A Porous Carbon with Excellent Gas Storage Properties from Waste Polystyrene
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Vibrational Characterization
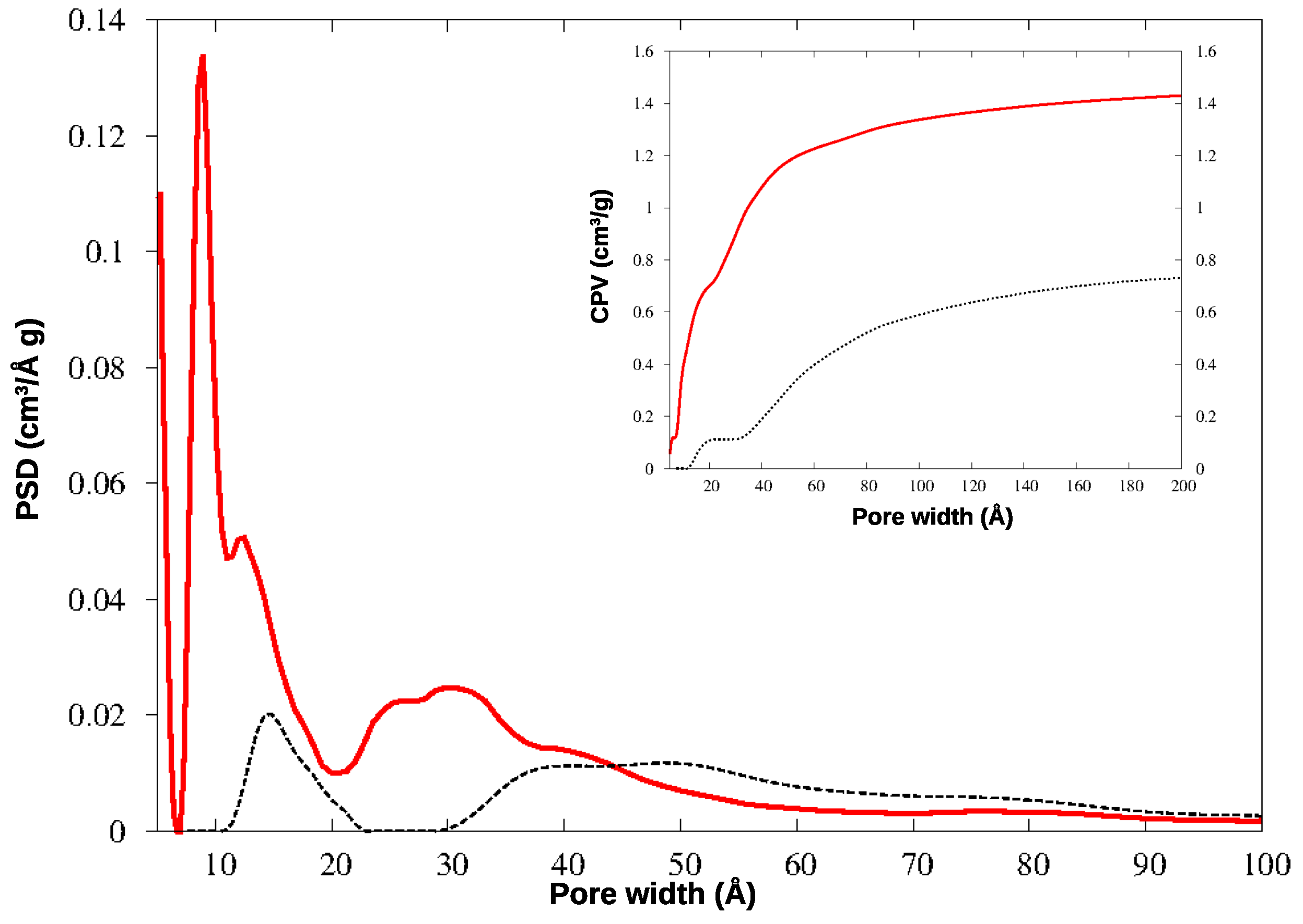
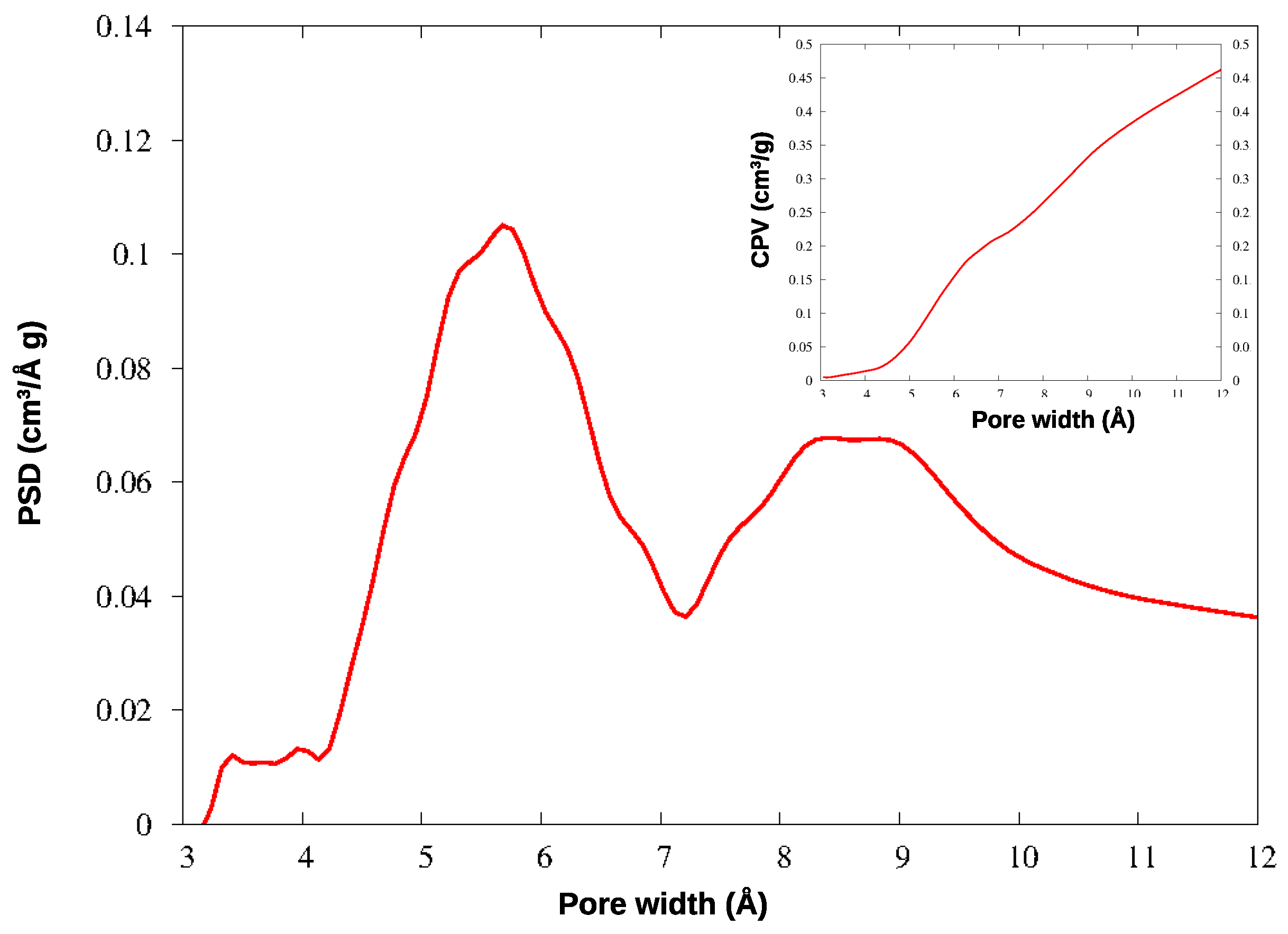
3.2. Pore-Size Analysis
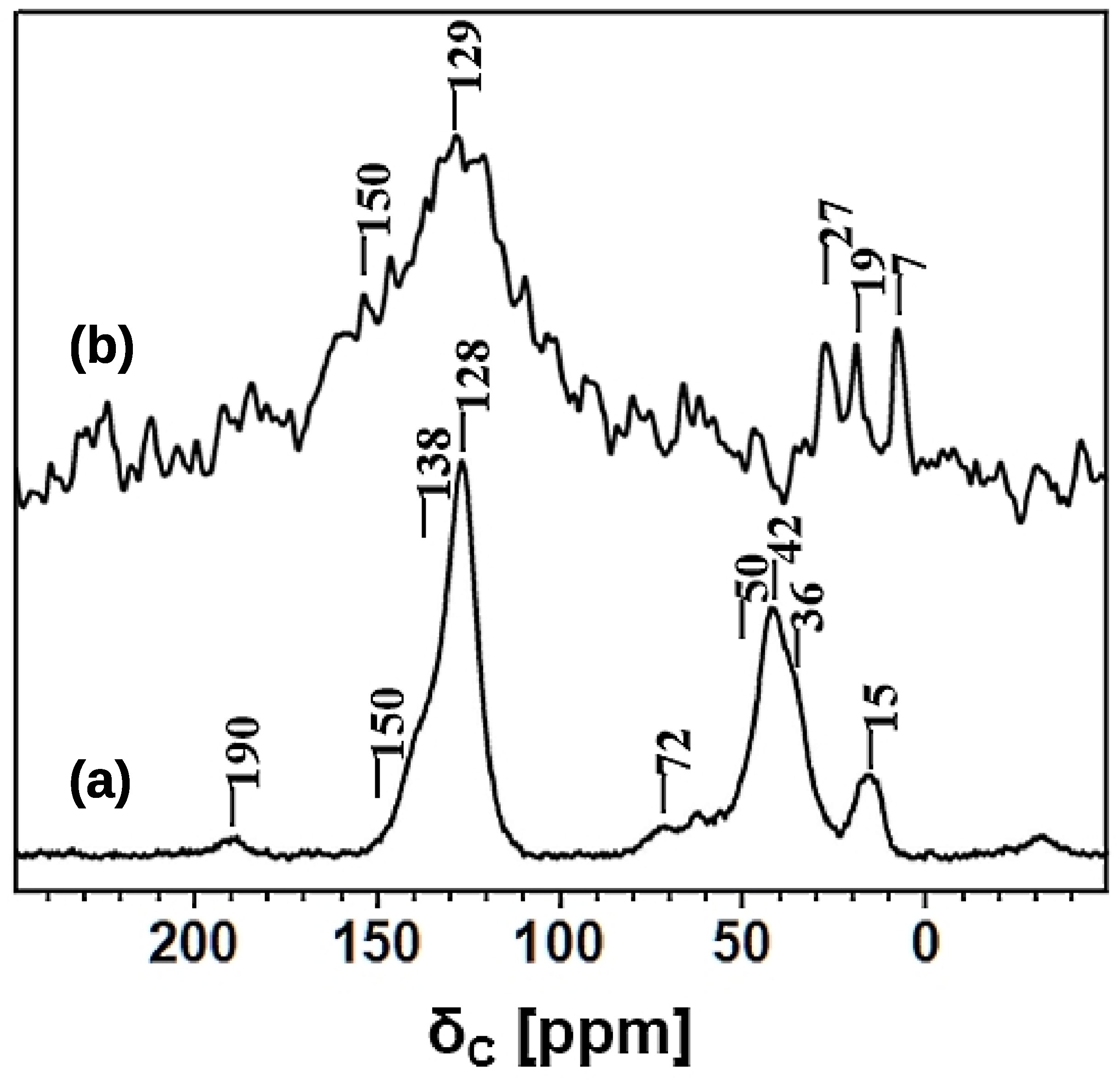
3.3. Solid State NMR
3.4. Methane Adsorption
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Byamba-Ochir, N.; Shim, W.; Balathanigaimani, M.; Moon, H. High Density Mongolian Anthracite Based Porous Carbon Monoliths for Methane Storage By Adsorption. Appl. Energy 2017, 190, 257–265. [Google Scholar] [CrossRef]
- Rash, T.; Gillespie, A.; Holbrook, B.; Hiltzik, L.; Romanos, J.; Soo, Y.; Sweany, S.; Pfeifer, P. Microporous Carbon Monolith Synthesis and Production for Methane Storage. Fuel 2017, 200, 371–379. [Google Scholar] [CrossRef]
- Kizzie, A.; Dailly, A.; Perry, L.; Lail, M.; Lu, W.; Nelson, T.; Cai, M.; Zhou, H.C. Enhanced Methane Sorption in Densified Forms of a Porous Polymer Network. Mater. Sci. Appl. 2014, 5, 387–394. [Google Scholar] [CrossRef]
- Casco, M.; Martínez-Escandell, M.; Kaneko, K.; Silvestre-Albero, J.; Rodríguez-Reinoso, F. Very High Methane Uptake on Activated Carbons Prepared from Mesophase Pitch: A Compromise Between Microporosity and Bulk Density. Carbon 2015, 93, 11–21. [Google Scholar] [CrossRef]
- Simon, C.; Kim, J.; Gomez-Gualdron, D.; Camp, J.; Chung, Y.; Martin, R.; Mercado, R.; Deem, M.; Gunter, D.; Haranczyk, M.; et al. The Materials Genome in Action: Identifying the Performance Limits for Methane Storage. Energy Environ. Sci. 2015, 8, 1190–1199. [Google Scholar] [CrossRef]
- Makal, T.; Li, J.R.; Lu, W.; Zhou, H.C. Methane Storage in Advanced Porous Materials. Chem. Soc. Rev. 2012, 41, 7761–7779. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhou, W.; Yildirim, T.; Chen, B. A Series of Metal-organic Frameworks with High Methane Uptake and An Empirical Equation for Predicting Methane Storage Capacity. Energy Environ. Sci. 2013, 6, 2735–2744. [Google Scholar] [CrossRef]
