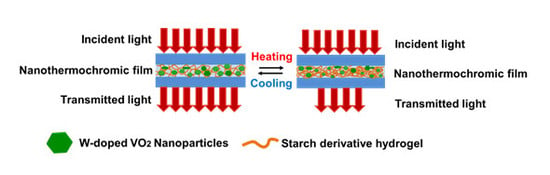
Tungsten-Doped VO2/Starch Derivative Hybrid Nanothermochromic Hydrogel for Smart Window
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of HBPS and HBPS Hydrogel Films
2.3. Preparation of W-Doped VO2 NPs and VO2/PU Composite Film
2.4. Preparation of W-doped VO2/HBPS Hydrogel Composite Films
3. Characterization Methods
4. Results and Discussion
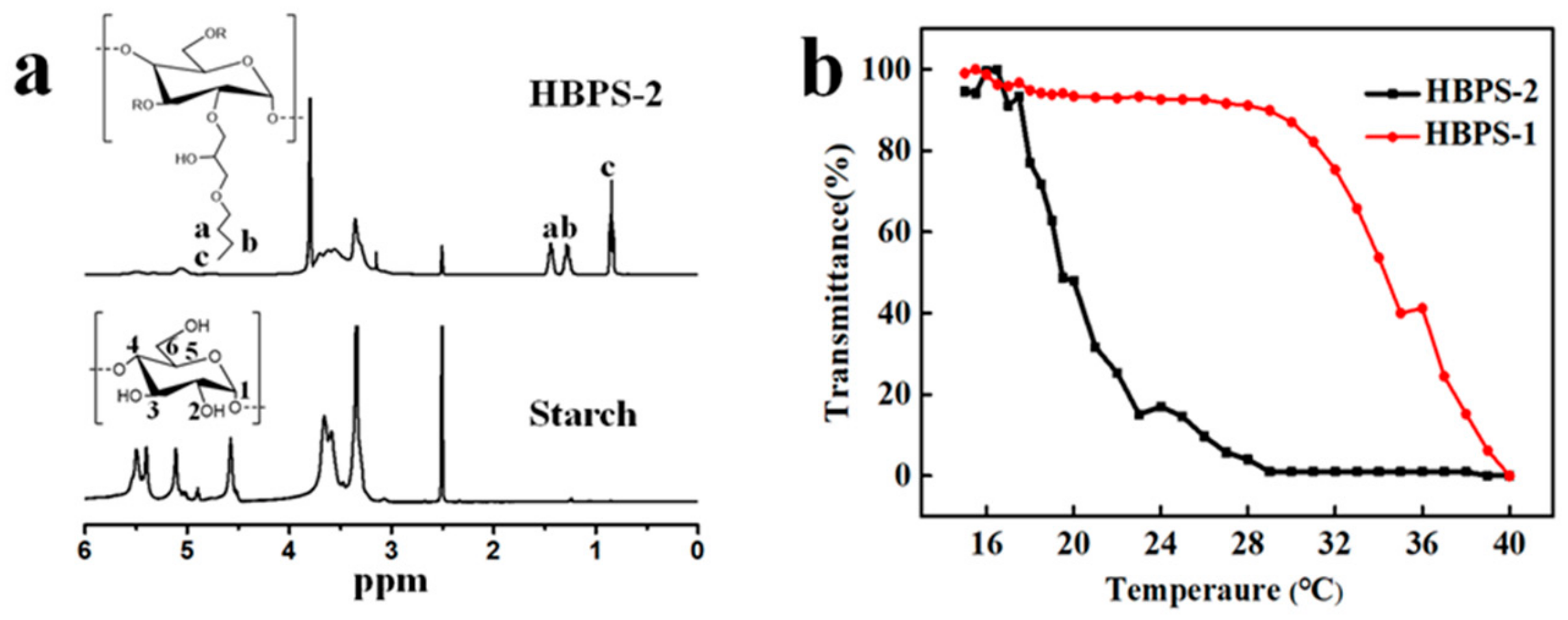
4.1. Structure Analysis and Thermoresponse Properties of HBPS
4.2. Optical Transmittance Properties of HBPS Films
4.3. Optical Transmittance Properties of Composite Films
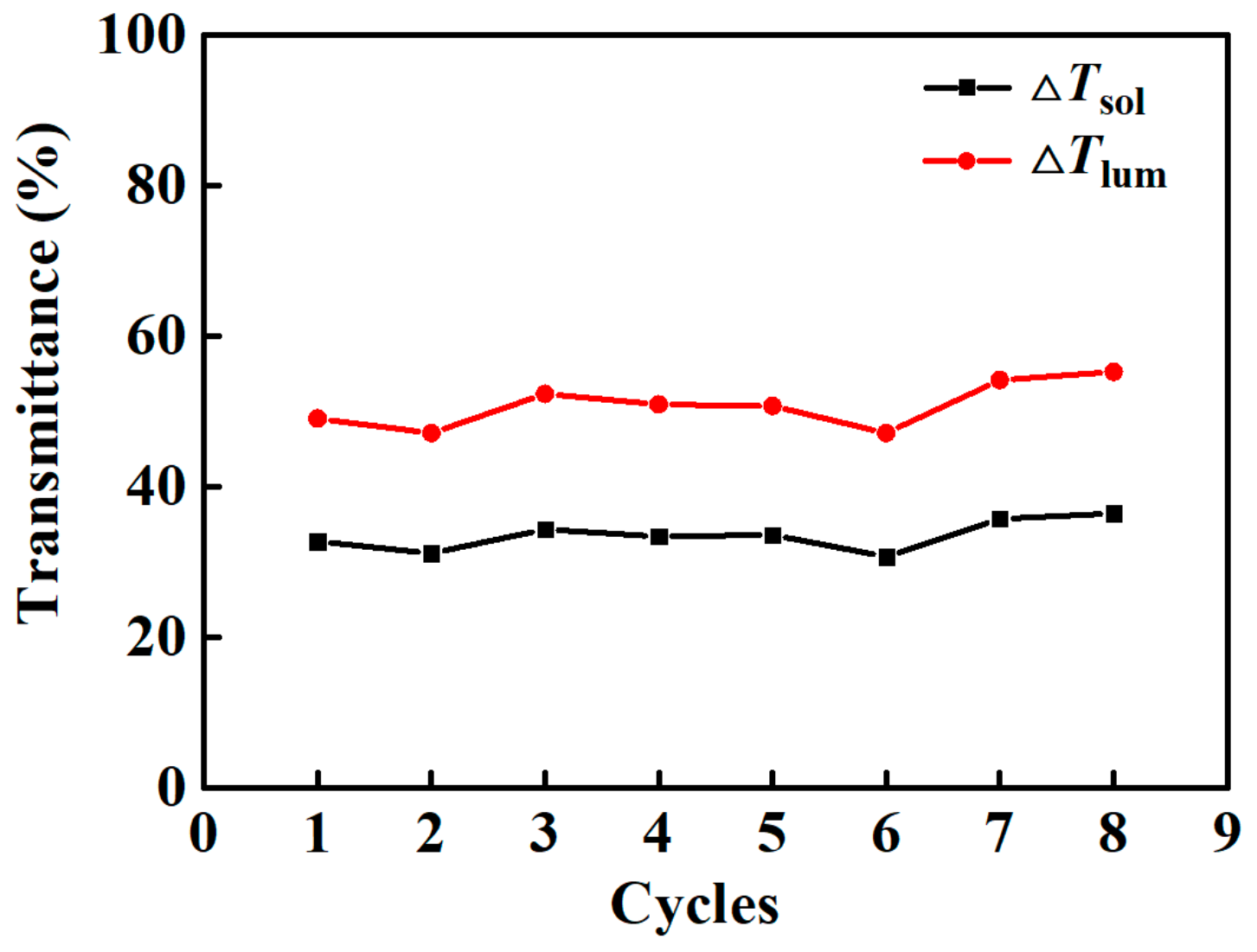
4.4. Cyclic Stability of Composite Films
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wang, Y.; Wang, J.; Zhang, K.; Li, L.; Chen, Z.; Gao, Y.; Guo, X. Application of thermochromic hydrogels as novel materials of energy chemical engineering in smart window. J. East China Univ. Sci. Technol. 2018, 44, 1–8. [Google Scholar]
- Cui, Y.; Ke, Y.; Liu, C.; Chen, Z.; Wang, N.; Zhang, L.; Zhou, Y.; Wang, S.; Gao, Y.; Long, Y. Thermochromic VO2 for Energy-Efficient Smart Windows. Joule 2018, 2, 1–40. [Google Scholar] [CrossRef]
- Chen, Z.; Gao, Y.; Kang, L.; Du, J.; Zhang, Z.; Luo, H.; Miao, H.; Tan, G. VO2-based double-layered films for smart windows: Optical design, all-solution preparation and improved properties. Sol. Energy Mater. Sol. Cells 2011, 95, 2677–2684. [Google Scholar] [CrossRef]
- Ke, Y.; Zhou, C.; Zhou, Y.; Wang, S.; Chan, S.H.; Long, Y. Emerging Thermal-Responsive Materials and Integrated Techniques Targeting the Energy-Efficient Smart Window Application. Adv. Funct. Mater. 2018, 28, 1800113. [Google Scholar] [CrossRef]
- Azens, A.; Granqvist, C. Electrochromic smart windows: Energy efficiency and device aspects. J. Solid State Electrochem. 2003, 64, 64–68. [Google Scholar] [CrossRef]
- Wittwer, V.; Datz, M.; Ell, J.; Georg, A.; Graf, W.; Walze, G. Gasochromic windows. Sol. Energy Mater. Sol. Cells 2004, 84, 305–314. [Google Scholar] [CrossRef]
- Kang, L.; Gao, Y.; Luo, H.; Chen, Z.; Du, J.; Zhang, Z. Nanoporous thermochromic VO2 films with low optical constants, enhanced luminous transmittance and thermochromic properties. ACS Appl. Mater. Interfaces 2011, 3, 135–138. [Google Scholar] [CrossRef]
