Efficient One-Pot Synthesis of a Hexamethylenetetramine-Doped Cu-BDC Metal-Organic Framework with Enhanced CO2 Adsorption
Abstract
:1. Introduction
2. Materials and Methods
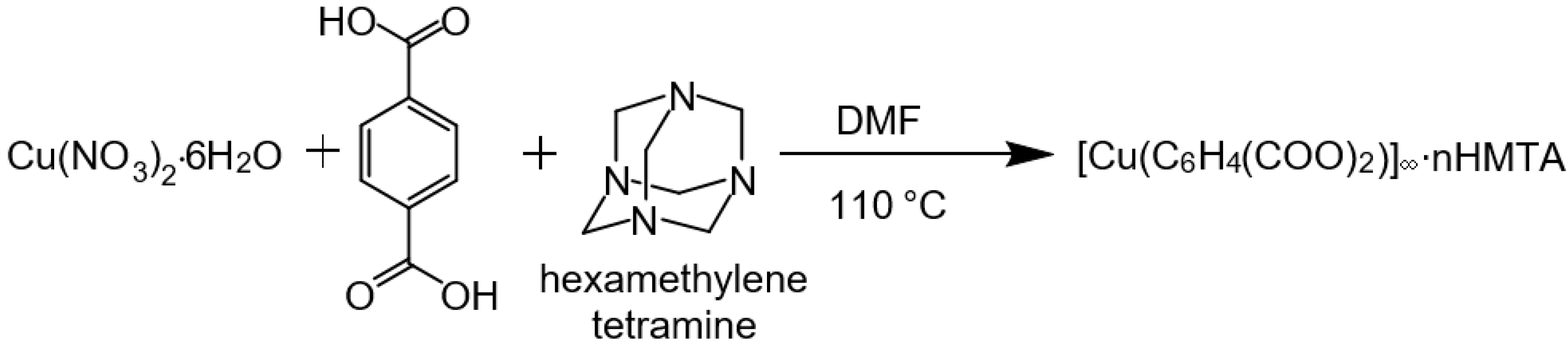
2.1. Synthesis
2.2. Characterization
3. Results and Discussion
3.1. PXRD Patterns of Cu-BDC and Cu-BDC⊃HMTA
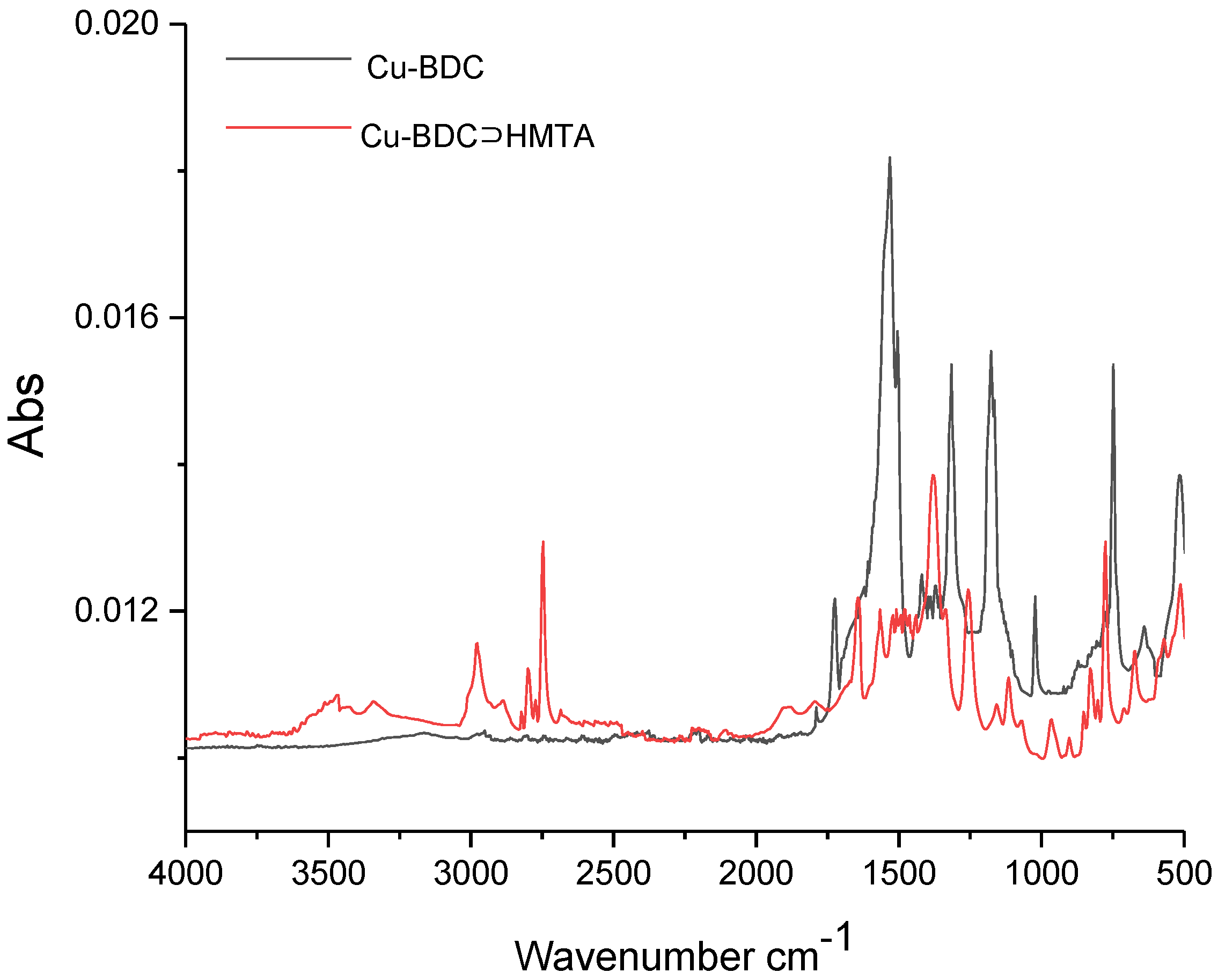
3.2. FTIR of Cu-BDC and Cu-BDC⊃HMTA
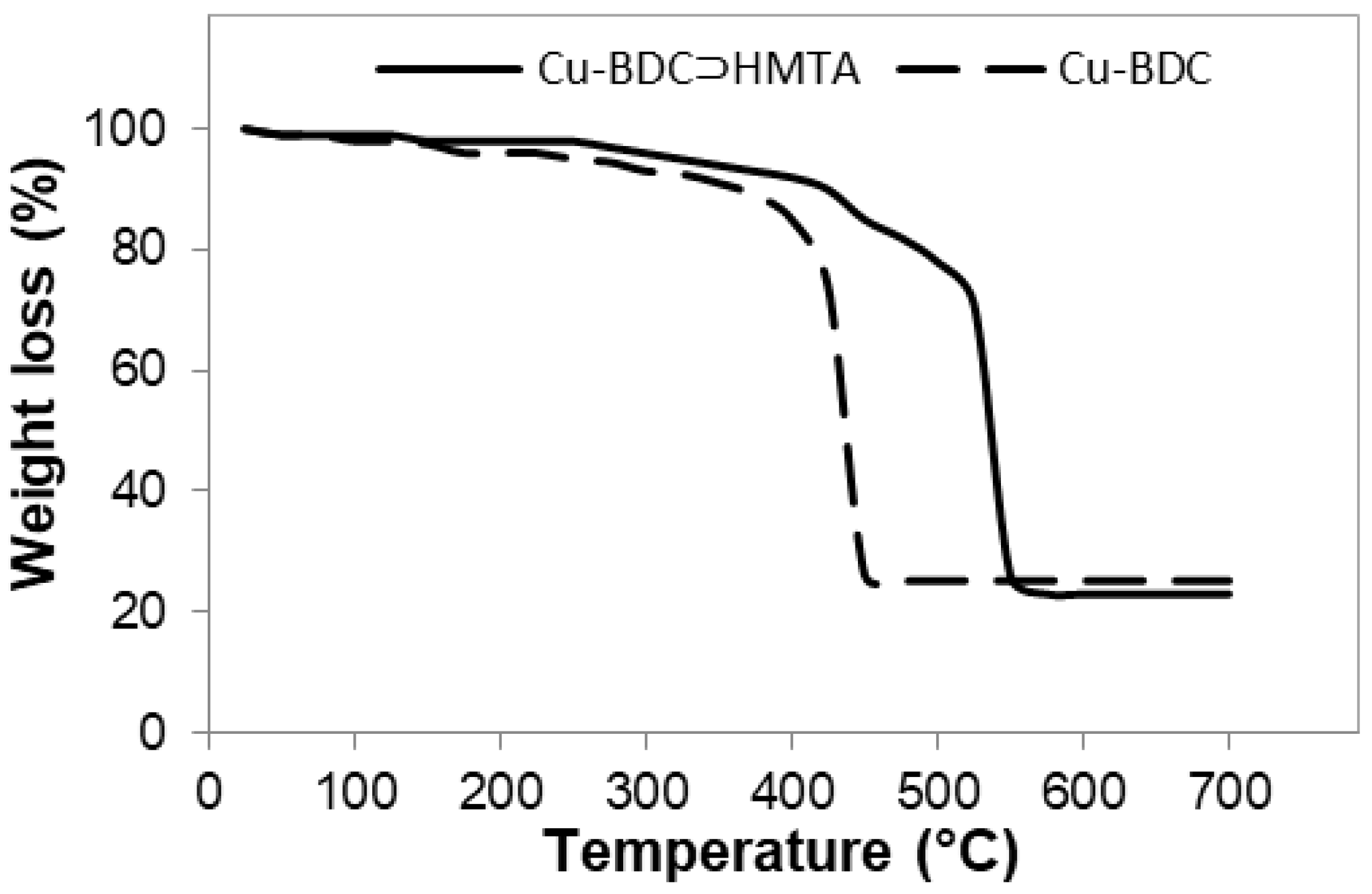
3.3. Thermal Stability of Cu-BDC and Cu-BDC⊃HMTA
