, P.; Permyakova, A.; Skrylnyk, O.; Steunou, N.; Degrez, M.; Frère, M. Further improvement of the synthesis of silica gel and CaCl2 composites: Enhancement of energy storage density and stability over cycles for solar heat storage coupled with space heating applications. Sol. Energy 2017, 157, 532–541. [Google Scholar] [CrossRef]
- Ristić, A.; Maučec, D.; Henninger, S.K.; Kaučič, V. New two-component water sorbent CaCl2-FeKIL2 for solar thermal energy storage. Microporous Mesoporous Mater. 2012, 164, 266–272. [Google Scholar] [CrossRef]
- Ponomarenko, I.V.; Glaznev, I.S.; Gubar, A.V.; Aristov, Y.I.; Kirik, S.D. Synthesis and water sorption properties of a new composite “CaCl2 confined into SBA-15 pores”. Microporous Mesoporous Mater. 2010, 129, 243–250. [Google Scholar] [CrossRef]
- Jänchen, J.; Ackermann, D.; Stach, H.; Brösicke, W. Studies of the water adsorption on Zeolites and modified mesoporous materials for seasonal storage of solar heat. Sol. Energy 2004, 76, 339–344. [Google Scholar] [CrossRef]
- Nonnen, T.; Beckert, S.; Gleichmann, K.; Brandt, A.; Unger, B.; Kerskes, H.; Mette, B.; Bonk, S.; Badenhop, T.; Salg, F.; et al. A Thermochemical Long-Term Heat Storage System Based on a Salt/Zeolite Composite. Chem. Eng. Technol. 2016, 39, 2427–2434. [Google Scholar] [CrossRef]
- Jabbari-Hichri, A.; Bennici, S.; Auroux, A. CaCl2-containing composites as thermochemical heat storage materials. Sol. Energy Mater. Sol. Cells 2017, 172, 177–185. [Google Scholar] [CrossRef]
- Casey, S.P.; Elvins, J.; Riffat, S.; Robinson, A. Salt impregnated desiccant matrices for “open” thermochemical energy storage—Selection, synthesis and characterisation of candidate materials. Energy Build. 2014, 84, 412–425. [Google Scholar] [CrossRef]
- Permyakova, A.; Wang, S.; Courbon, E.; Nouar, F.; Heymans, N.; D’Ans, P.; Barrier, N.; Billemont, P.; De Weireld, G.; Steunou, N.; et al. Design of salt-metal organic framework composites for seasonal heat storage applications. J. Mater. Chem. A 2017, 5, 12889–12898. [Google Scholar] [CrossRef]
- Palomba, V.; Frazzica, A. Recent advancements in sorption technology for solar thermal energy storage applications. Sol. Energy 2018, 1–37. [Google Scholar] [CrossRef]
- N’Tsoukpoe, K.E.; Rammelberg, H.U.; Lele, A.F.; Korhammer, K.; Watts, B.A.; Schmidt, T.; Ruck, W.K.L. A review on the use of calcium chloride in applied thermal engineering. Appl. Therm. Eng. 2015, 75, 513–531. [Google Scholar] [CrossRef]
- Ng, E.P.; Mintova, S. Nanoporous materials with enhanced hydrophilicity and high water sorption capacity. Microporous Mesoporous Mater. 2008, 114, 1–26. [Google Scholar] [CrossRef]
- Maaz, S.; Rose, M.; Palkovits, R. Systematic investigation of the pore structure and surface properties of SBA-15 by water vapor physisorption. Microporous Mesoporous Mater. 2016, 220, 183–187. [Google Scholar] [CrossRef]
- Tokarev, M.; Gordeeva, L.; Romannikov, V.; Glaznev, I.; Aristov, Y. New composite sorbent CaCl2 in mesopores for sorption cooling/heating. Int. J. Therm. Sci. 2002, 41, 470–474. [Google Scholar] [CrossRef]
- Glaznev, I.; Ponomarenko, I.; Kirik, S.; Aristov, Y. Composites CaCl2/SBA-15 for adsorptive transformation of low temperature heat: Pore size effect. Int. J. Refrig. 2011, 34, 1244–1250. [Google Scholar] [CrossRef]
- Ristić, A.; Henninger, S.K. Sorption composite materials for solar thermal energy storage. Energy Procedia 2014, 48, 977–981. [Google Scholar] [CrossRef]
