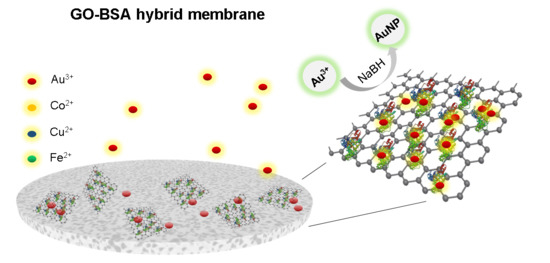
Removing Metal Ions from Water with Graphene–Bovine Serum Albumin Hybrid Membrane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Synthesis of GO–BSA Nanohybrids
2.3. Fabrication of GO–BSA Membranes
2.4. Removing Metallic Ions with Membranes
2.5. Characterization Techniques
3. Results and Discussion
3.1. Morphological Characterization of GO–BSA Membranes
3.2. Structural and Property Characterizations of GO–BSA Membranes
3.3. Separation of Metal Ions by GO–BSA Membranes
3.4. Reduction of Au3+ on GO–BSA Membranes
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shannon, M.A.; Bohn, P.W.; Elimelech, M.; Georgiadis, J.G.; Marinas, B.J.; Mayes, A.M. Science and technology for water purification in the coming decades. Nature 2008, 452, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Ivanets, A.I.; Kitikova, N.V.; Shashkova, I.L.; Oleksiienko, O.V.; Levchuk, I.; Sillanpää, M. Removal of Zn 2+, Fe 2+, Cu 2+, Pb 2+, Cd 2+, Ni 2+ and Co 2+ ions from aqueous solutions using modified phosphate dolomite. J. Environ. Chem. Eng. 2014, 2, 981–987. [Google Scholar] [CrossRef]
- Lin, S.; Reddy, D.H.K.; Bediako, J.K.; Song, M.H.; Wei, W.; Kim, J.A.; Yun, Y.S. Effective adsorption of Pd(II), Pt(IV) and Au(III) by Zr(IV)-based metal-organic frameworks from strongly acidic solutions. J. Mater. Chem. A 2017, 5, 13557–13564. [Google Scholar] [CrossRef]
- Zhang, W.S.; Yu, X.Q.; Li, Y.; Su, Z.Q.; Jandt, K.D.; Wei, G. Protein-mimetic peptide nanofibers: Motif design, self-assembly synthesis, and sequence-specific biomedical applications. Prog. Polym. Sci. 2018, 80, 94–124. [Google Scholar] [CrossRef]
- Zhang, W.S.; Lin, D.M.; Wang, H.X.; Li, J.F.; Nienhaus, G.U.; Su, Z.Q.; Wei, G.; Shang, L. Supramolecular self-assembly bioinspired synthesis of luminescent gold nanocluster-embedded peptide nanofibers for temperature sensing and cellular imaging. Bioconjugate Chem. 2017, 28, 2224–2229. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Su, Z.Q.; Reynolds, N.P.; Arosio, P.; Hamley, I.W.; Gazit, E.; Mezzenga, R. Self-assembling peptide and protein amyloids: from structure to tailored function in nanotechnology. Chem. Soc. Rev. 2017, 46, 4661–4708. [Google Scholar] [CrossRef] [PubMed]
- Bolisetty, S.; Reinhold, N.; Zeder, C.; Orozco, M.N.; Mezzenga, R. Efficient purification of arsenic-contaminated water using amyloid-carbon hybrid membranes. Chem. Commun. 2017, 53, 5714–5717. [Google Scholar] [CrossRef] [PubMed]
- Bolisetty, S.; Mezzenga, R. Amyloid-carbon hybrid membranes for universal water purification. Nat. Nanotechnol. 2016, 11, 365–367. [Google Scholar] [CrossRef]
- Xie, J.P.; Zheng, Y.G.; Ying, J.Y. Protein-directed synthesis of highly fluorescent gold nanoclusters. J. Am. Chem. Soc. 2009, 131, 888–889. [Google Scholar] [CrossRef]