- Feng, W.; Zou, L.; Gao, G.; Wu, G.; Shen, J.; Li, W. Gasochromic smart window: Optical and thermal properties, energy simulation and feasibility analysis. Sol. Energy Mater. Sol. Cells 2016, 144, 316–323. [Google Scholar] [CrossRef]
- Drosos, C.; Vernardou, D. Perspectives of energy materials grown by APCVD. Sol. Energy Mater. Sol. Cells 2015, 140, 1–8. [Google Scholar] [CrossRef]
- Louloudakis, D.; Vernardou, D.; Spanakis, E.; Katsarakis, N.; Koudoumas, E. Thermochromic vanadium oxide coatings grown by APCVD at low temperatures. Phys. Procedia 2013, 46, 137–141. [Google Scholar] [CrossRef]
- Chen, Z.; Gao, Y.; Kang, L.; Cao, C.; Chen, S.; Luo, H. Fine crystalline VO2 nanoparticles: Synthesis, abnormal phase transition temperatures and excellent optical properties of a derived VO2 nanocomposite foil. J. Mater. Chem. A 2014, 2, 2718–2727. [Google Scholar] [CrossRef]
- Zhang, Q.; Hoogenboom, R. Polymers with upper critical solution temperature behavior in alcohol/water solvent mixtures. Prog. Polym. Sci. 2015, 48, 122–142. [Google Scholar] [CrossRef]
- Zhou, Y.; Cai, Y.; Hu, X.; Long, Y. Temperature-responsive hydrogel with ultra-large solar modulation and high luminous transmission for “smart window” applications. J. Mater. Chem. A 2014, 2, 13550–13555. [Google Scholar] [CrossRef]
- Wang, M.; Gao, Y.; Cao, C.; Chen, K.; Wen, Y.; Fang, D.; Li, L.; Guo, X. Binary solvent colloids of thermosensitive poly (n-isopropylacrylamide) microgel for smart windows. Ind. Eng. Chem. Res. 2014, 53, 18462–18472. [Google Scholar] [CrossRef]
- Zhou, Y.; Cai, Y.; Hu, X.; Long, Y. VO2/hydrogel hybrid nanothermochromic material with ultra-high solar modulation and luminous transmission. J. Mater. Chem. A 2015, 3, 1121–1126. [Google Scholar] [CrossRef]
- Zhu, J.; Huang, A.; Ma, H.; Chen, Y.; Zhang, S.; Ji, S.; Bao, S.; Jin, P. Hybrid films of VO2 nanoparticles and a nickel(ii)-based ligand exchange thermochromic system: Excellent optical performance with a temperature responsive colour change. New J. Chem. 2017, 41, 830–835. [Google Scholar] [CrossRef]
