A Novel Polymeric Adsorbent Embedded with Phase Change Materials (PCMs) Microcapsules: Synthesis and Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
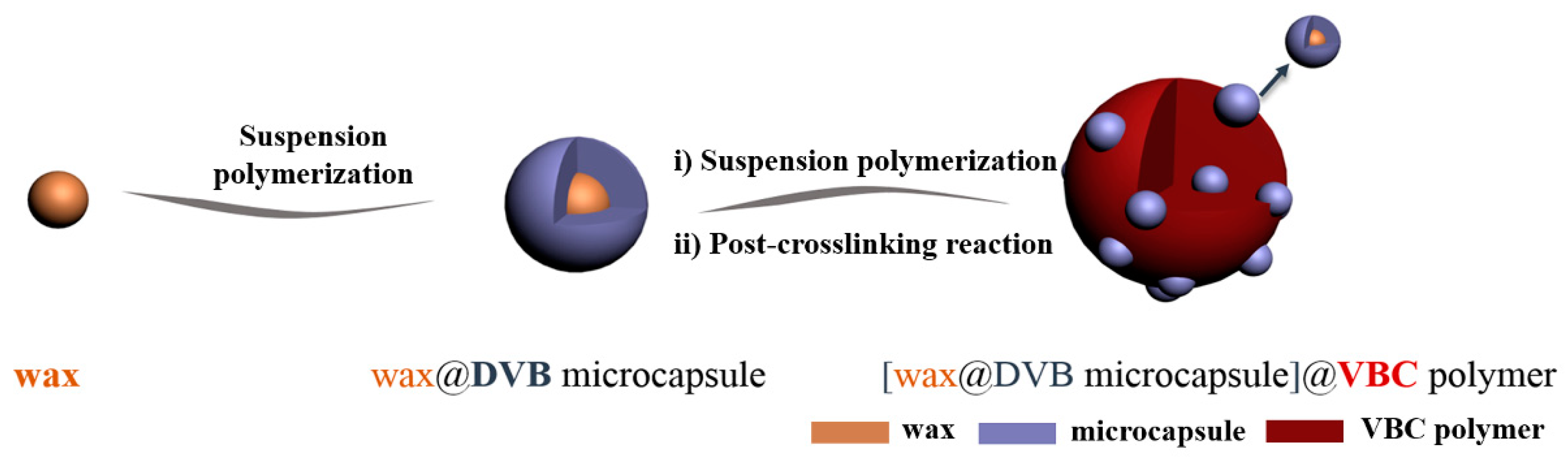
2.2. Preparation of DVB-Wax Microcapsules
2.3. Characterization of DVB-Wax Microcapsules
2.4. Preparation of VBC-DVB Copolymer Embedded with Microcapsules (VPM)
2.5. Preparation of Hypercrosslinked VBC-DVB Beads (HVPM)
2.6. Characterization of Polymeric Beads (VPM and HVPM)
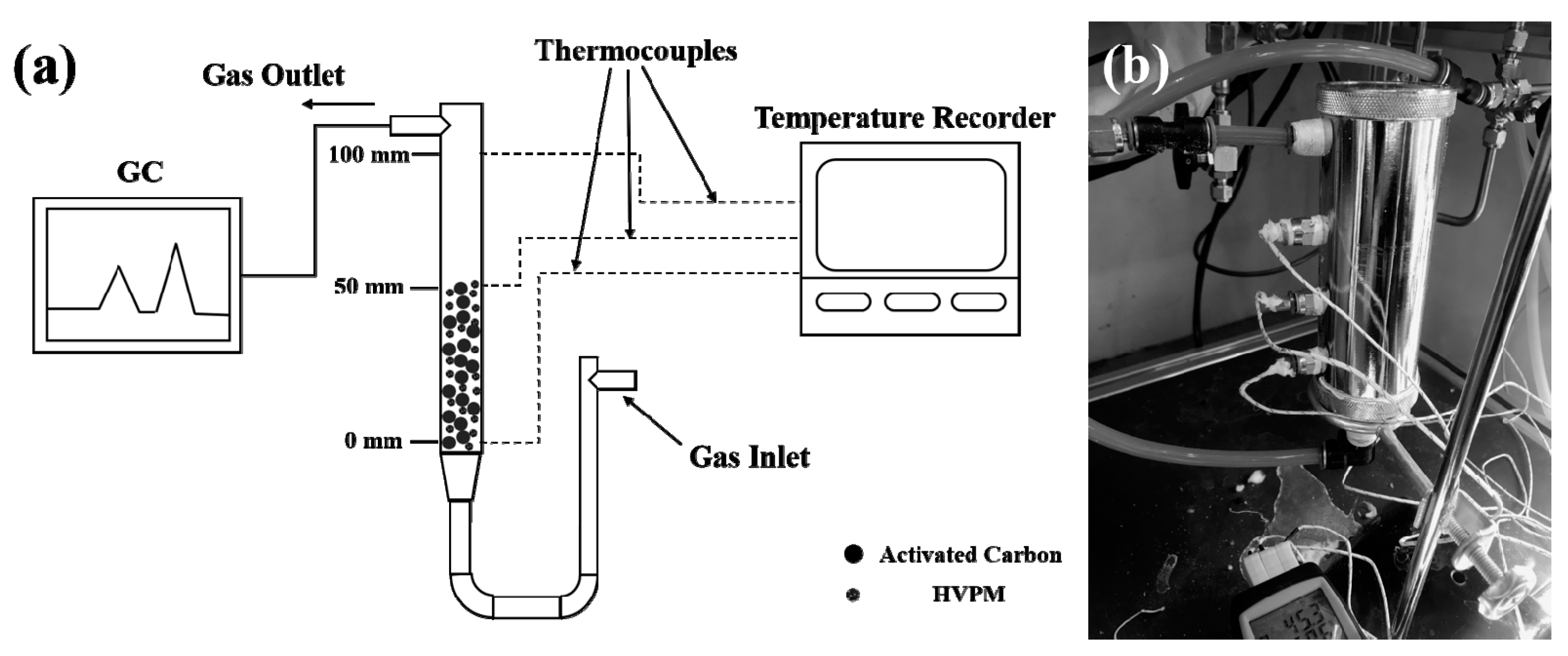
2.7. Dynamic Adsorption and Temperature Control Tests
3. Results and Discussion
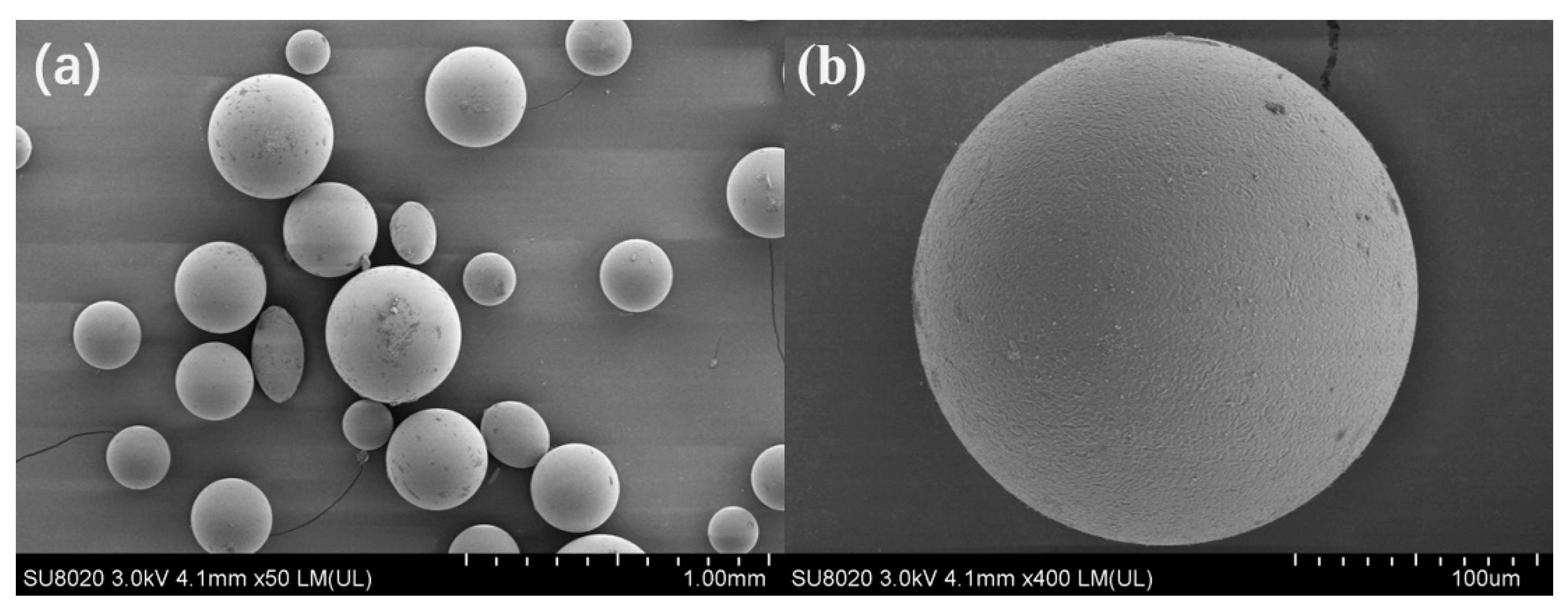
3.1. Characterization of Microcapsules
3.2. Characterization of Polymeric Adsorbents (VPM and HVPM)
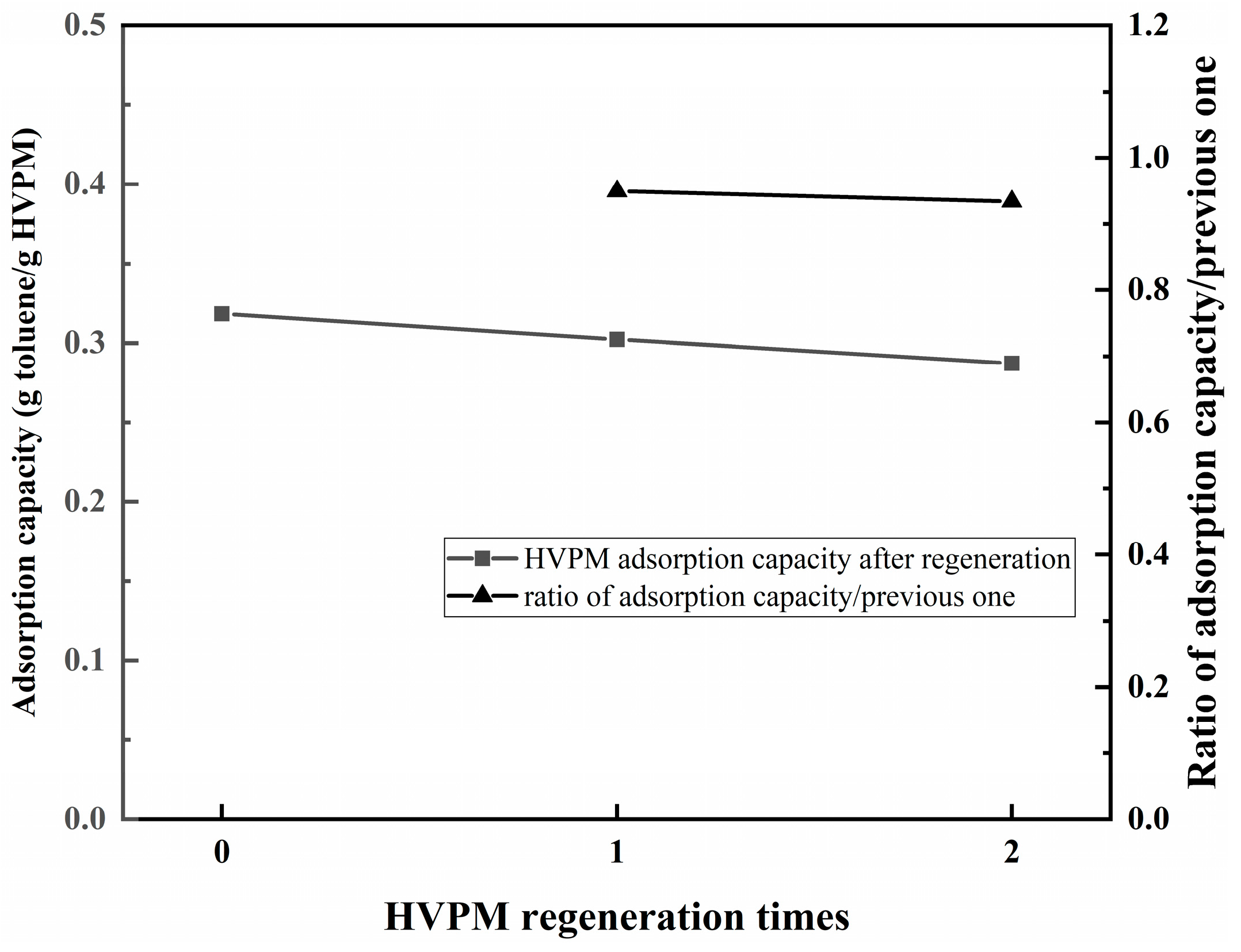
3.3. Dynamic Adsorption and Temperature Control Tests
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Atkinson, R. Atmospheric chemistry of VOCs and NOx. Atmos. Environ. 2000, 34, 2063–2101. [Google Scholar] [CrossRef]
- Gupta, V.K.; Verma, N. Removal of volatile organic compounds by cryogenic condensation followed by adsorption. Chem. Eng. Sci. 2002, 57, 2679–2696. [Google Scholar] [CrossRef]
- Choi, B.S.; Yi, J. Simulation and optimization on the regenerative thermal oxidation of volatile organic compounds. Chem. Eng. J. 2000, 76, 103–114. [Google Scholar] [CrossRef]
- Heneghan, C.S.; Hutchings, G.J.; O’Leary, S.R.; Taylor, S.H.; Boyd, V.J.; Hudson, I.D. A temporal analysis of products study of the mechanism of VOC catalytic oxidation using uranium oxide catalysts. Catal. Today 1999, 54, 3–12. [Google Scholar] [CrossRef]