3.4. Elemental Composition of Cu-BDC and Cu-BDC⊃HMTA
3.5. Morphology of Cu-BDC and Cu-BDC⊃HMTA
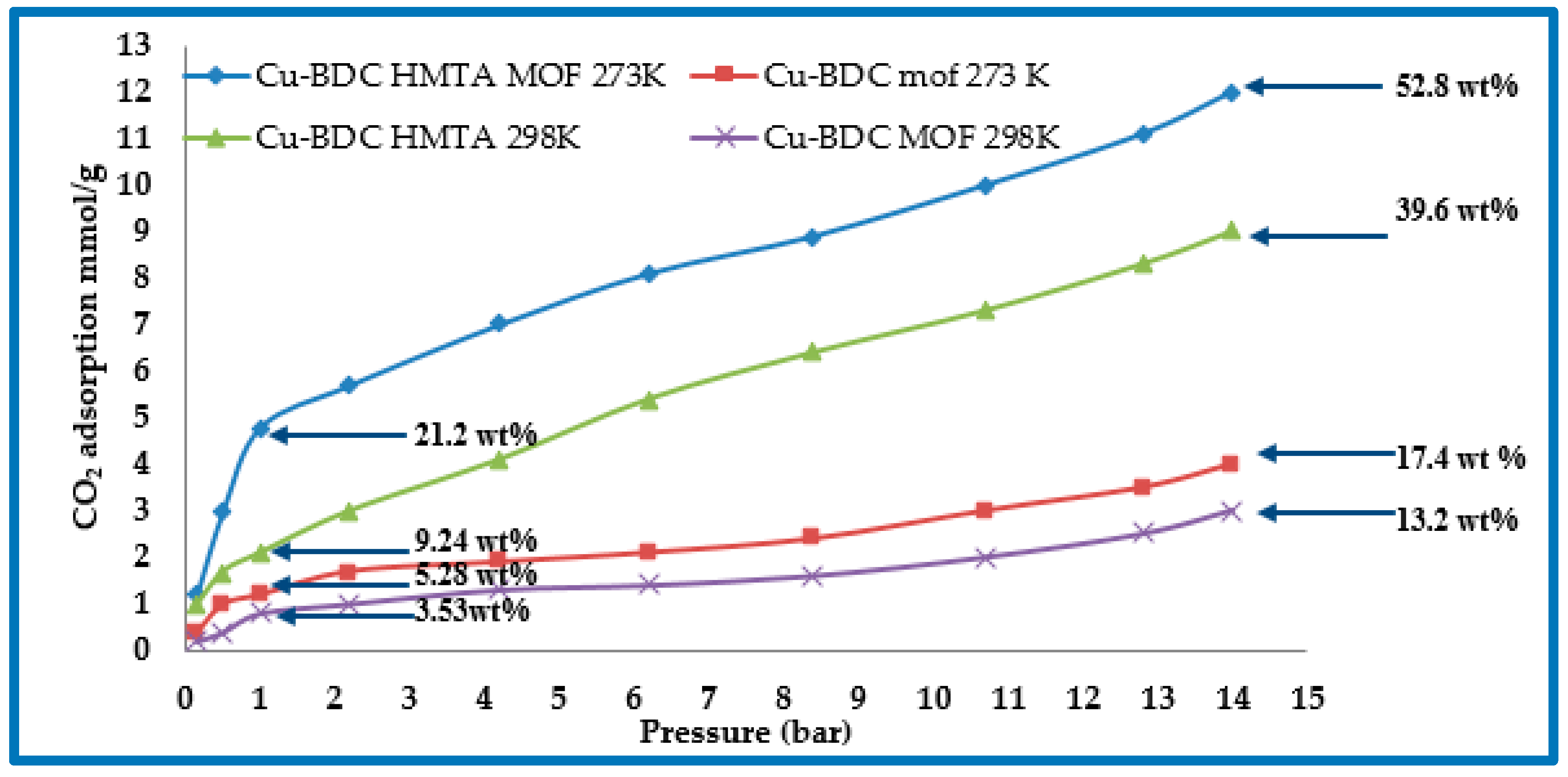
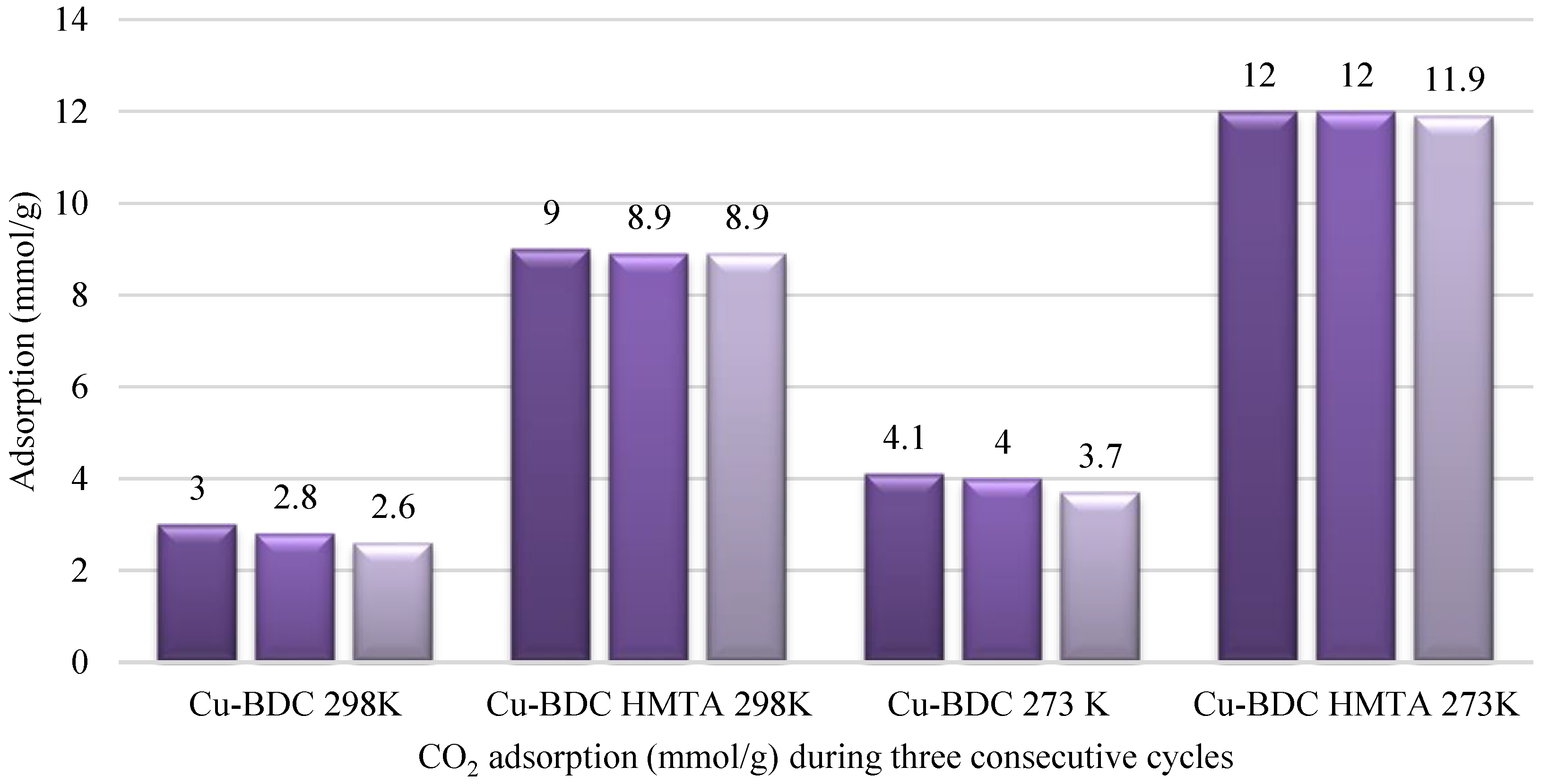
3.6. CO2 Adsorption Studies of Cu-BDC MOF and Cu-BDC⊃HMTA
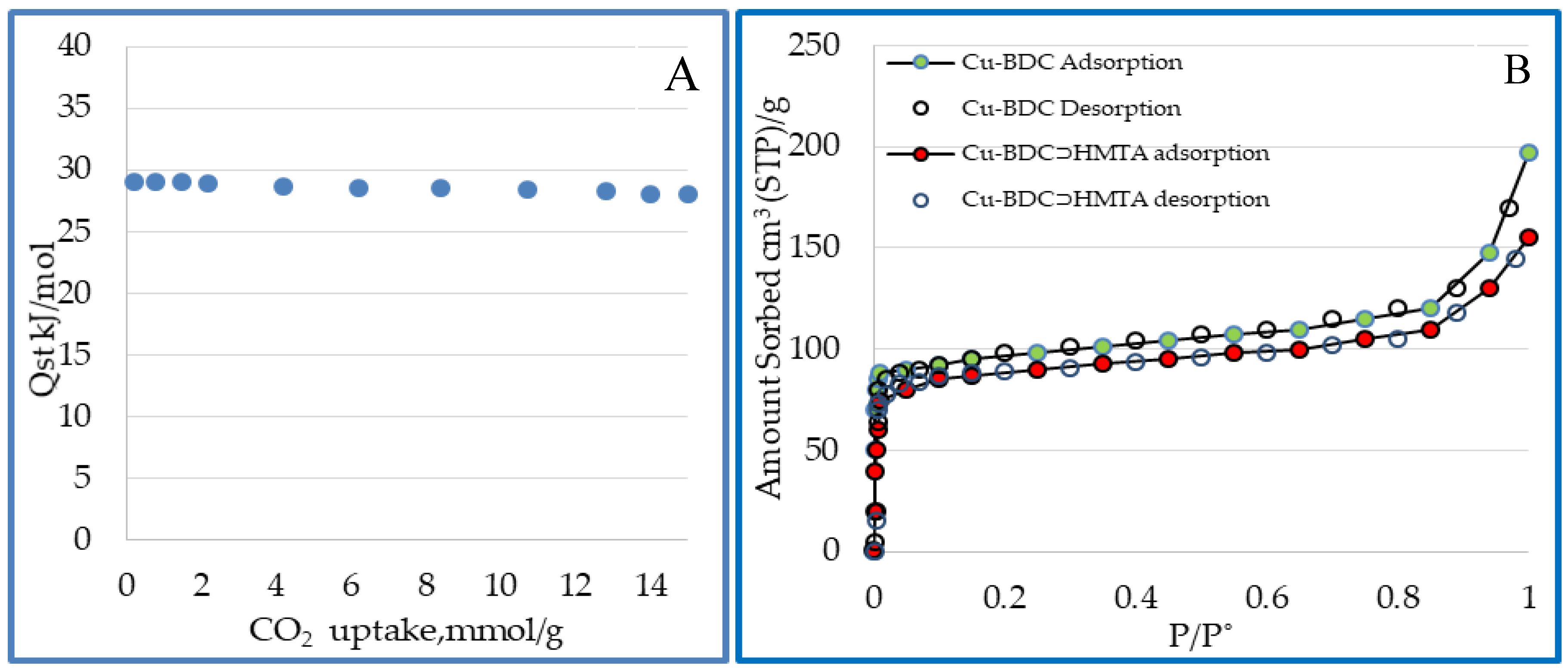
3.7. Surface Area and Porosity of Cu-BDC⊃HMTA MOF
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bao, Z.; Yu, L.; Ren, Q.; Lu, X.; Deng, S. Adsorption of CO2 and CH4 on a magnesium-based metal organic framework. J. Colloid Interface