- Houska, J.; Kolenaty, D.; Vlcek, J.; Barta, T.; Rezek, J.; Cerstvy, R. Significant improvement of the performance of ZrO2/V1-xWxO2/ZrO2 thermochromic coatings by utilizing a second-order interference. Sol. Energy Mater. Sol. Cells 2019, 191, 365–371. [Google Scholar] [CrossRef]
- Peng, Z.; Jiang, W.; Liu, H. Synthesis and electrical properties of tungsten-doped vanadium dioxide nanopowders by thermolysis. J. Phys. Chem. C 2007, 111, 1119–1122. [Google Scholar] [CrossRef]
- Hu, L.; Tao, H.; Chen, G.; Pan, R.; Wan, M.; Xiong, D.; Zhao, X. Porous W-doped VO2 films with simultaneously enhanced visible transparency and thermochromic properties. J. Sol Gel Sci. Technol. 2016, 77, 85–93. [Google Scholar] [CrossRef]
- Zhang, K.; Shi, Y.; Wu, L.; Chen, L.; Wei, T.; Jia, X.; Chen, Z.; Li, M.; Xu, Y.; Wang, Y.; et al. Thermo- and pH-responsive starch derivatives for smart window. Carbohydr. Polym. 2018, 196, 209–216. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Y.; Xu, M.; Wu, L.; Jia, X.; Wei, T.; Zhang, S.; Guo, X. Smart flocculant with temperature and pH response derived from starch. RSC Adv. 2016, 6, 44383–44391. [Google Scholar] [CrossRef]
- Ju, B.; Yan, D.; Zhang, S. Micelles self-assembled from thermoresponsive 2-hydroxy-3-butoxypropyl starches for drug delivery. Carbohydr. Polym. 2012, 87, 1404–1409. [Google Scholar] [CrossRef]
- Shen, N.; Bong, B.; Cao, C.; Chen, Z.; Liu, J.; Luo, H.; Gao, Y. Lowered phase transition temperature and excellent solar heat shielding properties of well-crystallized VO2 by W doping. Phys. Chem. Chem. Phys. 2016, 18, 28010–28017. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, S.; Luo, H.; Dai, L.; Cao, C.; Liu, Y.; Chen, Z.; Kanehira, M. Enhanced chemical stability of VO2 nanoparticles by the formation of SiO2/VO2 core/shell structures and the application to transparent and flexible VO2-based composite foils with excellent thermochromic properties for solar heat control. Energy Environ. Sci. 2012, 5, 6104–6110. [Google Scholar] [CrossRef]