- Kennes, C.; Veiga, M.C. Fungal biocatalysts in the biofiltration of VOC-polluted air. J. Biotechnol. 2004, 113, 305–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, C.L.; Huang, W.Q.; Wang, Y.X.; Shi, L. Experimental Study on Adsorption of Gasoline Vapor on Activated Carbon at High Concentration. Adv. Mater. Res. 2013, 807–809, 549–552. [Google Scholar] [CrossRef]
- Huang, C.C.; Fair, J.R. Study of the Adsorption and Desorption of Multiple Adsorbates in a Fixed Bed. AICHE J. 2010, 34, 1861–1877. [Google Scholar] [CrossRef]
- Tan, L.; Tan, B. Hypercrosslinked porous polymer materials: design, synthesis, and applications. Chem. Soc. Rev. 2017, 46, 3322. [Google Scholar] [CrossRef]
- Jing, H.; Turner, S.R. Hypercrosslinked Polymers: A Review. Polym. Rev. 2017, 58, 1–41. [Google Scholar] [CrossRef]
- Grassie, N.; Gilks, J. Friedel-Crafts crosslinking of polystyrene. J. Polym. Sci. Pol. Chem. 1973, 11, 1531–1552. [Google Scholar] [CrossRef]
- Saito, T.; Brown, S.; Chatterjee, S.; Kim, J.; Tsouris, C.; Mayes, R.T.; Kuo, L.J.; Gill, G.; Oyola, Y.; Janke, C.J. Uranium recovery from seawater: Development of fiber adsorbents prepared via atom-transfer radical polymerization. J. Mater. Chem. A 2014, 2, 14674–14681. [Google Scholar] [CrossRef]
- Rose, M.; Böhlmann, W.; Sabo, M.; Kaskel, S. Element-organic frameworks with high permanent porosity. Chem. Commun. 2008, 21, 2462–2464. [Google Scholar] [CrossRef]