Sci. 2011, 353, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Thallapally, P.K.; McGrail, B.P.; Brown, D.R.; Liu, J. Progress in adsorption-based CO2 capture by metal-organic frameworks. Chem. Soc. Rev. 2012, 41, 2308–2322. [Google Scholar] [CrossRef] [PubMed]
- Sumida, K.; Rogow, D.L.; Mason, J.A.; McDonald, T.M.; Bloch, E.D.; Herm, Z.R.; Bae, T.-H.; Long, J.R. Carbon Dioxide Capture in Metal–Organic Frameworks. Chem. Rev. 2012, 112, 724–781. [Google Scholar] [CrossRef] [PubMed]
- Easun, T.L.; Nevin, A.N. Metal nodes and metal sites in metal–organic frameworks. Organomet. Chem. 2019, 42, 54–79. [Google Scholar]
- Li, J.R.; Sculley, J.; Zhou, H.C. Metal–Organic Frameworks for Separations. Chem. Rev. 2012, 112, 869–932. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Jiang, F.L.; Wu, M.Y.; Chen, L.; Chen, Q.H.; Hong, M.C. A non-interpenetrated porous metal-organic framework with high gas-uptake capacity. Chem. Commun. 2011, 47, 9861–9866. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Muller, U.; Yaghi, O.M. “Heterogeneity within order” in metal-organic frameworks. Angew. Chem. Int. Ed. 2015, 54, 3417–3430. [Google Scholar] [CrossRef]
- Mason, J.A.; McDonald, T.M.; Bae, T.-H.; Bachman, J.E.; Sumida, K.; Dutton, J.J.; Kaye, S.S.; Long, J.R. Application of a high-throughput analyzer in evaluating solid adsorbents for post combustion carbon capture via multicomponent adsorption of CO2, N2, and H2O. J. Am. Chem. Soc. 2015, 137, 4787–4803. [Google Scholar] [CrossRef]
- Carson, C.G.; Hardcastle, K.; Schwartz, J.; Liu, X.; Hoffmann, C.; Gerhardt, R.A.; Tannenbaum, R. Synthesis and structure characterization of copper terephthalate metal-organic frameworks. Eur. J. Inorg. Chem. 2009, 16, 2338–2343. [Google Scholar] [CrossRef]
- Hexamethylenetetraamine; Sigma-Aldrich Corporation online (UK) pricing 11th April 2019 of £62.80 for 4 kg, ReagentPlus®, 99%. Available online: https://www.sigmaaldrich.com/catalog/product/sial/h11300?lang=en®ion=PK (accessed on 28 April 2019).