- Konstas, K.; Osl, T.; Yang, Y.; Batten, M.; Burke, N.; Hill, A.; Hill, M. Methane Storage in Metal Organic Frameworks. J. Mater. Chem. 2012, 22, 16698–16708. [Google Scholar] [CrossRef]
- Furukawa, H.; Yaghi, O. Storage of Hydrogen, Methane, and Carbon Dioxide in Highly Porous Covalent Organic Frameworks for Clean Energy Applications. J. Am. Chem. Soc. 2009, 131, 8875–8883. [Google Scholar] [CrossRef]
- Feng, X.; Ding, X.; Jiang, D. Covalent Organic Frameworks. Chem. Soc. Rev. 2012, 41, 6010–6022. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Cao, D. Porous Covalent-organic Materials: Synthesis, Clean Energy Application and Design. J. Mater. Chem. A 2013, 1, 2691–2718. [Google Scholar] [CrossRef]
- Yuan, D.; Lu, W.; Zhao, D.; Zhou, H.C. Highly Stable Porous Polymer Networks with Exceptionally High Gas-uptake Capacities. Adv. Mater. 2011, 23, 3723–3725. [Google Scholar] [CrossRef]
- Ben, T.; Pei, C.; Zhang, D.; Xu, J.; Deng, F.; Jing, X.; Qiu, S. Gas Storage in Porous Aromatic Frameworks (PAFs). Energy Environ. Sci. 2011, 4, 3991–3999. [Google Scholar] [CrossRef]
- Wood, C.; Tan, B.; Trewin, A.; Su, F.; Rosseinsky, M.; Bradshaw, D.; Sun, Y.; Zhou, L.; Cooper, A. Microporous Organic Polymers for Methane Storage. Adv. Mater. 2008, 20, 1916–1921. [Google Scholar] [CrossRef]
- Cooper, A. Conjugated Microporous Polymers. Adv. Mater. 2009, 21, 1291–1295. [Google Scholar] [CrossRef]
- Lee, S.J.; Bae, Y.S. Review of Molecular Simulations of Methane Storage in Metal-organic Frameworks. J. Nanosci. Nanotech. 2016, 16, 4284–4290. [Google Scholar] [CrossRef]
- Marco-Lozar, J.; Kunowsky, M.; Carruthers, J.; Linares-Solano, A. Gas Storage Scale-up at Room Temperature on High Density Carbon Materials. Carbon 2014, 76, 123–132. [Google Scholar] [CrossRef]