- Lu, F.; Zhang, S.H.; Gao, H.J.; Jia, H.; Zheng, L.Q. Protein-decorated reduced oxide graphene composite and its application to SERS. ACS Appl. Mater. Interfaces 2012, 4, 3278–3284. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Kim, H.; Lee, Y.; Xu, X.F.; Park, J.S.; Zheng, Y.; Balakrishnan, J.; Lei, T.; Kim, H.R.; Song, Y.I.; et al. Roll-to-roll production of 30-inch graphene films for transparent electrodes. Nat. Nanotechnol. 2010, 5, 574–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Zhang, Y.J.; Wu, A.G.; Wei, G. Designed graphene-peptide nanocomposites for biosensor applications: A review. Anal. Chim. Acta 2017, 985, 24–40. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, A.G.; Wei, G. Graphene-based aptasensors: from molecule-interface interactions to sensor design and biomedical diagnostics. Analyst 2018, 143, 1526–1543. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Q.; Ciacchi, L.C.; Wei, G. Recent advances in the synthesis of graphene-based nanomaterials for controlled drug delivery. Appl. Sci.-Basel 2017, 7, 1175. [Google Scholar] [CrossRef]
- Zhang, H.C.; Gruner, G.; Zhao, Y.L. Recent advancements of graphene in biomedicine. J. Mater. Chem. B 2013, 1, 2542–2567. [Google Scholar] [CrossRef]
- Wang, H.X.; Sun, D.M.; Zhao, N.N.; Yang, X.C.; Shi, Y.Z.; Li, J.F.; Su, Z.Q.; Wei, G. Thermo-sensitive graphene oxide-polymer nanoparticle hybrids: Synthesis, characterization, biocompatibility and drug delivery. J. Mater. Chem. B 2014, 2, 1362–1370. [Google Scholar] [CrossRef]
- Li, X.L.; Zhi, L.J. Graphene hybridization for energy storage applications. Chem. Soc. Rev. 2018, 47, 3189–3216. [Google Scholar] [CrossRef]
- Perreault, F.; de Faria, A.F.; Elimelech, M. Environmental applications of graphene-based nanomaterials. Chem. Soc. Rev. 2015, 44, 5861–5896. [Google Scholar] [CrossRef]
- Li, A.H.; Liu, J.Q.; Feng, S.Y. Applications of graphene based materials in energy and environmental science. Sci. Adv. Mater. 2014, 6, 209–234. [Google Scholar] [CrossRef]
- Zhao, X.N.; Zhang, P.P.; Chen, Y.T.; Su, Z.Q.; Wei, G. Recent advances in the fabrication and structure-specific applications of graphene-based inorganic hybrid membranes. Nanoscale 2015, 7, 5080–5093. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, P.P.; Ouyang, Z.F.; Zhang, M.F.; Lin, Z.J.; Li, J.F.; Su, Z.Q.; Wei, G. Nanoscale graphene doped with highly dispersed silver nanoparticles: Quick synthesis, facile fabrication of 3D membrane-modified electrode, and super performance for electrochemical sensing. Adv. Funct. Mater. 2016, 26, 2122–2134. [Google Scholar] [CrossRef]
- Yu, X.Q.; Zhang, W.S.; Zhang, P.P.; Su, Z.Q. Fabrication technologies and sensing applications of graphene-based composite films: Advances and challenges. Biosens. Bioelectron. 2017, 89, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, Y.; Su, X.G. A small-molecule-linked DNA-graphene oxide-based fluorescence-sensing system for detection of biotin. Anal. Biochem. 2013, 442, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Zhao, X.J.; Zhang, P.P.; Ning, J.; Li, J.F.; Su, Z.Q.; Wei, G. A facile fabrication of large-scale reduced graphene oxide-silver nanoparticle hybrid film as a highly active surface-enhanced Raman scattering substrate. J. Mater. Chem. C 2015, 3, 4126–4133. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, Y.; Zhen, S.J.; Huang, C.Z. A graphene oxide enhanced fluorescence anisotropy strategy for DNAzyme-based assay of metal ions. Chem. Commun. 2013, 49, 1942–1944. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.W.; Murali, S.; Cai, W.W.; Li, X.S.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, W.; Lu, Z.; Li, J.; Su, Z.; Gang, W. Sequence-designed peptide nanofibers bridged conjugation of graphene quantum dots with graphene oxide for high performance electrochemical hydrogen peroxide biosensor. Adv. Mater. Interfaces 2017, 4, 1600895. [Google Scholar]