- Aristov, Y.I. Challenging offers of material science for adsorption heat transformation: A review. Appl. Therm. Eng. 2013, 50, 1610–1618. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Van Der Voort, P.; Ravikovitch, P.I.; De Jong, K.P.; Benjelloun, M.; Van Bavel, E.; Janssen, A.H.; Neimark, A.V.; Weckhuysen, B.M.; Vansant, E.F. A new templated ordered structure with combined micro- and mesopores and internal silica nanocapsules. J. Phys. Chem. B 2002, 106, 5873–5877. [Google Scholar] [CrossRef]
- Celer, E.B.; Kruk, M.; Zuzek, Y.; Jaroniec, M. Hydrothermal stability of SBA-15 and related ordered mesoporous silicas with plugged pores. J. Mater. Chem. 2006, 16, 2824. [Google Scholar] [CrossRef]
- Zhao, D.; Huo, Q.; Feng, J.; Chmelka, B.F.; Stucky, G.D. Nonionic Triblock and Star Diblock Copolymer and Oligomeric Surfactant Syntheses of Highly Ordered, Hydrothermally Stable, Mesoporous Silica Structures. J. Am. Chem. Soc. 1998, 120, 6024–6036. [Google Scholar] [CrossRef]
- Munnik, P.; De Jongh, P.E.; De Jong, K.P. Recent Developments in the Synthesis of Supported Catalysts. Chem. Rev. 2015, 115, 6687–6718. [Google Scholar] [CrossRef] [PubMed]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- de Lange, M.F.; Verouden, K.J.F.M.; Vlugt, T.J.H.; Gascon, J.; Kapteijn, F. Adsorption-Driven Heat Pumps: The Potential of Metal–Organic Frameworks. Chem. Rev. 2015, 115, 12205–12250. [Google Scholar] [CrossRef] [PubMed]
- Bering, B.P.; Dubinin, M.M.; Serpinsky, V.V. Theory of volume filling for vapor adsorption. J. Colloid Interface Sci. 1966, 21, 378–393. [Google Scholar] [CrossRef]
- Krajnc, A.; Varlec, J.; Mazaj, M.; Ristić, A.; Logar, N.Z.; Mali, G. Superior Performance of Microporous Aluminophosphate with LTA Topology in Solar-Energy Storage and Heat Reallocation. Adv. Energy Mater. 2017, 7, 1601815. [Google Scholar] [CrossRef]
- Mazaj, M.; Stevens, W.J.J.; Logar, N.Z.; Ristić, A.; Tušar, N.N.; Arčon, I.; Daneu, N.; Meynen, V.; Cool, P.; Vansant, E.F.; et al. Synthesis and structural investigations on aluminium-free Ti-Beta/SBA-15 composite. Microporous Mesoporous Mater. 2009, 117, 458–465. [Google Scholar] [CrossRef]
- Tasbihi, M.; Štangar, U.L.; Škapin, A.S.; Ristić, A.; Kaučič, V.; Tušar, N.N. Titania-containing mesoporous silica powders: Structural properties and photocatalytic activity towards isopropanol degradation. J. Photochem. Photobiol. A Chem. 2010, 216, 167–178. [Google Scholar] [CrossRef]
- Šuligoj, A.; Štangar, U.L.; Ristić, A.; Mazaj, M.; Verhovšek, D.; Tušar, N.N. TiO2-SiO2films from organic-free colloidal TiO2anatase nanoparticles as photocatalyst for removal of volatile organic compounds from indoor air. Appl. Catal. B Environ. 2016, 184, 119–131. [Google Scholar] [CrossRef]
- Lemaire, A.; Rooke, J.C.; Chen, L.H.; Su, B.L. Direct observation of macrostructure formation of hierarchically structured meso-macroporous aluminosilicates with 3D interconnectivity by optical microscope. Langmuir 2011, 27, 3030–3043. [Google Scholar] [CrossRef] [PubMed]
- Lukens, W.W.; Schmidt-Winkel, P.; Zhao, D.; Feng, J.; Stucky, G.D. Evaluating pore sizes in mesoporous materials: A simplified standard adsorption method and a simplified Broekhoff-de Boer method. Langmuir 1999, 15, 5403–5409. [Google Scholar] [CrossRef]
- Tanev, P.T.; Vlaev, L.T. An attempt at a more precise evaluation of the approach to mesopore size distribution calculations depending on the degree of pore blocking. J. Colloid Interface Sci. 1993, 160, 110–116. [Google Scholar] [CrossRef]