- Casco, M.; Martínez-Escandell, M.; Gadea-Ramos, E.; Kaneko, K.; Silvestre-Albero, J.; Rodríguez-Reinoso, F. High-pressure Methane Storage in Porous Materials: Are Carbon Materials in the Pole Position? Chem. Mater. 2015, 27, 959–964. [Google Scholar] [CrossRef]
- Alonso, A.; Moral-Vico, J.; Abo Markeb, A.; Busquets-Fité, M.; Komilis, D.; Puntes, V.; Sánchez, A.; Font, X. Critical Review of Existing Nanomaterial Adsorbents to Capture Carbon Dioxide and Methane. Sci. Total Environ. 2017, 595, 51–62. [Google Scholar] [CrossRef]
- Marco-Lozar, J.; Kunowsky, M.; Suárez-García, F.; Carruthers, J.; Linares-Solano, A. Activated Carbon Monoliths for Gas Storage At Room Temperature. Energy Environ. Sci. 2012, 5, 9833–9842. [Google Scholar] [CrossRef]
- Marco-Lozar, J.; Juan-Juan, J.; Suárez-García, F.; Cazorla-Amorós, D.; Linares-Solano, A. MOF-5 and Activated Carbons as Adsorbents for Gas Storage. Int. J. Hydrogen Energy 2012, 37, 2370–2381. [Google Scholar] [CrossRef]
- Xu, S.; Luo, Y.; Tan, B. Recent Development of Hypercrosslinked Microporous Organic Polymers. Macromol. Rapid Commun. 2013, 34, 471–484. [Google Scholar] [CrossRef]
- Li, B.; Gong, R.; Wang, W.; Huang, X.; Zhang, W.; Li, H.; Hu, C.; Tan, B. A New Strategy to Microporous Polymers: Knitting Rigid Aromatic Building Blocks By External Cross-linker. Macromolecules 2011, 44, 2410–2414. [Google Scholar] [CrossRef]
- Ding, L.; Gao, H.; Xie, F.; Li, W.; Bai, H.; Li, L. Porosity-Enhanced Polymers from Hyper-Cross-Linked Polymer Precursors. Macromolecules 2017, 50, 956–962. [Google Scholar] [CrossRef]
- Davankov, V.A.; Tsyurupa, M.P. Comprehensive Analytical Chemistry. In Hypercrosslinked Polymeric Networks and Adsorbing Materials; Elsevier: Amsterdam, The Netherlands, 2011; Volume 56. [Google Scholar]