- Su, Z.; Shen, H.; Wang, H.; Wang, J.; Li, J.; Nienhaus, G.U.; Li, S.; Gang, W. Motif-designed peptide nanofibers decorated with graphene quantum dots for simultaneous targeting and imaging of tumor cells. Adv. Funct. Mater. 2015, 25, 5472–5478. [Google Scholar] [CrossRef]
- Ali, I.; Alharbi, O.M.L.; Tkachev, A.; Galunin, E.; Burakov, A.; Grachev, V.A. Water treatment by new-generation graphene materials: Hope for bright future. Environ. Sci. Pollut. R 2018, 25, 7315–7329. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Bazylewski, P.; Fanchini, G. Porous graphene-based membranes for water purification from metal ions at low differential pressures. Nanoscale 2016, 8, 9563–9571. [Google Scholar] [CrossRef] [PubMed]
- Tabish, T.A.; Memon, F.A.; Gomez, D.E.; Horsell, D.W.; Zhang, S.W. A facile synthesis of porous graphene for efficient water and wastewater treatment. Sci. Rep. 2018, 8, 1817–1828. [Google Scholar] [CrossRef] [PubMed]
- Suarez-Iglesias, O.; Collado, S.; Oulego, P.; Diaz, M. Graphene-family nanomaterials in wastewater treatment plants. Chem. Eng. J. 2017, 313, 121–135. [Google Scholar] [CrossRef]
- Han, L.; Mao, D.; Huang, Y.C.; Zheng, L.J.; Yuan, Y.; Su, Y.; Sun, S.Y.; Fang, D. Fabrication of unique Tin(IV) Sulfide/Graphene Oxide for photocatalytically treating chromium(VI)-containing wastewater. J. Clean. Prod. 2017, 168, 519–525. [Google Scholar] [CrossRef]
- Kim, S.; Nham, J.; Jeong, Y.S.; Lee, C.S.; Ha, S.H.; Park, H.B.; Lee, Y.J. Biomimetic selective ion transport through graphene oxide membranes functionalized with ion recognizing peptides. Chem. Mater. 2015, 27, 1255–1261. [Google Scholar] [CrossRef]
- Zhang, M.F.; Li, Y.; Su, Z.Q.; Wei, G. Recent advances in the synthesis and applications of graphene-polymer nanocomposites. Polym. Chem. 2015, 6, 6107–6124. [Google Scholar] [CrossRef]
- Yu, X.Q.; Wang, Z.P.; Su, Z.Q.; Wei, G. Design, fabrication, and biomedical applications of bioinspired peptide-inorganic nanomaterial hybrids. J. Mater. Chem. B 2017, 5, 1130–1142. [Google Scholar] [CrossRef]
- Li, D.P.; Zhang, W.S.; Yu, X.Q.; Wang, Z.P.; Su, Z.Q.; Wei, G. When biomolecules meet graphene: From molecular level interactions to material design and applications. Nanoscale 2016, 8, 19491–19509. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, H.M.; Chen, S.; Yu, H.T.; Quan, X. Stimuli-responsive peroxidase mimicking at a smart graphene interface. Chem. Commun. 2012, 48, 7055–7057. [Google Scholar] [CrossRef]
- Yu, X.Q.; Liu, W.; Deng, X.L.; Yan, S.Y.; Su, Z.Q. Gold nanocluster embedded bovine serum albumin nanofibers-graphene hybrid membranes for the efficient detection and separation of mercury ion. Chem. Eng. J. 2018, 335, 176–184. [Google Scholar] [CrossRef]
