In Situ Synthesis of a Stable Fe3O4@Cellulose Nanocomposite for Efficient Catalytic Degradation of Methylene Blue
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pretreatment of Cellulose
2.3. Preparation of Cellulose@Fe3O4 Nanocomposite
2.4. Characterization
2.5. Degradation Experiments
2.6. Recyclability Experiments
3. Results and Discussion
3.1. Structure of the Cellulose Solutions
3.2. Analysis of the Interaction between Cellulose and Fe3O4
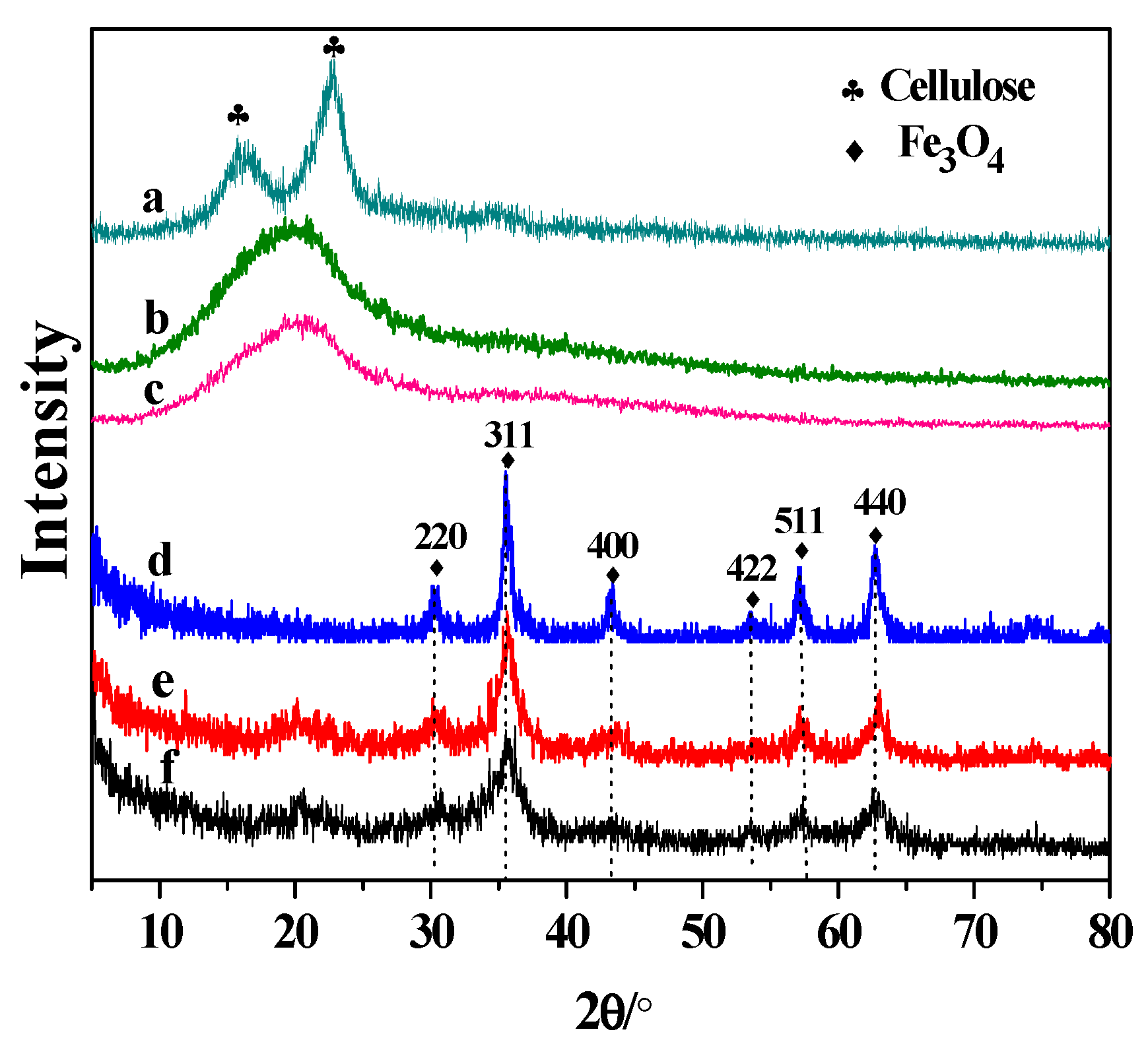
3.2.1. XRD Analysis
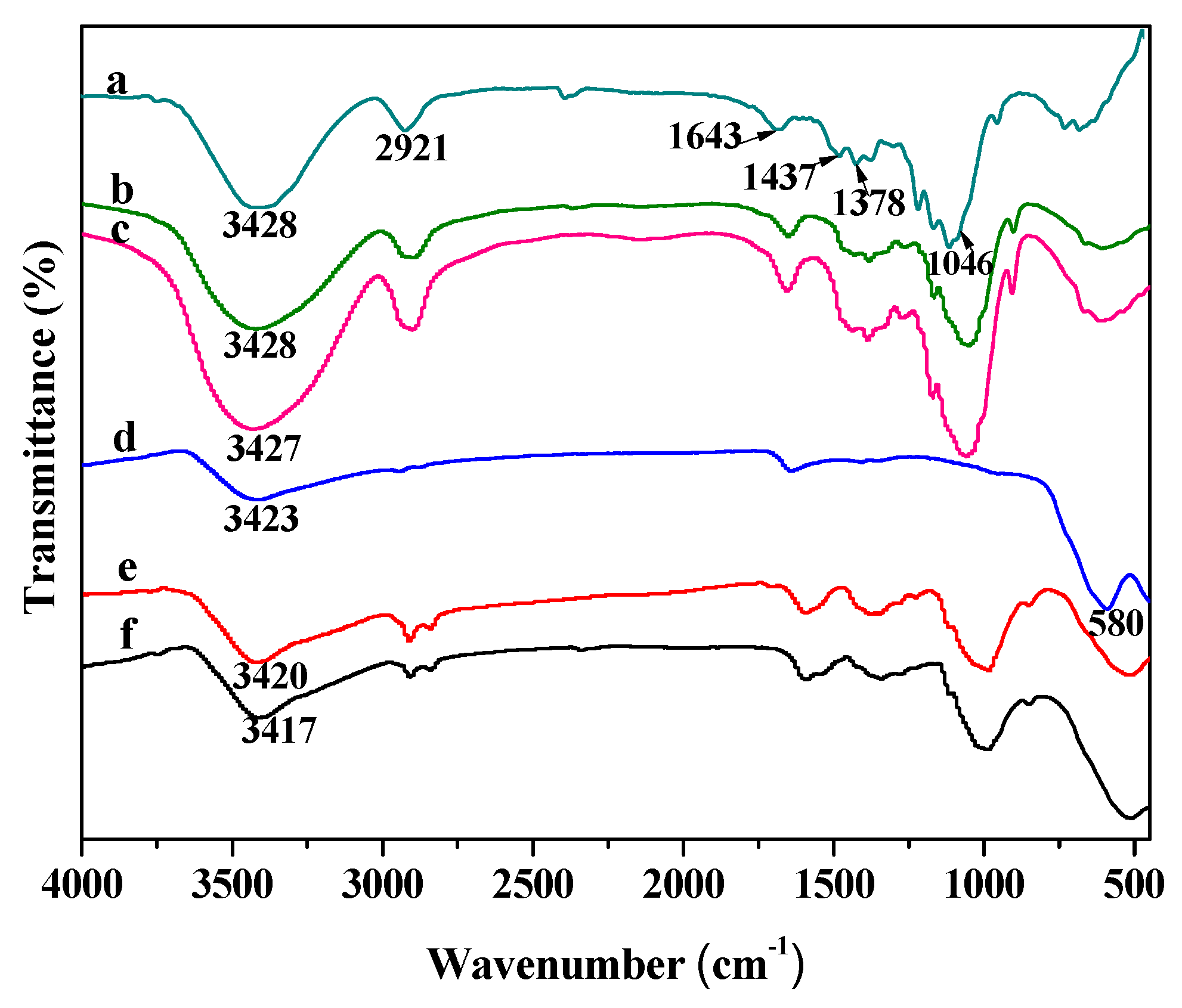
3.2.2. FTIR Analysis
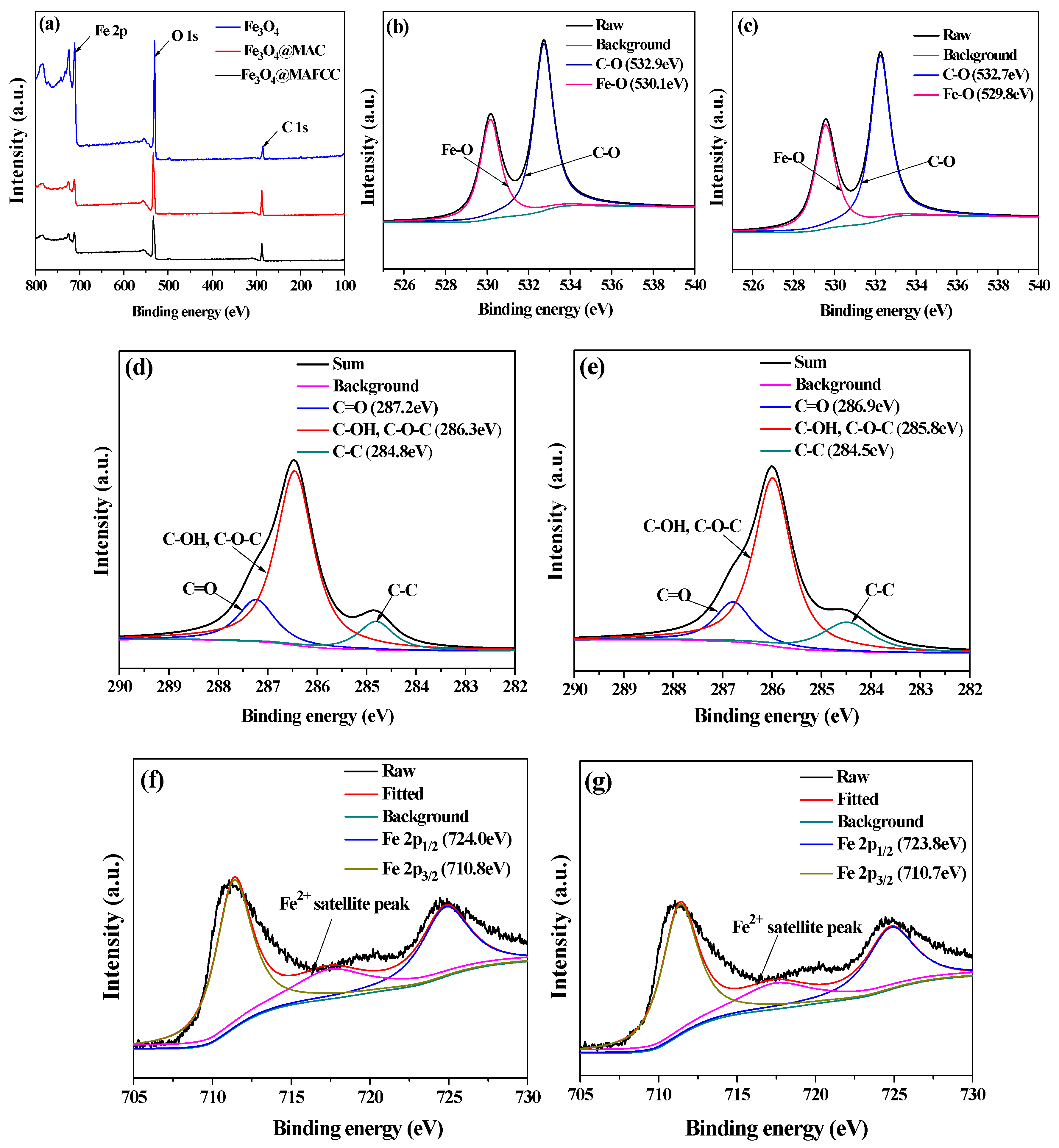
3.2.3. Surface Element Composition Analysis
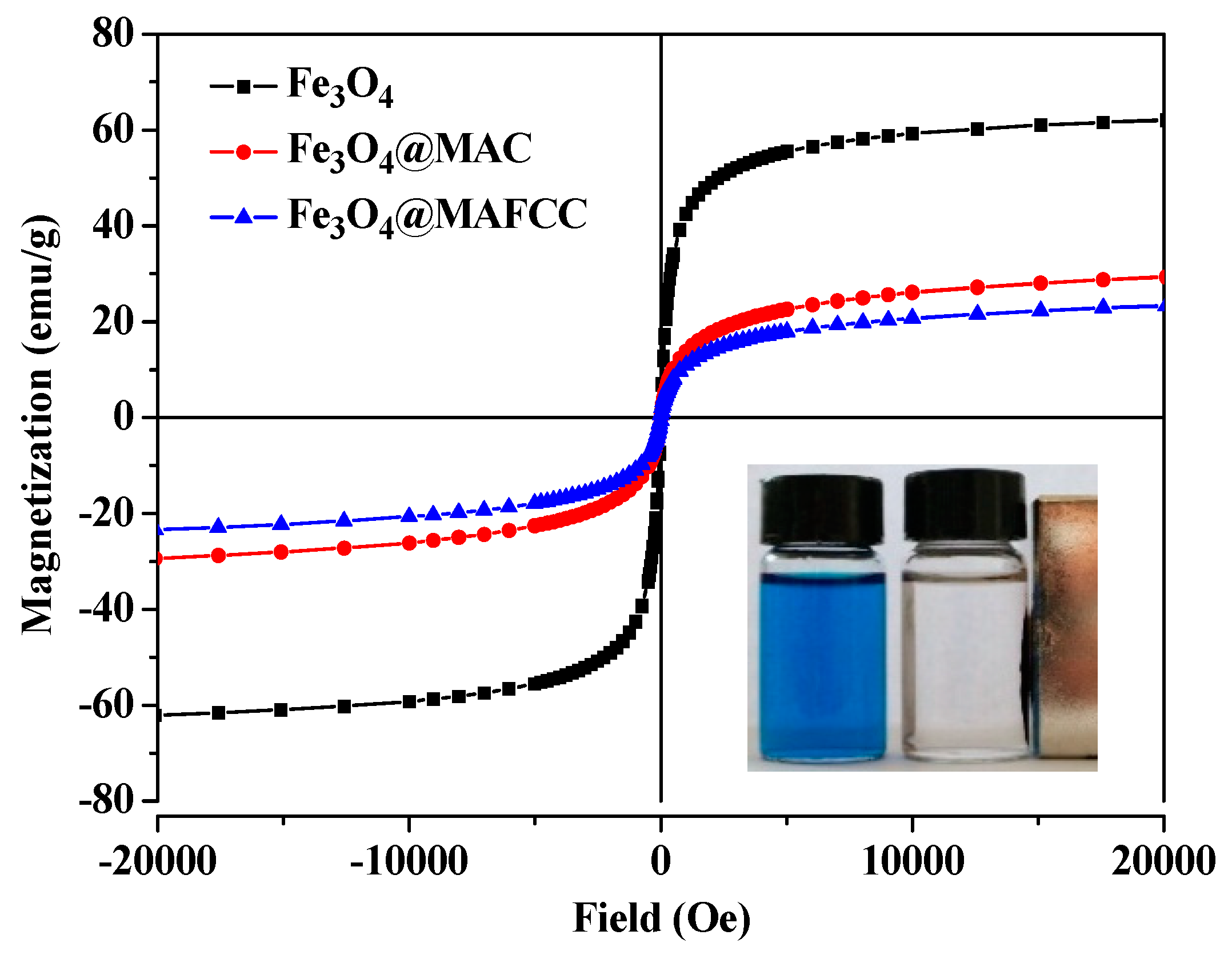
3.2.4. Magnetic Behaviors
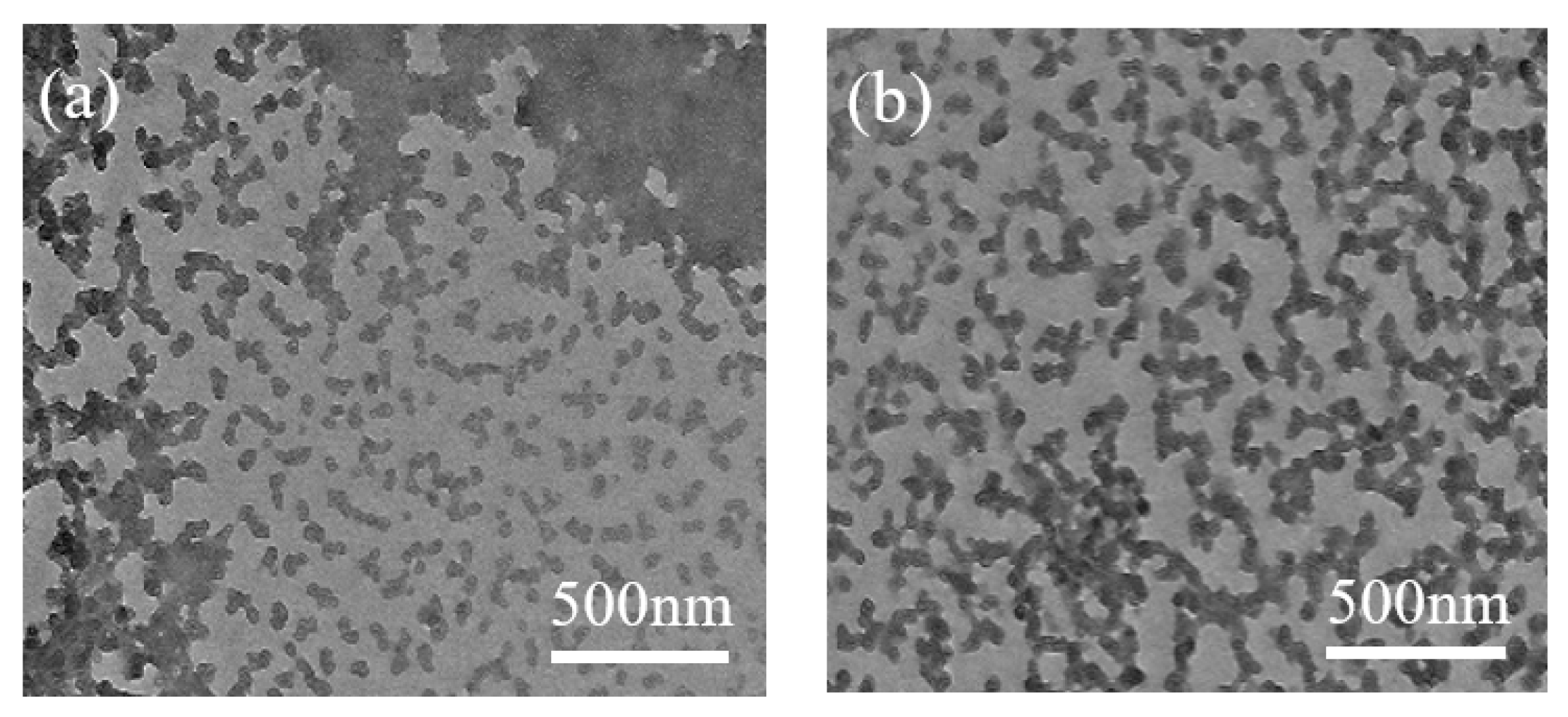
3.2.5. Surface Morphology Analysis
3.2.6. Process of the Combination of Cellulose and Fe3O4 Nanoparticles
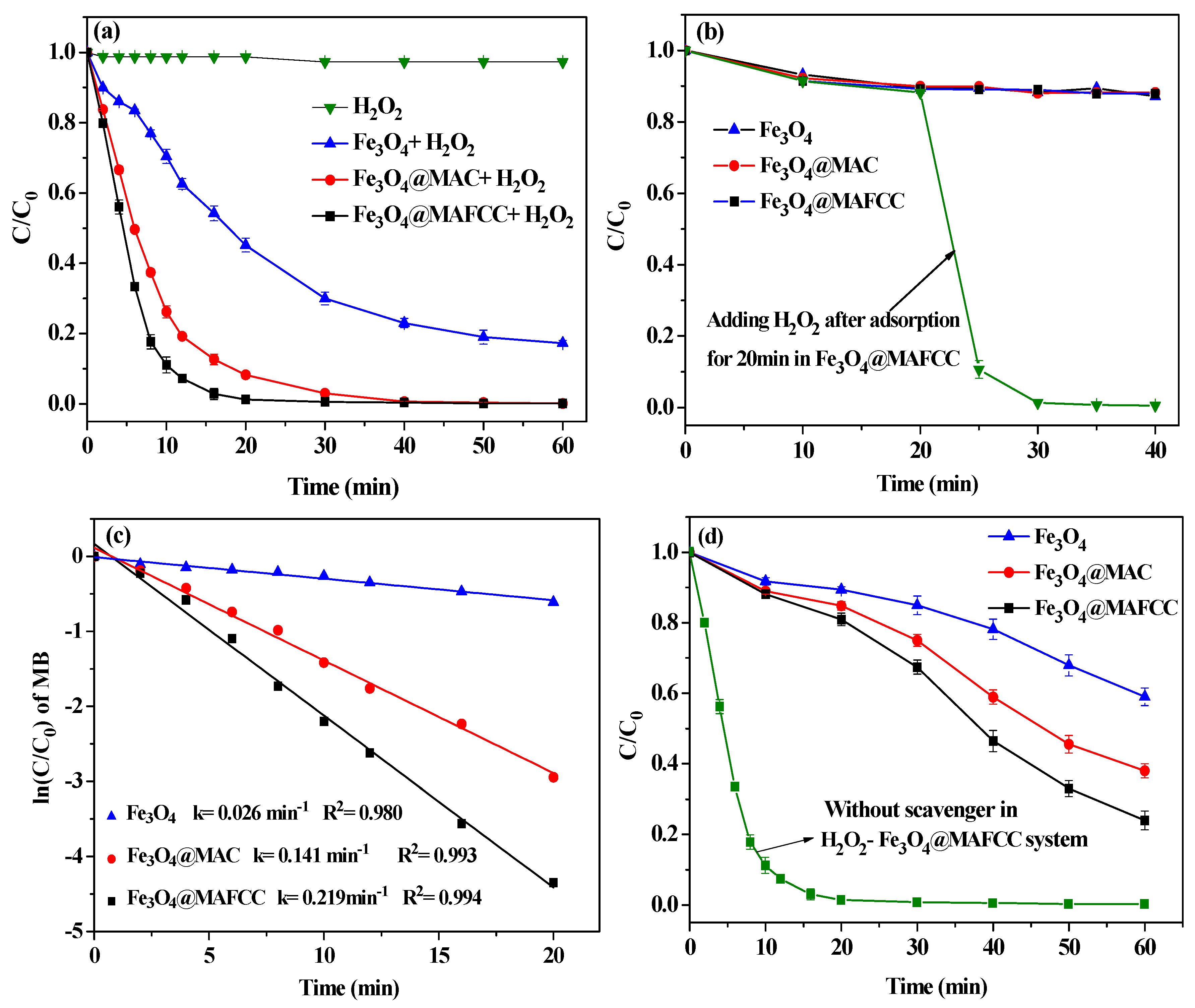
3.3. Catalytic Degradation of MB
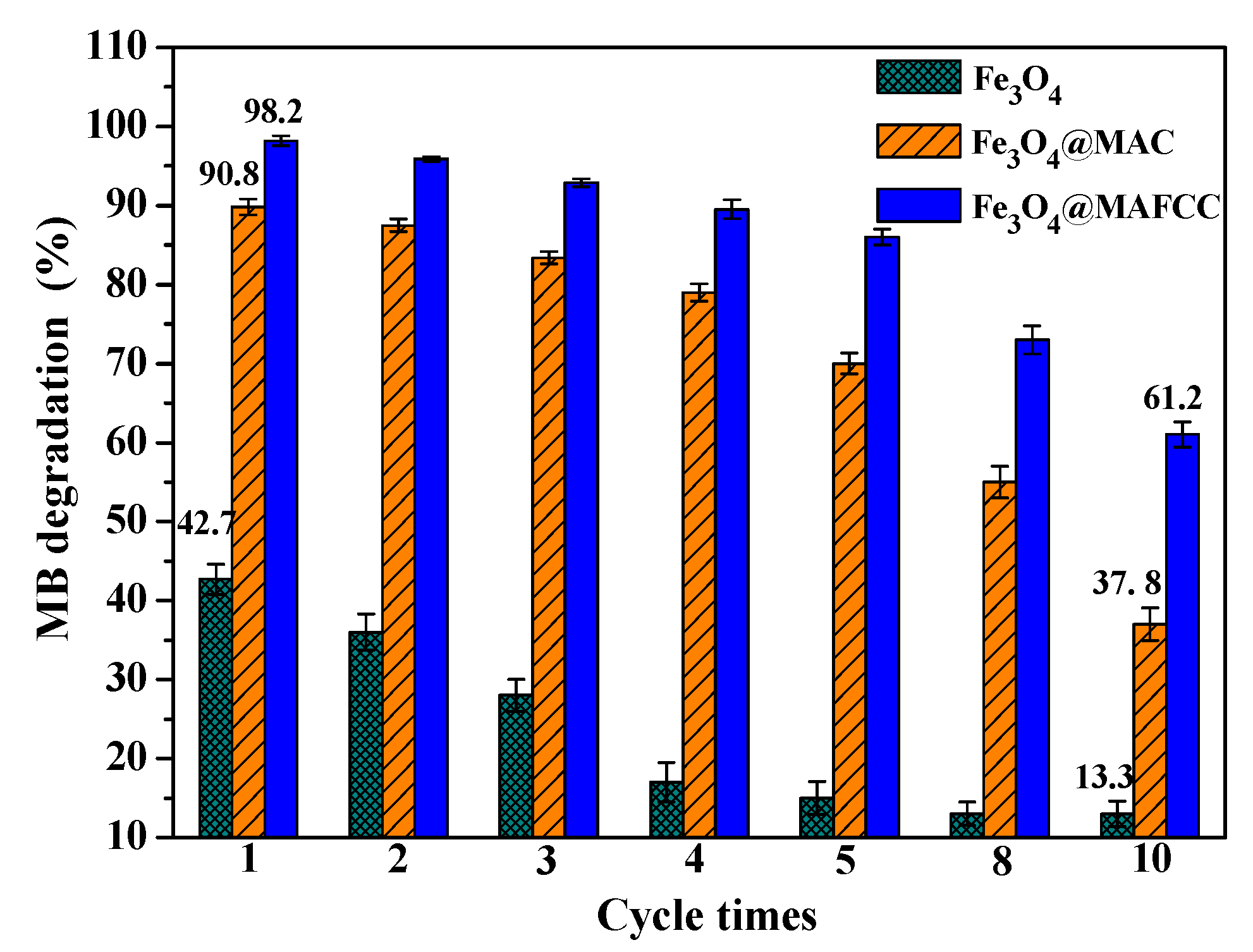
3.4. Reusability Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lee, J.; Kim, J.; Chang, Y.; Chang, Y. Steel dust catalysis for Fenton-like oxidation of polychlorinated dibenzo-p-dioxins. J. Hazard. Mater. 2009, 163, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Sable, S.S.; Panchangam, S.C.; Lo, S. Abatement of clofibric acid by Fenton-like process using iron oxide supported sulfonated-ZrO2: Efficient heterogeneous catalysts. J. Water Process Eng. 2018, 26, 92–99. [Google Scholar] [CrossRef]