- Slater, A.; Cooper, A. Function-led Design of New Porous Materials. Science 2015, 348, aaa8075. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Tan, B. Hypercrosslinked Porous Polymer Materials: Design , Synthesis, and Applications. Chem. Soc. Rev. 2017, 46, 3322–3356. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Tan, B.; Wood, C. Solution-processable Hypercrosslinked Polymers By Low Cost Strategies: A Promising Platform for Gas Storage and Separation. J. Mater. Chem. A 2016, 4, 15072–15080. [Google Scholar] [CrossRef]
- Dawson, R.; Stöckel, E.; Holst, J.; Adams, D.; Cooper, A. Microporous Organic Polymers for Carbon Dioxide Capture. Energy Environ. Sci. 2011, 4, 4239–4245. [Google Scholar] [CrossRef]
- Jing, X.; Zou, D.; Cui, P.; Ren, H.; Zhu, G. Facile Synthesis of Cost-effective Porous Aromatic Materials with Enhanced Carbon Dioxide Uptake. J. Mater. Chem. A 2013, 1, 13926–13931. [Google Scholar] [CrossRef]
- Errahali, M.; Gatti, G.; Tei, L.; Paul, G.; Rolla, G.A.; Canti, L.; Fraccarollo, A.; Cossi, M.; Comotti, A.; Sozzani, P.; et al. Microporous Hyper-Cross-Linked Aromatic Polymers Designed for Methane and Carbon Dioxide Adsorption. J. Phys. Chem. C 2014, 118, 28699–28710. [Google Scholar] [CrossRef]
- Ratvijitvech, T.; Barrow, M.; Cooper, A.; Adams, D. The Effect of Molecular Weight on the Porosity of Hypercrosslinked Polystyrene. Polym. Chem. 2015, 6, 7280–7285. [Google Scholar] [CrossRef]
- Fu, Z.; Jia, J.; Li, J.; Liu, C. Transforming Waste Expanded Polystyrene Foam Into Hyper-crosslinked Polymers for Carbon Dioxide Capture and Separation. Chem. Eng. J. 2017, 323, 557–564. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, Z.; Yu, Y.; Liu, L.; Wang, G.; Chen, A. Porous Carbon Derived from Waste Polystyrene Foam for Supercapacitor. J. Mater. Sci. 2018, 53, 12115–12122. [Google Scholar] [CrossRef]