- Kikkinides, E.S.; Yang, R.T. Gas separation and purification by polymeric adsorbents: Flue gas desulfurization and SO[sub 2] recovery with styrenic polymers. Ind. Eng. Chem. Res. 1993, 32, 2365–2372. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, Y.; Zheng, S.; Park, Y.; Frost, R.L. Preparation and thermal energy storage properties of paraffin/calcined diatomite composites as form-stable phase change materials. Thermochim. Acta 2013, 558, 16–21. [Google Scholar] [CrossRef] [Green Version]
- Guarino, F.; Athienitis, A.; Cellura, M.; Bastien, D. PCM thermal storage design in buildings: Experimental studies and applications to solaria in cold climates. Appl. Energy 2017, 185, 95–106. [Google Scholar] [CrossRef]
- Ürge-Vorsatz, D.; Cabeza, L.F.; Serrano, S.; Barreneche, C.; Petrichenko, K. Heating and cooling energy trends and drivers in buildings. Renew. Sust. Energ. Rev. 2015, 41, 85–98. [Google Scholar] [CrossRef] [Green Version]
- He, B.; Setterwall, F. Technical grade paraffin waxes as phase change materials for cool thermal storage and cool storage systems capital cost estimation. Energy Conv. Manag. 2002, 43, 1709–1723. [Google Scholar] [CrossRef]
- Snoeck, D.; Priem, B.; Dubruel, P.; Belie, N.D. Encapsulated Phase-Change Materials as additives in cementitious materials to promote thermal comfort in concrete constructions. Mater. Struct. 2016, 49, 225–239. [Google Scholar] [CrossRef]
- Luzzi, L.A. Microencapsulation. J. Pharm. Sci. 1970, 59, 1367–1376. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Castellón, C.; Nogués, M.; Medrano, M.; Leppers, R.; Zubillaga, O. Use of microencapsulated PCM in concrete walls for energy savings. Energy Build. 2007, 39, 113–119. [Google Scholar] [CrossRef]
