Enhanced Phase Transition Properties of VO2 Thin Films on 6H-SiC (0001) Substrate Prepared by Pulsed Laser Deposition
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of VO2/6H-SiC Films
2.2. Materials Characterization
3. Results and Discussion
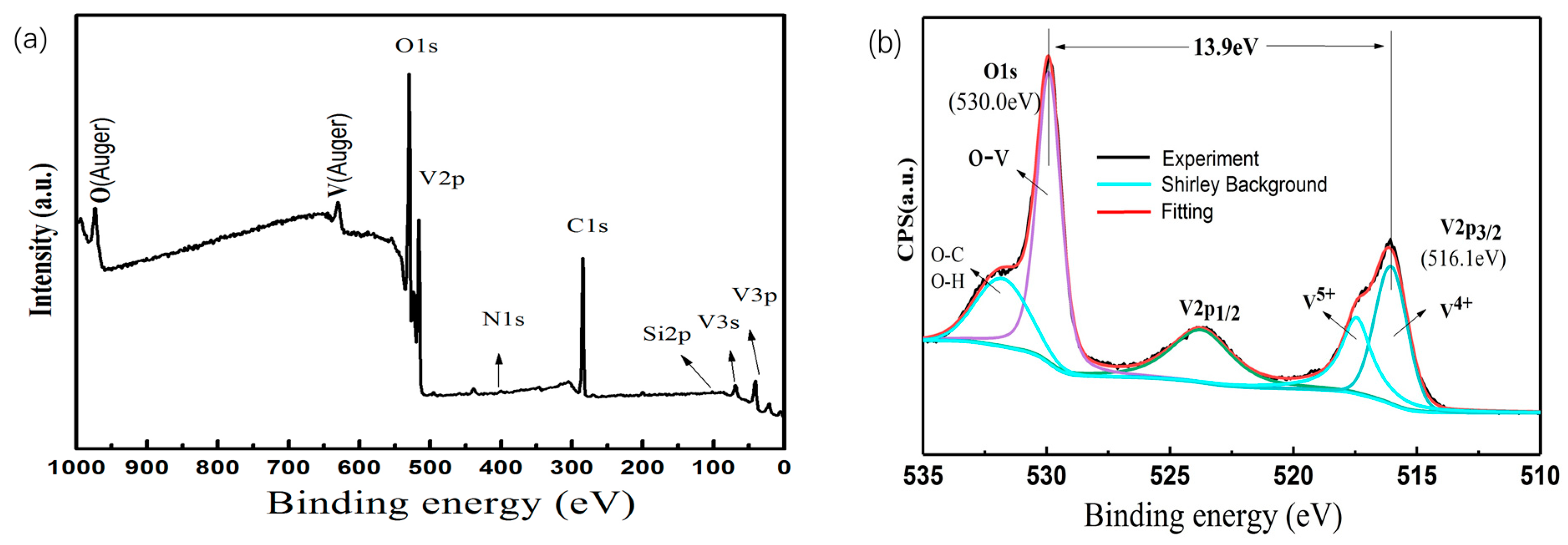
3.1. Influence of Substrate Temperatures and Deposition Time on the Properties of VO2/6H-SiC Films
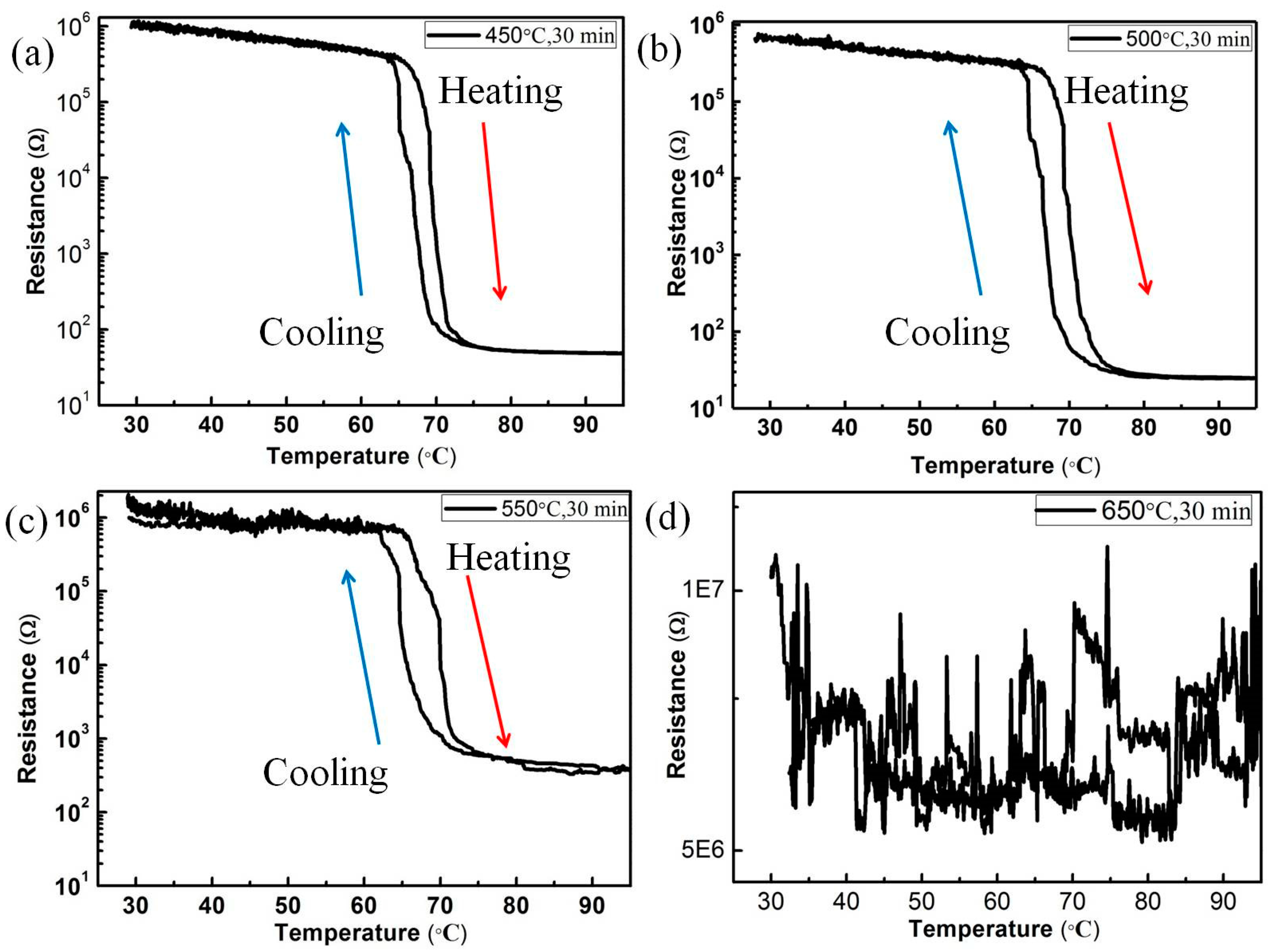
3.2. Variation of VO2/6H-SiC Thin Films Resistance in S1 and S2 Groups During MIT
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Morin, F.J. Oxides Which Show a Metal-to-Insulator Transition at the Neel Temperature. Phys. Rev. Lett. 1959, 3, 34–36. [Google Scholar] [CrossRef]
- Sohn, J.I.; Yang, J.H.; Jang, J.E.; Cha, S.N.; Kim, J. Unusual M2-mediated metal-insulator transition in epitaxial VO2 thin films on GaN substrates. Europhys. Lett. 2015, 109. [Google Scholar] [CrossRef]
- Yang, Z.; Ko, C.; Ramanathan, S. Oxide Electronics Utilizing Ultrafast Metal-Insulator Transitions. Annu. Rev. Mater. Res. 2010, 41, 337–367. [Google Scholar] [CrossRef]
- Vitale, W.A.; Casu, E.A.; Biswas, A.; Rosca, T.; Alper, C.; Krammer, A.; Luong, G.V.; Zhao, Q.-T.; Mantl, S.; Schüler, A.; et al. A Steep-Slope Transistor Combining Phase-Change and Band-to-Band-Tunneling to Achieve a sub-Unity Body Factor. Sci. Rep. 2017, 7, 355. [Google Scholar] [CrossRef] [PubMed]
- Teeslink, T.S.; Torres, D.; Ebel, J.L.; Sepúlveda, N.; Anagnostou, D.E. Reconfigurable Bowtie Antenna using Metal-Insulator Transition in Vanadium Dioxide. IEEE Antennas Wirel. Propag. Lett. 2015, 14, 1. [Google Scholar] [CrossRef]
- Kang, C.Y.; Zhang, C.; Zhang, L.W.; Liang, S.S.; Geng, C.C.; Cao, G.H.; Zong, H.T.; Li, M. Transformation of crystalline structure and photoelectric properties in VO2/glass thin films by inserting TiO2 buffer layers. Appl. Surf. Sci. 2019, 463, 704–712. [Google Scholar] [CrossRef]