- De Paula, F.; de Castro, M.; Ortega, P.; Blanco, C.; Lavall, R.; Santamaría, R. High Value Activated Carbons from Waste Polystyrene Foams. Micropor. Mesopor. Mater. 2018, 267, 181–184. [Google Scholar] [CrossRef]
- Zhang, Y.; Qiang, Z.; Vogt, B.D. Relationship between Crosslinking and Ordering Kinetics for the Fabrication of Soft Templated (FDU-16) Mesoporous Carbon Thin Films. RSC Adv. 2014, 4, 44858–44867. [Google Scholar] [CrossRef]
- Deng, G.; Zhang, Y.; Ye, C.; Qiang, Z.; Stein, G.E.; Cavicchi, K.A.; Vogt, B.D. Bicontinuous Mesoporous Carbon Thin Films Via An Order–order Transition. Chem. Commun. 2014, 50, 12684–12687. [Google Scholar] [CrossRef]
- Zhang, C.; Kong, R.; Wang, X.; Xu, Y.; Wang, F.; Ren, W.; Wang, Y.; Su, F.; Jiang, J.X. Porous Carbons Derived from Hypercrosslinked Porous Polymers for Gas Adsorption and Energy Storage. Carbon 2017, 114, 608–618. [Google Scholar] [CrossRef]
- Modak, A.; Bhaumik, A. Porous Carbon Derived Via KOH Activation of a Hypercrosslinked Porous Organic Polymer for Efficient CO2, CH4, H2 Adsorptions and High CO2/N2 Selectivity. J. Solid State Chem. 2015, 232, 157–162. [Google Scholar] [CrossRef]
- Vinodh, R.; Abidov, A.; Peng, M.; Babu, C.; Palanichamy, M.; Cha, W.; Jang, H.T. Synthesis and Characterization of Semiconducting Porous Carbon for Energy Applications and CO2 Adsorption. J. Ind. Eng. Chem. 2015, 32, 273–281. [Google Scholar] [CrossRef]
- Canti, L.; Fraccarollo, A.; Gatti, G.; Errahali, M.; Marchese, L.; Cossi, M. An Atomistic Model of a Disordered Nanoporous Solid: Interplay Between Monte Carlo Simulations and Gas Adsorption Experiments. AIP Adv. 2017, 7, 045013. [Google Scholar] [CrossRef]
- Gatti, G.; Olivas Olivera, D.; Sacchetto, V.; Cossi, M.; Braschi, I.; Marchese, L.; Bisio, C. Experimental Determination of the Molar Absorption Coefficient of N-Hexane Adsorbed on High-Silica Zeolites. ChemPhysChem 2017, 18, 2374–2380. [Google Scholar] [CrossRef]