- Shin, Y.; Yoo, D.I.; Son, K. Development of thermoregulating textile materials with microencapsulated Phase Change Materials (PGM). IV. Performance properties and hand of fabrics treated with PCM microcapsules. J. Appl. Polym. Sci. 2010, 97, 910–915. [Google Scholar] [CrossRef]
- Hoang, H.M.; Leducq, D.; Pérez-Masia, R.; Lagaron, J.M.; Gogou, E.; Taoukis, P.; Alvarez, G. Heat transfer study of submicro-encapsulated PCM plate for food packaging application. Int. J. Refrig.-Rev. Int. Froid 2015, 52, 151–160. [Google Scholar] [CrossRef]
- Wei, L.; Rong, Z.; Nan, J.; Tang, X.; Shi, H.; Zhang, X.; Zhang, Y.; Lin, D.; Zhang, N. Composite macrocapsule of phase change materials/expanded graphite for thermal energy storage. Energy 2013, 57, 607–614. [Google Scholar] [CrossRef]
- Xu, M.C.; Shi, Z.Q.; Shi, R.; Liu, J.X.; Lu, Y.; He, B. Synthesis of the adsorbent based on macroporous copolymer MA–DVB beads and its application in purification for the extracts from Ginkgo biloba leaves. React. Funct. Polym. 2000, 43, 297–304. [Google Scholar] [CrossRef]
- Scampini, Z.G.; Aguiar, A.P.D.; Aguiar, M.R.M.P.; Maria, L.C.D.S. Oxime groups introduction in copolymer networks based on acrolein. Mater. Lett. 2004, 58, 3933–3938. [Google Scholar] [CrossRef]
- Chen, Z.H.; Wang, J.; Fei, Y.; Zhang, Z.G.; Gao, X. Preparation and properties of graphene oxide-modified Poly(melamine-formaldehyde) microcapsules containing phase change materials n-dodecanol for thermal energy storage. J. Mater. Chem. A 2015, 3, 11624–11630. [Google Scholar] [CrossRef]
- Sui, H.; An, P.; Li, X.; Cong, S.; He, L. Removal and recovery of o-xylene by silica gel using vacuum swing adsorption. Chem. Eng. J. 2017, 316, 232–242. [Google Scholar] [CrossRef]