- Chen, S.; Wang, Z.; Fan, L.; Chen, Y.; Ren, H.; Ji, H.; Natelson, D.; Huang, Y.; Jiang, J.; Zou, C. Sequential insulator-metal-insulator phase transitions of VO2 triggered by hydrogen doping. Phys. Rev. B 2017, 96, 125130. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, S.; Lv, C.; Huang, Y.; Lai, J. Infrared characteristics of VO2 thin films for smart window and laser protection applications. Appl. Phys. Lett. 2012, 101, 191905. [Google Scholar] [CrossRef]
- Fan, L.L.; Chen, S.; Wu, Y.F.; Chen, F.H.; Chu, W.S.; Chen, X.; Zou, C.W.; Wu, Z.Y. Growth and phase transition characteristics of pure M-phase VO2 epitaxial film prepared by oxide molecular beam epitaxy. Appl. Phys. Lett. 2013, 103, 131914. [Google Scholar] [CrossRef]
- Ren, H.; Chen, S.; Chen, Y.L.; Luo, Z.J.; Zou, C.W. Wet-Etching Induced Abnormal Phase Transition in Highly Strained VO2/TiO2 (001) Epitaxial Film. Phys. Status Solidi 2018, 12, 1700320. [Google Scholar] [CrossRef]
- Li, X.; Schaak, R.E. Size- and Interface-Modulated Metal-Insulator Transition in Solution-Synthesized Nanoscale VO2 -TiO2 -VO2 Heterostructures. Angew. Chem. 2017, 129, 15756–15760. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, H.; Li, C.; Qi, H.; Zhang, D.; Lv, W. Thickness-modulated thermochromism of vanadium dioxide thin films grown by magnetron sputtering. Surf. Coat. Technol. 2019, 359, 396–402. [Google Scholar] [CrossRef]
- Kang, C.; Zhang, C.; Yao, Y.; Yang, Y.; Zong, H.; Zhang, L.; Li, M. Enhanced thermochromic properties of vanadium dioxide (VO2)/glass heterostructure by inserting a Zr-based thin film metallic glasses (Cu50Zr50) buffer layer. Appl. Sci. 2018, 8, 1751. [Google Scholar] [CrossRef]
- Hoshino, H.; Okimura, K.; Yamaguchi, I.; Tsuchiya, T. Infrared-light switching in highly oriented VO2 films on ZnO-buffered glasses with controlled phase transition temperatures. Sol. Energy Mater. Sol. Cells 2019, 191, 9–14. [Google Scholar] [CrossRef]