- Gao, M.; Jia, X.; Kuang, G.; Li, Y.; Liang, D.; Wei, Y. Thermo- and pH-Responsive Dendronized Copolymers of Styrene and Maleic Anhydride Pendant with Poly(amidoamine) Dendrons as Side Groups. Macromolecules 2009, 42, 4273–4281. [Google Scholar] [CrossRef]
- Ju, B.; Zhang, C.; Zhang, S. Thermoresponsive starch derivates with widely tuned LCSTs by introducing short oligo(ethylene glycol) spacers. Carbohydr. Polym. 2014, 108, 307–312. [Google Scholar] [CrossRef]
- Lee, E.; Kim, D.; Yoon, J. Stepwise Activation of Switchable Glazing by Compositional Gradient of Copolymers. ACS Appl. Mater. Interfaces 2016, 8, 26359–26364. [Google Scholar] [CrossRef]






| Sample | BGE:AGU 1 | DS 2 | LCST (°C) 3 |
|---|---|---|---|
| HBPS-1 | 0.55 | 0.31 | 32 |
| HBPS-2 | 0.65 | 0.42 | 21 |
| Sample | Tlum, low (%) | Tlum, high (%) | ΔTlum (%) | Tlum, average (%) | ΔTIR (%) | ΔTsol (%) |
|---|---|---|---|---|---|---|
| PNIPAm hydrogel [11] | 88.5 | 58.8 | 29.7 | 73.6 | 9.5 | 21.4 |
| VO2/hydrogel [13] | 82.1 | 43.2 | 38.9 | 62.6 | 29.9 | 34.7 |
| VO2 [9] | 45.6 | 40 | 5.6 | 42.8 | --- | 22.3 |
| HBPS-1 (5 g/L) | 97.9 | 53.1 | 44.8 | 75.5 | 5.0 | 28.9 |
| HBPS-1 (10 g/L) | 95.8 | 49.6 | 46.2 | 72.7 | 6.3 | 31.0 |
| HBPS-2 (5 g/L) | 94.9 | 28.9 | 68.0 | 60.9 | 20.7 | 58.0 |
| HBPS-2 (10 g/L) | 96.2 | 7.8 | 88.4 | 52.0 | 26.2 | 74.6 |
| Composite 1 | 88.2 | 56.9 | 37.6 | 72.6 | 11.6 | 24.7 |
| Composite 2 | 92.0 | 43.6 | 48.5 | 67.8 | 15.1 | 32.5 |
| Composite 3 | 78.7 | 29.0 | 49.8 | 53.9 | 8.5 | 34.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhao, F.; Wang, J.; Li, L.; Zhang, K.; Shi, Y.; Gao, Y.; Guo, X. Tungsten-Doped VO2/Starch Derivative Hybrid Nanothermochromic Hydrogel for Smart Window. Nanomaterials 2019, 9, 970. https://doi.org/10.3390/nano9070970
Wang Y, Zhao F, Wang J, Li L, Zhang K, Shi Y, Gao Y, Guo X. Tungsten-Doped VO2/Starch Derivative Hybrid Nanothermochromic Hydrogel for Smart Window. Nanomaterials. 2019; 9(7):970. https://doi.org/10.3390/nano9070970
Chicago/Turabian StyleWang, Yu, Fang Zhao, Jie Wang, Li Li, Kaiqiang Zhang, Yulin Shi, Yanfeng Gao, and Xuhong Guo. 2019. "Tungsten-Doped VO2/Starch Derivative Hybrid Nanothermochromic Hydrogel for Smart Window" Nanomaterials 9, no. 7: 970. https://doi.org/10.3390/nano9070970