- Rubeena, K.K.; Hari Prasad Reddy, P.; Laiju, A.R.; Nidheesh, P.V. Iron impregnated biochars as heterogeneous Fenton catalyst for the degradation of acid red 1 dye. J. Environ. Manag. 2018, 226, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Keihan, A.H.; Veisi, H.; Biabri, P.M. Facile synthesis of PEG-coated magnetite (Fe3O4) and embedment of gold nanoparticle as a nontoxic antimicrobial agent. Appl. Organomet. Chem. 2017, 31, e3873. [Google Scholar] [CrossRef]
- Kakavandi, B.; Jonidi, J.A.; Rezaei, K.R.; Nasseri, S.; Ameri, A.; Esrafily, A. Synthesis and properties of Fe3O4-activated carbon magnetic nanoparticles for removal of aniline from aqueous solution: Equilibrium, kinetic and thermodynamic studies. Iran. J. Environ. Health Sci. Eng. 2013, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Li, L.; Cui, Y.; Cui, F. New branch on old tree: Green-synthesized RGO/Fe3O4 composite as a photo-Fenton catalyst for rapid decomposition of methylene blue. Ceram. Int. 2017, 43, 14361–14368. [Google Scholar] [CrossRef]
- Chang, J.; Ma, J.; Ma, Q.; Zhang, D.; Qiao, N.; Hu, M.; Ma, H. Adsorption of methylene blue onto Fe3O4/activated montmorillonite nanocomposite. Appl. Clay Sci. 2016, 119, 132–140. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Q.; Su, J.; Lin, Y.; Huang, Z.; Lu, Y.; Sun, G.; Yang, M.; Huang, A.; Hu, H.; et al. A green and efficient technology for the degradation of cellulosic materials: Structure changes and enhanced enzymatic hydrolysis of natural cellulose pretreated by synergistic interaction of mechanical activation and metal salt. Bioresour. Technol. 2015, 177, 176–181. [Google Scholar] [CrossRef]