- Wang, M.; Bian, J.; Sun, H.; Liu, W.; Zhang, Y.; Luo, Y. n-VO2/p-GaN based nitride-oxide heterostructure with various thickness of VO2 layer grown by MBE. Appl. Surf. Sci. 2016, 389, 199–204. [Google Scholar] [CrossRef]
- Sato, K.; Hoshino, H.; Mian, M.S.; Okimura, K. Low-temperature growth of VO2 films on transparent ZnO/glass and Al-doped ZnO/glass and their optical transition properties. Thin Solid Films 2018, 651, 91–96. [Google Scholar] [CrossRef]
- Dettmer, E.S.; Romenesko, B.M.; Charles, H.K.; Carkhuff, B.G.; Merrill, D.J. Steady-state thermal conductivity measurements of AlN and SiC substrate materials. IEEE Trans. Compon. Hybrids Manuf. Technol. 1989, 12, 543–547. [Google Scholar] [CrossRef]
- Lin, S.; Chen, Z.; Peng, L.; Ba, Y.; Liu, S. Formation and suppression of misoriented grains in 6H-SiC crystals. CrystEngComm 2011, 13, 2709–2713. [Google Scholar] [CrossRef]
- Liao, G.M.; Chen, S.; Fan, L.L.; Chen, Y.L.; Wang, X.Q.; Ren, H.; Zhang, Z.M.; Zou, C.W. Dynamically tracking the joule heating effect on the voltage induced metal-insulator transition in VO2 crystal film. AIP Adv. 2016, 6, 1039. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, W.; Feng, Q.; Zheng, J.; Liu, X.; Tang, B.; Li, J.; Xue, J.; Peng, S. Irradiation effects in 6H–SiC induced by neutron and heavy ions: Raman spectroscopy and high-resolution XRD analysis. J. Nucl. Mater. 2016, 478, 215–221. [Google Scholar] [CrossRef]
- Burton, J.C.; Sun, L.; Pophristic, M.; Li, J.; Long, F.H.; Feng, Z.C.; Ferguson, I. Spatial characterization of Doped SiC Wafers. J. Appl. Phys. 1998, 84, 6268–6273. [Google Scholar] [CrossRef]
- Bian, J.; Wang, M.; Sun, H.; Liu, H.; Li, X.; Luo, Y.; Zhang, Y. Thickness-modulated metal-insulator ransition of VO2 film grown on sapphire substrate by MBE. J. Mater. Sci. 2016, 51, 6149–6155. [Google Scholar] [CrossRef]