- Landers, J.; Gor, G.; Neimark, A. Density Functional Theory Methods for Characterization of Porous Materials. Colloids Surf. A 2013, 437, 3–32. [Google Scholar] [CrossRef]
- Thommes, M.; Cychosz, K.A. Physical Adsorption Characterization of Nanoporous Materials: Progress and Challenges. Adsorption 2014, 20, 233–250. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, L.; Li, F.; Yu, R.; Jin, C. Structural Evolution in the Graphitization Process of Activated Carbon by High-pressure Sintering. Carbon 2009, 47, 744–751. [Google Scholar] [CrossRef]
- Cançado, L.; Takai, K.; Enoki, T.; Endo, M.; Kim, Y.; Mizusaki, H.; Speziali, N.; Jorio, A.; Pimenta, M. Measuring the Degree of Stacking Order in Graphite by Raman Spectroscopy. Carbon 2008, 46, 272–275. [Google Scholar] [CrossRef]
- Joseph, R.; Ford, W.T.; Zhang, S.; Tsyurupa, M.P.; Pastukhov, A.V.; Davankov, V.A. Solid-state 13C-NMR Analysis of Hypercrosslinked Polystyrene. J. Polym. Sci. A Polym. Chem. 1997, 35, 695–701. [Google Scholar] [CrossRef]
- Conte, P.; Carotenuto, G.; Piccolo, A.; Perlo, P.; Nicolais, L. NMR-investigation of the Mechanism of Silver Mercaptide Thermolysis in Amorphous Polystyrene. J. Mater. Chem. 2007, 17, 201–205. [Google Scholar] [CrossRef]
- Woodward, R.T.; Stevens, L.A.; Dawson, R.; Vijayaraghavan, M.; Hasell, T.; Silverwood, I.P.; Ewing, A.V.; Ratvijitvech, T.; Exley, J.D.; Chong, S.Y.; et al. Swellable, Water- and Acid-Tolerant Polymer Sponges for Chemoselective Carbon Dioxide Capture. J. Am. Chem. Soc. 2014, 136, 9028–9035. [Google Scholar] [CrossRef]
- Wang, Z.; Opembe, N.; Kobayashi, T.; Nelson, N.C.; Slowing, I.; Pruski, M. Quantitative Atomic-scale Structure Characterization of Ordered Mesoporous Carbon Materials By Solid State NMR. Carbon 2018, 131, 102–110. [Google Scholar] [CrossRef]