- Arantes, A.C.C.; Almeida, C.D.G.; Dauzacker, L.C.L.; Bianchi, M.L.; Wood, D.F.; Williams, T.G.; Orts, W.J.; Tonoli, G.H.D. Renewable hybrid nanocatalyst from magnetite and cellulose for treatment of textile effluents. Carbohydr. Polym. 2017, 163, 101–107. [Google Scholar] [CrossRef]
- Mahmoud, K.A.; Male, K.B.; Hrapovic, S.; Luong, J.H.T. Cellulose Nanocrystal/Gold Nanoparticle Composite as a Matrix for Enzyme Immobilization. ACS Appl. Mater. Interfaces 2009, 1, 1383–1386. [Google Scholar] [CrossRef] [Green Version]
- Jiao, Y.; Wan, C.; Bao, W.; Gao, H.; Liang, D.; Li, J. Facile hydrothermal synthesis of Fe3O4@cellulose aerogel nanocomposite and its application in Fenton-like degradation of Rhodamine B. Carbohydr. Polym. 2018, 189, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Qin, Z.; Liu, Y.; Cheng, M.; Qian, P.; Wang, Q.; Zhu, M. Superparamagnetic iron oxide coated on the surface of cellulose nanospheres for the rapid removal of textile dye under mild condition. Appl. Surf. Sci. 2015, 357, 2103–2111. [Google Scholar] [CrossRef]
- Zhu, H.Y.; Fu, Y.Q.; Jiang, R.; Jiang, J.H.; Xiao, L.; Zeng, G.M.; Zhao, S.L.; Wang, Y. Adsorption removal of congo red onto magnetic cellulose/Fe3O4/activated carbon composite: Equilibrium, kinetic and thermodynamic studies. Chem. Eng. J. 2011, 173, 494–502. [Google Scholar] [CrossRef]