- Liang, Z.; Song, X.; Jian, W.; Chao, L.; Li, A.; Zhang, Q. Preparation and characterization of micro-mesoporous hypercrosslinked polymeric adsorbent and its application for the removal of VOCs. Chem. Eng. J. 2012, 192, 8–12. [Google Scholar] [CrossRef]







| Tonset a/°C | Tend b/°C | Tonset c/°C | Tend d/°C | △H e/J·g−1 | Percentage of Precursor f/% | |
|---|---|---|---|---|---|---|
| Wax | 27.0 | 37.3 | 39.1 | 54.4 | 147.6 | |
| Microcapsule | 31.3 | 38.7 | 38.9 | 51.8 | 33.8 | 22.9 |
| HVPM | 32.0 | 34.6 | 35.0 | 48.2 | 11.1 | 32.8 |
| Sample | SBET a/m2·g−1 | Smicro b/m2·g−1 | Smeso c/m2·g−1 | Vmicro d/cm3·g−1 | Vmeso e/cm3·g−1 | Vtotal f/cm3·g−1 |
|---|---|---|---|---|---|---|
| Value | 665 | 501 | 162 | 0.24 | 0.08 | 0.38 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Sun, L.; Sui, H.; He, L.; Yuan, W.; Han, Z. A Novel Polymeric Adsorbent Embedded with Phase Change Materials (PCMs) Microcapsules: Synthesis and Application. Nanomaterials 2019, 9, 736. https://doi.org/10.3390/nano9050736
Li X, Sun L, Sui H, He L, Yuan W, Han Z. A Novel Polymeric Adsorbent Embedded with Phase Change Materials (PCMs) Microcapsules: Synthesis and Application. Nanomaterials. 2019; 9(5):736. https://doi.org/10.3390/nano9050736
Chicago/Turabian StyleLi, Xingang, Lingyu Sun, Hong Sui, Lin He, Wei Yuan, and Zhenwei Han. 2019. "A Novel Polymeric Adsorbent Embedded with Phase Change Materials (PCMs) Microcapsules: Synthesis and Application" Nanomaterials 9, no. 5: 736. https://doi.org/10.3390/nano9050736