- Chiu, N.F.; Fan, S.Y.; Yang, C.D.; Huang, T.Y. Carboxyl-functionalized graphene oxide composites as SPR biosensors with enhanced sensitivity for immunoaffinity detection. Biosens. Bioelectron. 2017, 89, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Lv, R.D.; Li, L.; Wang, Y.G.; Chen, Z.D.; Liu, S.C.; Wang, X.; Wang, J.; Li, Y.F. Carboxyl graphene oxide solution saturable absorber for femtosecond mode-locked erbium-doped fiber laser. Chin. Phys. B 2018, 27, 114214. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, X.; Cai, H.; Chen, Z.; Wang, T.; Jia, L.; Wang, J.; Wan, Q.; Pei, X. Osteogenic activity and antibacterial effect of zinc oxide/carboxylated graphene oxide nanocomposites: Preparation and in vitro evaluation. Colloids Surf. B 2016, 147, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.J.; Hofmann, M.; Cheng, Y.C.; Huang, C.W.; Chang, K.W.; Lee, J.Y. Characterization of graphene edge functionalization by grating enhanced Raman spectroscopy. RSC Adv. 2016, 6, 12398–12401. [Google Scholar] [CrossRef]
- Zhao, X.J.; Li, Y.; Wang, J.H.; Ouyang, Z.F.; Li, J.F.; Wei, G.; Su, Z.Q. Interactive oxidation-reduction reaction for the in situ synthesis of graphene-phenol formaldehyde composites with enhanced properties. ACS Appl. Mater. Interfaces 2014, 6, 4254–4263. [Google Scholar] [CrossRef] [PubMed]
- Stobinski, L.; Lesiak, B.; Malolepszy, A.; Mazurkiewicz, M.; Mierzwa, B.; Zemek, J.; Jiricek, P.; Bieloshapka, I. Graphene oxide and reduced graphene oxide studied by the XRD, TEM and electron spectroscopy methods. J. Electron. Spectrosc. Relat. Phenom. 2014, 195, 145–154. [Google Scholar] [CrossRef]
- Zhang, B.; Li, P.; Zhang, H.; Li, X.; Tian, L.; Wang, H.; Chen, X.; Ali, N.; Ali, Z.; Zhang, Q. Red-blood-cell-like BSA/Zn3(PO4)2 hybrid particles: preparation and application to adsorption of heavy metal ions. Appl. Surf. Sci. 2016, 366, 328–338. [Google Scholar] [CrossRef]
- Wright, A.K.; Thompson, M.R. Hydrodynamic structure of BSA determined by transient electric birefringence. Biophys. J. 1975, 15, 137–141. [Google Scholar] [CrossRef]
- Liu, J.B.; Fu, S.H.; Yuan, B.; Li, Y.L.; Deng, Z.X. Toward a universal "adhesive nanosheet" for the assembly of multiple nanoparticles based on a protein-induced reduction/decoration of graphene oxide. J. Am. Chem. Soc. 2010, 132, 7279–7281. [Google Scholar] [CrossRef]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Sun, S.; Zhou, L.; Miao, Z.; Zhang, X.; Su, Z.; Wei, G. Removing Metal Ions from Water with Graphene–Bovine Serum Albumin Hybrid Membrane. Nanomaterials 2019, 9, 276. https://doi.org/10.3390/nano9020276
Yu X, Sun S, Zhou L, Miao Z, Zhang X, Su Z, Wei G. Removing Metal Ions from Water with Graphene–Bovine Serum Albumin Hybrid Membrane. Nanomaterials. 2019; 9(2):276. https://doi.org/10.3390/nano9020276
Chicago/Turabian StyleYu, Xiaoqing, Shuwei Sun, Lin Zhou, Zhicong Miao, Xiaoyuan Zhang, Zhiqiang Su, and Gang Wei. 2019. "Removing Metal Ions from Water with Graphene–Bovine Serum Albumin Hybrid Membrane" Nanomaterials 9, no. 2: 276. https://doi.org/10.3390/nano9020276