- Abel, M.L.; Carley, A.; Watts, J.F.; Hryha, E.; Rutqvist, E.; Nyborg, L. Stoichiometric vanadium oxides studied by XPS. Surf. Interface Anal. 2012, 44, 1022–1025. [Google Scholar]
- Chen, Y.L.; Fan, L.L.; Fang, Q.; Xu, W.; Chen, S.; Zan, G.; Ren, H.; Song, L.; Zou, C. Free-standing SWNTs/VO2/Mica hierarchical films for high-performance thermochromic devices. Nano Energy 2017, 31, 144–151. [Google Scholar] [CrossRef]
- Zhou, Y.; Ramanathan, S. Heteroepitaxial VO2 thin films on GaN: Structure and metal-insulator transition characteristics. J. Appl. Phys. 2012, 112, 74114. [Google Scholar] [CrossRef]
- Gu, D.; Zheng, H.; Ma, Y.; Xu, S.; Zhou, X. A highly-efficient approach for reducing phase transition temperature of VO2 polycrystalline thin films through Ru4+-doping. J. Alloys Compd. 2019, 790, 602–609. [Google Scholar] [CrossRef]
- Liu, S.J.; Fang, H.W.; Su, Y.T.; Hsieh, J.H. Metal-insulator transition characteristics of Mo-and Mn-doped VO2 films fabricated by magnetron cosputtering technique. Jpn. J. Appl. Phys. 2014, 53, 063201. [Google Scholar] [CrossRef]
- Madiba, I.; Chaker, M.; Khanyile, B.; Tadadjeu, S.; Zolliker, P.; Nkosi, M. Effect of neutron irradiation on the structural, electrical and optical properties evolution of RPLD VO2 films. Beam Interact. Mater. Atoms 2019, 443, 25–30. [Google Scholar] [CrossRef]
- Bian, J.; Wang, M.; Miao, L.; Li, X.; Luo, Y.; Dong, Z.; Zhang, Y. Growth and characterization of VO2/p-GaN/sapphire heterostructure with phase transition properties. Appl. Surf. Sci. 2015, 357, 282–286. [Google Scholar] [CrossRef]
- Aggarwal, R.; Gupta, P.; Narayan, R.J.; Narayan, J.; Gupta, A.; Dutta, T. Semiconductor to metal transition characteristics of VO2 thin films grown epitaxially on Si (001). Appl. Phys. Lett. 2009, 95, 111915. [Google Scholar]