- Sriplai, N.; Mongkolthanaruk, W.; Eichhorn, S.J.; Pinitsoontorn, S. Magnetically responsive and flexible bacterial cellulose membranes. Carbohydr. Polym. 2018, 192, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Jia, S.; Wan, T.; Jia, Y.; Yang, H.; Li, J.; Yan, L.; Zhong, C. Biosynthesis of spherical Fe3O4/bacterial cellulose nanocomposites as adsorbents for heavy metal ions. Carbohydr. Polym. 2011, 86, 1558–1564. [Google Scholar] [CrossRef]
- An, X.; Cheng, D.; Dai, L.; Wang, B.; Ocampo, H.J.; Nasrallah, J.; Jia, X.; Zou, J.; Long, Y.; Ni, Y. Synthesis of nano-fibrillated cellulose/magnetite/titanium dioxide (NFC@Fe3O4@TNP) nanocomposites and their application in the photocatalytic hydrogen generation. Appl. Catal. B-Environ. 2017, 206, 53–64. [Google Scholar] [CrossRef]
- Qi, H.; Chang, C.; Zhang, L. Effects of temperature and molecular weight on dissolution of cellulose in NaOH/urea aqueous solution. Cellulose 2008, 15, 779–787. [Google Scholar] [CrossRef]
- Yang, Q.; Qi, H.; Lue, A.; Hu, K.; Cheng, G.; Zhang, L. Role of sodium zincate on cellulose dissolution in NaOH/urea aqueous solution at low temperature. Carbohydr. Polym. 2011, 83, 1185–1191. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, Y.; Liu, X.; Huang, Z.; Chen, Y.; Yang, M.; Qin, X.; Feng, Z. Structural changes and enhanced accessibility of natural cellulose pretreated by mechanical activation. Polym. Bull. 2014, 71, 453–464. [Google Scholar] [CrossRef]
- Gan, T.; Zhang, Y.; Chen, Y.; Hu, H.; Yang, M.; Huang, Z.; Chen, D.; Huang, A. Reactivity of main components and substituent distribution in esterified sugarcane bagasse prepared by effective solid phase reaction. Carbohydr. Polym. 2018, 181, 633–641. [Google Scholar] [CrossRef]