- Vieira, M.A.; Gonçalves, G.R.; Cipriano, D.F.; Schettino, M.A.; Filho, E.A.S.; Cunha, A.G.; Emmerich, F.G.; Freitas, J.C. Synthesis of Graphite Oxide from Milled Graphite Studied by Solid-state 13C Nuclear Magnetic Resonance. Carbon 2016, 98, 496–503. [Google Scholar] [CrossRef]
- Parvez, K.; Wu, Z.S.; Li, R.; Liu, X.; Graf, R.; Feng, X.; Müllen, K. Exfoliation of Graphite into Graphene in Aqueous Solutions of Inorganic Salts. J. Am. Chem. Soc. 2014, 136, 6083–6091. [Google Scholar] [CrossRef]
- Cai, W.; Piner, R.D.; Stadermann, F.J.; Park, S.; Shaibat, M.A.; Ishii, Y.; Yang, D.; Velamakanni, A.; An, S.J.; Stoller, M.; et al. Synthesis and Solid-State NMR Structural Characterization of 13C-Labeled Graphite Oxide. Science 2008, 321, 1815–1817. [Google Scholar] [CrossRef]
- Gao, W.; Alemany, L.; Ci, L.; Ajayan, P. New Insights Into the Structure and Reduction of Graphite Oxide. Nat. Chem. 2009, 1, 403–408. [Google Scholar] [CrossRef]
- Huang, P.; Schwegler, E.; Galli, G. Water Confined in Carbon Nanotubes: Magnetic Response and Proton Chemical Shieldings. J. Phys. Chem. C 2009, 113, 8696–8700. [Google Scholar] [CrossRef]
- Kibalchenko, M.; Payne, M.C.; Yates, J.R. Magnetic Response of Single-Walled Carbon Nanotubes Induced by an External Magnetic Field. ACS Nano 2011, 5, 537–545. [Google Scholar] [CrossRef]
- Forse, A.C.; Griffin, J.M.; Presser, V.; Gogotsi, Y.; Grey, C.P. Ring Current Effects: Factors Affecting the NMR Chemical Shift of Molecules Adsorbed on Porous Carbons. J. Phys. Chem. C 2014, 118, 7508–7514. [Google Scholar] [CrossRef]
- Nguyen, H.; Espinal, L.; van Zee, R.; Thommes, M.; Toman, B.; Hudson, M.; Mangano, E.; Brandani, S.; Broom, D.; Benham, M.; et al. A Reference High-pressure CO2 Adsorption Isotherm for Ammonium ZSM-5 Zeolite: Results of An Interlaboratory Study. Adsorption 2018, 24, 531–539. [Google Scholar] [CrossRef]