- Zhang, P.; Zhang, W.; Wang, J.; Jiang, K.; Zhang, J.; Li, W.; Wu, J.; Hu, Z.; Chu, J. The electro-optic mechanism and infrared switching dynamic of the hybrid multilayer VO2/Al: ZnO heterojunctions. Sci. Rep. 2017, 7, 4425. [Google Scholar] [CrossRef] [PubMed]
- Hong, B.; Hu, K.; Tao, Z.; Zhao, J.; Pan, N.; Wang, X.; Lu, M.; Yang, Y.; Luo, Z.; Gao, C. Polymorph separation induced by angle distortion and electron delocalization effect via orbital modification in VO2 epitaxial thin films. Phys. Rev. B 2017, 95, 7. [Google Scholar] [CrossRef]




| S2 Group | Resistance Change | Hysteresis Width (°C) |
|---|---|---|
| S2-1 | 2.60 × 104 | 4.1 |
| S2-2 | 2.71 × 104 | 3.4 |
| S2-3 | 3.68 × 104 | 3.6 |
| S2-4 | 5.66 × 104 | 3.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, X.; Gao, Q.; Li, K.; Liu, Z.; Liu, Q.; Liu, Q.; Zhang, Y.; Li, B. Enhanced Phase Transition Properties of VO2 Thin Films on 6H-SiC (0001) Substrate Prepared by Pulsed Laser Deposition. Nanomaterials 2019, 9, 1061. https://doi.org/10.3390/nano9081061
Cheng X, Gao Q, Li K, Liu Z, Liu Q, Liu Q, Zhang Y, Li B. Enhanced Phase Transition Properties of VO2 Thin Films on 6H-SiC (0001) Substrate Prepared by Pulsed Laser Deposition. Nanomaterials. 2019; 9(8):1061. https://doi.org/10.3390/nano9081061
Chicago/Turabian StyleCheng, Xiankun, Qiang Gao, Kaifeng Li, Zhongliang Liu, Qinzhuang Liu, Qiangchun Liu, Yongxing Zhang, and Bing Li. 2019. "Enhanced Phase Transition Properties of VO2 Thin Films on 6H-SiC (0001) Substrate Prepared by Pulsed Laser Deposition" Nanomaterials 9, no. 8: 1061. https://doi.org/10.3390/nano9081061
APA StyleCheng, X., Gao, Q., Li, K., Liu, Z., Liu, Q., Liu, Q., Zhang, Y., & Li, B. (2019). Enhanced Phase Transition Properties of VO2 Thin Films on 6H-SiC (0001) Substrate Prepared by Pulsed Laser Deposition. Nanomaterials, 9(8), 1061. https://doi.org/10.3390/nano9081061