- Burton, A.W.; Ong, K.; Rea, T.; Chan, I.Y. On the estimation of average crystallite size of zeolites from the Scherrer equation: A critical evaluation of its application to zeolites with one-dimensional pore systems. Microporous Mesoporous Mat. 2009, 117, 75–90. [Google Scholar] [CrossRef]
- Xu, L.; Wang, J. Fenton-like degradation of 2,4-dichlorophenol using Fe3O4 magnetic nanoparticles. Appl. Catal. B-Environ. 2012, 123, 117–126. [Google Scholar] [CrossRef]
- Gan, T.; Zhang, Y.; Su, Y.; Hu, H.; Huang, A.; Huang, Z.; Chen, D.; Yang, M.; Wu, J. Esterification of bagasse cellulose with metal salts as efficient catalyst in mechanical activation-assisted solid phase reaction system. Cellulose 2017, 24, 5371–5387. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, L.; Liu, S.; Liu, Y.; Xu, X.; Chen, X.; Chu, B.; Guo, X.; Xu, J.; Cheng, H.; et al. Dynamic Self-Assembly Induced Rapid Dissolution of Cellulose at Low Temperatures. Macromolecules 2008, 41, 9345–9351. [Google Scholar] [CrossRef]
- Li, X.; He, Y.; Sui, H.; He, L. One-Step Fabrication of Dual Responsive Lignin Coated Fe3O4 Nanoparticles for Efficient Removal of Cationic and Anionic Dyes. Nanomaterials 2018, 8, 162. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Yang, J.; Zheng, W.; Wang, X.; Xiang, C.; Tang, L.; Zhang, W.; Shiyan Chen, H.W. Synthesis of flexible magnetic nanohybrid based on bacterial cellulose under ultrasonic irradiation. Mater. Sci. Eng. C 2013, 33, 2407–2412. [Google Scholar] [CrossRef] [PubMed]