- Ilyes, E.; Florea, M.; Madalan, A.M.; Haiduc, I.; Parvulescu, V.I.; Andruh, M. A Robust Metal–Organic Framework Constructed from Alkoxo-Bridged Binuclear Nodes and Hexamethylenetetramine Spacers: Crystal Structure and Sorption Studies. Inorg. Chem. 2012, 51, 7954–7956. [Google Scholar] [CrossRef] [PubMed]
- Caskey, S.R.; Wong-Foy, A.G.; Matzger, A.J. Dramatic tuning of carbon dioxide uptake via metal substitution in a coordination polymer with cylindrical pores. J. Am. Chem. Soc. 2008, 130, 10870–10871. [Google Scholar] [CrossRef]
- Yang, Y.; Lin, R.; Ge, L.; Hou, L.; Bernhardt, P.; Rufford, T.E.; Wang, S.; Rudolph, V.; Wang, Y.; Zhu, Z. Synthesis and characterization of three amino-functionalized metal-organic frameworks based on the 2-aminoterephthalic ligand. Dalton Trans. 2015, 44, 8190–8197. [Google Scholar] [CrossRef]
- Hu, Z.; Nalaparaju, A.; Peng, Y.; Jiang, J.; Zhao, D. Modulated hydrothermal synthesis of UIO-66(Hf) type Metal Organic Frameworks for Optimal Carbon Dioxide Separation. Inorg. Chem. 2016, 55, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Bai, J.; Duan, J.; Wojtas, L.; Zaworotko, M.J. Enhanced CO2 Binding Affinity of a High-Uptakerht-Type Metal−Organic Framework Decorated with Acylamide Groups. J. Am. Chem. Soc. 2011, 133, 748–751. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.F.; Wang, H.Z.; Wang, H.L.; Duan, X.; Wang, Z.Y.; Cui, Y.J.; Yang, Y.; Chen, B.L.; Qian, G.D. An amino-decorated NbO-type metal–organic framework for high C2H2 storage and selective CO2 capture. RSC Adv. 2015, 5, 77417–77422. [Google Scholar]
- Xiong, S.; He, Y.; Krishna, R.; Chen, B.; Wang, Z. Metal–Organic Framework With Functional Amide Groups For Highly Selective Gas Separation. Cryst. Growth Des. 2013, 13, 2670–2674. [Google Scholar] [CrossRef]
- Zheng, B.; Yang, Z.; Bai, J.; Li, Y.; Li, S. High and selective CO2 capture by two mesoporous acylamide-functionalized rht-type metal–organic frameworks. Chem. Commun. 2012, 48, 7025–7028. [Google Scholar] [CrossRef]
- Duan, J.; Yang, Z.; Bai, J.; Zheng, B.; Li, Y.; Li, S. Highly selective CO2 capture of an agw-type metal–organic framework with inserted amides: Experimental and theoretical studies. Chem. Commun. 2012, 48, 3058–3060. [Google Scholar] [CrossRef]
- Park, J.; Li, J.R.; Chen, Y.P.; Yu, J.; Yakovenko, A.; Wang, Z.U.; Sun, L.B.; Balbuena, P.B.; Zhou, H.C. A Versatile Metal–Organic Framework For Carbon Dioxide Capture and Cooperative Catalysis. Chem. Commun. 2012, 48, 9995–9997. [Google Scholar] [CrossRef]
- Lu, Z.; Xing, H.; Sun, R.; Bai, J.; Zheng, B.; Li, Y. Water Stable Metal–Organic Framework Evolutionally Formed from a Flexible Multidentate Ligand with Acylamide Groups for Selective CO2 Adsorption. Cryst. Growth Des. 2012, 12, 1081–1084. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, B.; Liu, H.; Lin, X.; Yu, X.; Yi, P.; Yun, R. High Capacity Gas Storage by a Microporous Oxalamide Functionalized NbO-Type Metal–Organic Framework. Cryst. Growth Des. 2013, 13, 5001–5006. [Google Scholar] [CrossRef]
- Demessence, A.; D’Alessandro, D.M.; Foo, M.L.; Long, J.R. Strong CO2 binding in a water stable, triazolate-bridged metal-organic framework functionalized with ethylenediamine. J. Am. Chem. Soc. 2009, 131, 8784–8786. [Google Scholar] [CrossRef] [PubMed]