- Li, Y.; Ben, T.; Zhang, B.; Fu, Y.; Qiu, S. Ultrahigh Gas Storage Both At Low and High Pressures in KOH-activated Carbonized Porous Aromatic Frameworks. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef]
- Hilton, R.; Bick, P.; Tekeei, A.; Leimkuehler, E.; Pfeifer, P.; Suppes, G. Mass Balance and Performance Analysis of Potassium Hydroxide Activated Carbon. Ind. Eng. Chem. Res. 2012, 51, 9129–9135. [Google Scholar] [CrossRef]







| Material | Total | Microporous | Mesoporous |
|---|---|---|---|
| HCP-PS | 0.74 | 0.11 | 0.63 |
| KPS-1 | 1.42 | 0.70 | 0.72 |
| PC from ref. [34] | 0.7 | – | 0.7 |
| AC800 from ref. [35] | 1.20 | 0.93 | 0.27 |
| Material | Pressure (Bar) | Mass-Based Uptake (g/g) | Volume-Based Uptake (cm3(STP)/cm3) | Bulk Density (g/cm3) | Ref. |
|---|---|---|---|---|---|
| KPS-1 | 30 | 0.209 | 146 | 0.50 | this work |
| LMA738 | 35 | 0.191 | 142 | 0.53 | [18] |
| PY100_700 | 35 | 0.178 | 150 | 0.60 | [4] |
| DO100_700 | 35 | 0.182 | 160 | 0.62 | [4] |
| K-PAF-1-750 | 35 | 0.207 | n. a. | n. a. | [59] |
| KOH-corncob | 35 | 0.25 | n. a. | n. a. | [60] |
| Activ. monolith | 30 | 0.136 | 154 | 0.80 | [17,20] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gatti, G.; Errahali, M.; Tei, L.; Mangano, E.; Brandani, S.; Cossi, M.; Marchese, L. A Porous Carbon with Excellent Gas Storage Properties from Waste Polystyrene. Nanomaterials 2019, 9, 726. https://doi.org/10.3390/nano9050726
Gatti G, Errahali M, Tei L, Mangano E, Brandani S, Cossi M, Marchese L. A Porous Carbon with Excellent Gas Storage Properties from Waste Polystyrene. Nanomaterials. 2019; 9(5):726. https://doi.org/10.3390/nano9050726
Chicago/Turabian StyleGatti, Giorgio, Mina Errahali, Lorenzo Tei, Enzo Mangano, Stefano Brandani, Maurizio Cossi, and Leonardo Marchese. 2019. "A Porous Carbon with Excellent Gas Storage Properties from Waste Polystyrene" Nanomaterials 9, no. 5: 726. https://doi.org/10.3390/nano9050726