- Lan, W.; Liu, C.; Yue, F.; Sun, R.; Kennedy, J.F. Ultrasound-assisted dissolution of cellulose in ionic liquid. Carbohydr. Polym. 2011, 86, 672–677. [Google Scholar] [CrossRef]
- Luo, X.; Liu, S.; Zhou, J.; Zhang, L. In situ synthesis of Fe3O4/cellulose microspheres with magnetic-induced protein delivery. J. Mater. Chem. 2009, 19, 3538. [Google Scholar] [CrossRef]
- Huang, Z.; Ye, Y.; Zhu, S.; Yao, Y.; Lu, W.; Chen, W. Enhanced catalytic decoloration of Rhodamine B based on 4-aminopyridine iron coupled with cellulose fibers. J. Chem. Technol. Biotechnol. 2015, 90, 1144–1151. [Google Scholar] [CrossRef]
- Fan, T.; Zhao, Z.; Zhou, J.; Li, L.; Liu, Y.; Lu, M. Fabrication of magnetic cotton fabrics using surface micro-dissolving technology in ZnCl2 aqueous solution. Cellulose 2018, 25, 1437–1447. [Google Scholar] [CrossRef]
- Ma, J.; Xing, J.; Wang, K.; Yang, H.; Fei, B.; Liu, X. Inspired by efficient cellulose-dissolving system: Facile one-pot synthesis of biomass-based hydrothermal magnetic carbonaceous materials. Carbohydr. Polym. 2017, 164, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.; Zhang, J.; Lin, K.; Chen, J. Fe3O4 nanocubes assembled on RGO nanosheets: Ultrasound induced in situ and eco-friendly synthesis, characterization and their excellent catalytic performance for the production of liquid fuel in Fischer-tropsch synthesis. Ultrason. Sonochem. 2018, 42, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Wang, J.; Li, F.; Huang, X.; Tian, B.; Hao, H. Highly Efficient and Reusable Montmorillonite/Fe3O4/Humic Acid Nanocomposites for Simultaneous Removal of Cr(VI) and Aniline. Nanomaterials 2018, 8, 537. [Google Scholar] [CrossRef] [PubMed]