- Deshmane, V.G.; Adewuyi, Y.G. Mesoporous nanocrystalline sulfated zirconia synthesis and its application for FFA esterification in oils. Appl. Catal. A Gen. 2013, 462, 196–206. [Google Scholar] [CrossRef]
- Pikus, S.; Celer, E.B.; Jaroniec, M.; Solovyov, L.A.; Kozak, M. Studies of intrawall porosity in the hexagonally ordered mesostructures of SBA-15 by small angle X-ray scattering and nitrogen adsorption. Appl. Surf. Sci. 2010, 256, 5311–5315. [Google Scholar] [CrossRef]
- Jabbari-Hichri, A.; Bennici, S.; Auroux, A. Effect of aluminum sulfate addition on the thermal storage performance of mesoporous SBA-15 and MCM-41 materials. Sol. Energy Mater. Sol. Cells 2016, 149, 232–241. [Google Scholar] [CrossRef]
- Dubinin, M.M. The potential theory of adsorption of gases and vapors for adsorbents with energetically nonuniform surfaces. Chem. Rev. 1960, 60, 235–241. [Google Scholar] [CrossRef]
- Birsa Čelič, T.; Mazaj, M.; Guillou, N.; Elkaïm, E.; El Roz, M.; Thibault-Starzyk, F.; Mali, G.; Rangus, M.; Čendak, T.; Kaučič, V.; et al. Study of hydrothermal stability and water sorption characteristics of 3-dimensional Zn-based trimesate. J. Phys. Chem. C 2013, 117, 14608–14617. [Google Scholar] [CrossRef]
- Aristov, Y. Concept of adsorbent optimal for adsorptive cooling/heating. Appl. Therm. Eng. 2014, 72, 166–175. [Google Scholar] [CrossRef]











| Sample | SBET (m2/g) | Vtot (cm3/g) | Vmi (cm3/g) | Average Pore Size (nm) |
|---|---|---|---|---|
| PHTS | 810 | 0.705 | 0.122 | 5.7 |
| 4-CaCl2-PHTS | 461 | 0.492 | 0.037 | 5.6 |
| 10-CaCl2-PHTS | 322 | 0.377 | 0.022 | 5.8 |
| 20-CaCl2-PHTS | 163 | 0.189 | 0.016 | 6.2 |
| Sample After Water Sorption | SBET (m2/g) | Vtot (cm3/g) | Average Pore Size (nm) |
|---|---|---|---|
| PHTS | 640 | 0.624 | 5.5 |
| 4-CaCl2-PHTS | 227 | 0.394 | 6.7 |
| 10-CaCl2-PHTS | 133 | 0.195 | 5.2 |
| 20-CaCl2-PHTS | 50 | 0.039 | 5.5 |
| Sample After Cycling | SBET (m2/g) | Vtot (cm3/g) | Average Pore Size (nm) | EDX Analysis (wt.%) |
|---|---|---|---|---|
| PHTS | 620 | 0.560 | 5.7 | - |
| 4-CaCl2-PHTS | 256 | 0.400 | 6.4 | 4 |
| 10-CaCl2-PHTS | 165 | 0.304 | 6.5 | 10 |
| 20-CaCl2-PHTS | 90 | 0.119 | 6.0 | 20 |
| Sample | ∆w (kg/kg) | Qads (Wh/kg) | Qads (kJ/kg) |
|---|---|---|---|
| PHTS | 0.073 | 71 | 256 |
| 4-CaCl2-PHTS | 0.100 | 81 | 292 |
| 10-CaCl2-PHTS | 0.142 | 119 | 428 |
| 20-CaCl2-PHTS | 0.239 | 193 | 694 |
| Sample | ∆w (kg/kg) | Qads (Wh/kg) | Qads (kJ/kg) |
|---|---|---|---|
| PHTS | 0.125 | 117 | 421 |
| 4-CaCl2-PHTS | 0.150 | 131 | 472 |
| 10-CaCl2-PHTS | 0.250 | 205 | 738 |
| 20-CaCl2-PHTS | 0.430 | 333 | 1199 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ristić, A.; Zabukovec Logar, N. New Composite Water Sorbents CaCl2-PHTS for Low-Temperature Sorption Heat Storage: Determination of Structural Properties. Nanomaterials 2019, 9, 27. https://doi.org/10.3390/nano9010027
Ristić A, Zabukovec Logar N. New Composite Water Sorbents CaCl2-PHTS for Low-Temperature Sorption Heat Storage: Determination of Structural Properties. Nanomaterials. 2019; 9(1):27. https://doi.org/10.3390/nano9010027
Chicago/Turabian StyleRistić, Alenka, and Nataša Zabukovec Logar. 2019. "New Composite Water Sorbents CaCl2-PHTS for Low-Temperature Sorption Heat Storage: Determination of Structural Properties" Nanomaterials 9, no. 1: 27. https://doi.org/10.3390/nano9010027
APA StyleRistić, A., & Zabukovec Logar, N. (2019). New Composite Water Sorbents CaCl2-PHTS for Low-Temperature Sorption Heat Storage: Determination of Structural Properties. Nanomaterials, 9(1), 27. https://doi.org/10.3390/nano9010027