- Montoro, C.; Garcia, E.; Calero, S.; Perez-Fernandez, M.A.; Lopez, A.L.; Barea, E.; Navarro, J. Functionalization of MOF open metal sites with pendant amines for CO2 capture. J. Mater. Chem. 2012, 22, 10155–10158. [Google Scholar] [CrossRef]
- McDonald, T.M.; D’Alessandro, D.M.; Krishna, R.; Long, J.R. Enhanced carbon dioxide capture upon incorporation of N,N’-dimethylethylenediamine in the metal-organic framework CuBTTri. Chem. Sci. 2011, 2, 2022–2028. [Google Scholar] [CrossRef]
- Karmakar, A.; Desai, A.V.; Manna, B.; Joarder, B.; Ghosh, S.K. An Amide-Functionalized Dynamic Metal–Organic Framework Exhibiting Visual Colorimetric Anion Exchange and Selective Uptake of Benzene over Cyclohexane. Chem. A Eur. J. 2015, 21, 7071–7076. [Google Scholar] [CrossRef] [PubMed]


| Elemental Composition | Calculated by Elemental Analyzer | Calculated by EDS | |||||
|---|---|---|---|---|---|---|---|
| MOF Sample | C | H | N | C | O | N | Cu |
| Cu-BDC | 44.03 (44.07) | 3.64 (3.69) | 4.63 (4.67) | 45.94 (44.0) | 27.27 (26.62) | 5.70 (4.67) | 21.09 (21.13) |
| Cu-BDC⊃HMTA | 45.80 (45.77) | 4.4 (4.39) | 15.30 (15.26) | 47.81 (45.77) | 18.59 (17.41) | 16.30 (15.26) | 17.30 (17.27) |
| Material | BET (m2/g) | Temperature (K) | Pressure (bar) | CO2 Adsorption (wt %) | Qst (KJ mol−1) | Reference |
|---|---|---|---|---|---|---|
| Cu (TATB) | 3360 | 293 | - | - | 61 | [13] |
| [Cu3(TDPAT)] | 1938 | 273 | 1 | 25.8 | 42.2 | [14] |
| 2447 | 273 | 1 | 40.1 | 25.4 | [15] | |
| [Cu4(μ4-O)Cl2(COO)4N4] | 2690 | 273 | 10 | 27.3 | 36.5 | [16] |
| 285 | 296 | 1 | 5.5 | 30 | [17] | |
| 3288 | 273 | 20 | 157 | - | [18] | |
| 2690 | 273 | 1 | 27.3 | - | [19] | |
| 1372 | 273 | 1 | 28.6 | 26.3 | [20] | |
| 808 | 298 | 20 | 25.5 | 26.7 | [21] | |
| 1400 | 273 | 1 | 30.7 | 22.5 | [22] | |
| en-CuBTTri | 345 | 298 | 1 | 58.2 | 90 | [23] |
| en@CuBTC | - | 298 | 1 | 19.5 | 30 | [24] |
| mmen-CuBTTri | 870 | 298 | 1 | 15.4 | - | [25] |
| Cu-BDC | 708 | 273 | 1 (14) | 5.28 (17.4) | - | Present study |
| Cu-BDC⊃HMTA | 590 | 273 | 1 (14) | 21.2 (52.8) | 29.8 | Present study |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asghar, A.; Iqbal, N.; Noor, T.; Ali, M.; Easun, T.L. Efficient One-Pot Synthesis of a Hexamethylenetetramine-Doped Cu-BDC Metal-Organic Framework with Enhanced CO2 Adsorption. Nanomaterials 2019, 9, 1063. https://doi.org/10.3390/nano9081063
Asghar A, Iqbal N, Noor T, Ali M, Easun TL. Efficient One-Pot Synthesis of a Hexamethylenetetramine-Doped Cu-BDC Metal-Organic Framework with Enhanced CO2 Adsorption. Nanomaterials. 2019; 9(8):1063. https://doi.org/10.3390/nano9081063
Chicago/Turabian StyleAsghar, Aisha, Naseem Iqbal, Tayyaba Noor, Majid Ali, and Timothy L. Easun. 2019. "Efficient One-Pot Synthesis of a Hexamethylenetetramine-Doped Cu-BDC Metal-Organic Framework with Enhanced CO2 Adsorption" Nanomaterials 9, no. 8: 1063. https://doi.org/10.3390/nano9081063
APA StyleAsghar, A., Iqbal, N., Noor, T., Ali, M., & Easun, T. L. (2019). Efficient One-Pot Synthesis of a Hexamethylenetetramine-Doped Cu-BDC Metal-Organic Framework with Enhanced CO2 Adsorption. Nanomaterials, 9(8), 1063. https://doi.org/10.3390/nano9081063