- Grosvenor, A.P.; Kobe, B.A.; Biesinger, M.C.; McIntyre, N.S. Investigation of multiplet splitting of Fe 2p XPS spectra and bonding in iron compounds. Surf. Interface Anal. 2004, 36, 1564–1574. [Google Scholar] [CrossRef]
- Ruan, D.; Lue, A.; Zhang, L. Gelation behaviors of cellulose solution dissolved in aqueous NaOH/thiourea at low temperature. Polymer 2008, 49, 1027–1036. [Google Scholar] [CrossRef]
- Cai, J.; Zhang, L. Unique Gelation Behavior of Cellulose in NaOH/Urea Aqueous Solution. Biomacromolecules 2006, 7, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Hoque, M.; Guzman, M. Photocatalytic Activity: Experimental Features to Report in Heterogeneous Photocatalysis. Materials 2018, 11, 1990. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, B.; Deng, Y.; Chen, H.; Luo, S.; Sun, C.; Yang, P.; Yang, S. Adsorption and heterogeneous Fenton degradation of 17α-methyltestosterone on nano Fe3O4/MWCNTs in aqueous solution. Appl. Catal. B-Environ. 2011, 107, 274–283. [Google Scholar] [CrossRef]
- Luo, W.; Zhu, L.; Wang, N.; Tang, H.; Cao, M.; She, Y. Efficient Removal of Organic Pollutants with Magnetic Nanoscaled BiFeO3 as a Reusable Heterogeneous Fenton-Like Catalyst. Environ. Sci. Technol. 2010, 44, 1786–1791. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Y.; Li, T.; Zhou, M. Heterogeneous Fenton catalytic degradation of phenol based on controlled release of magnetic nanoparticles. Chem. Eng. J. 2014, 242, 1–9. [Google Scholar] [CrossRef]
- Zhou, B.; Zhao, X.; Liu, H.; Qu, J.; Huang, C.P. Visible-light sensitive cobalt-doped BiVO4 (Co-BiVO4) photocatalytic composites for the degradation of methylene blue dye in dilute aqueous solutions. Appl. Catal. B-Environ. 2010, 99, 214–221. [Google Scholar] [CrossRef]
- Luo, X.; Zhang, L. High effective adsorption of organic dyes on magnetic cellulose beads entrapping activated carbon. J. Hazard. Mater. 2009, 171, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Sabhi, S.; Kiwi, J. Degradation of 2, 4-dichlorophenol by immobilized iron catalysts. Water Res. 2001, 35, 1994–2002. [Google Scholar] [CrossRef]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Q.; Zhang, Y.; Hu, H.; Wang, W.; Huang, Z.; Chen, D.; Yang, M.; Liang, J. In Situ Synthesis of a Stable Fe3O4@Cellulose Nanocomposite for Efficient Catalytic Degradation of Methylene Blue. Nanomaterials 2019, 9, 275. https://doi.org/10.3390/nano9020275
Lu Q, Zhang Y, Hu H, Wang W, Huang Z, Chen D, Yang M, Liang J. In Situ Synthesis of a Stable Fe3O4@Cellulose Nanocomposite for Efficient Catalytic Degradation of Methylene Blue. Nanomaterials. 2019; 9(2):275. https://doi.org/10.3390/nano9020275
Chicago/Turabian StyleLu, Quan, Yanjuan Zhang, Huayu Hu, Wen Wang, Zuqiang Huang, Dong Chen, Mei Yang, and Jing Liang. 2019. "In Situ Synthesis of a Stable Fe3O4@Cellulose Nanocomposite for Efficient Catalytic Degradation of Methylene Blue" Nanomaterials 9, no. 2: 275. https://doi.org/10.3390/nano9020275





