Isothiocyanate-Functionalized Mesoporous Silica Nanoparticles as Building Blocks for the Design of Nanovehicles with Optimized Drug Release Profile
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Amino MSNs with CTAB (MSN–NH2 (CTAB))
2.3. Preparation of Amino MSNs without CTAB (MSN–(NH2))
2.4. Synthesis of Isothiocyanate MSNs (MSN–(NCS))
2.5. Functionalization of MSN–(NCS) with 4-(n-Butylamino)-N-(2-Aminoethyl)-1,8-Naphthalimide (MSN–(UNaph))
2.6. Synthesis of Azido MSNs (MSN–(N3))
2.7. Functionalization of (MSN–(N3)) with 4-(n-Butylamino)-N-(2-Propargyl)-1,8-Naphthalimide (MSN–(TNaph))
2.8. Synthesis of Bifunctionalized Amino-Isothiocyanate MSNs (MSN–(NH2)i(NCS)o)
2.9. Ataluren Loading in MSN–(NH2)i(NCS)o)
2.10. Synthesis of Tert-Butyl(2-((2-Isothiocyanatoethyl)Disulfanyl)Ethyl)Carbamate (6)
2.11. Synthesis of Tert-Butyl (51-Thioxo-2,5,8,11,14,17,20,23,26,29,32,35,38,41,44,47-Hexadecaoxa-55,56,Dithia-50,52-Diazaoctapentacontan-58-yl)Carbamate (7)
2.12. Synthesis of 1-(2-((2-Aminoethyl)Disulfanyl)Ethyl)-3-(2,5,8,11,14,1,20,23,26,29,32,35,38,41,44,47-Hexadecaoxanonatetracontan-49-yl)Thiourea (8)
2.13. Functionalization of MSN–(NH2)i(NCS)o with Aminotetraethylene Glycol Monomethyl Ether and Loading of Ataluren (MSN–(NH2)i(PEG)o (Ataluren))
2.14. Functionalization of MSN–(NH2)i(NCS)o with 1-(2-((2-Aminoethyl)Disulfanyl)Ethyl)-3-(2,5,8,11,14,1,20,23,26,29,32,35,38,41,44,47-Hexadecaoxanonatetracontan-49-yl)Thiourea and Loading of Ataluren (MSN–(NH2)i(SS-PEG)o (Ataluren))
2.15. Functionalization of MSN–(NH2)i(NCS)o with (2-Aminoethyl)Trimethylammonium Chloride Hydrochloride and Loading of Ataluren (MSN–(C+)o (Ataluren))
2.16. Release Experiments
2.17. Characterization
3. Results and Discussion
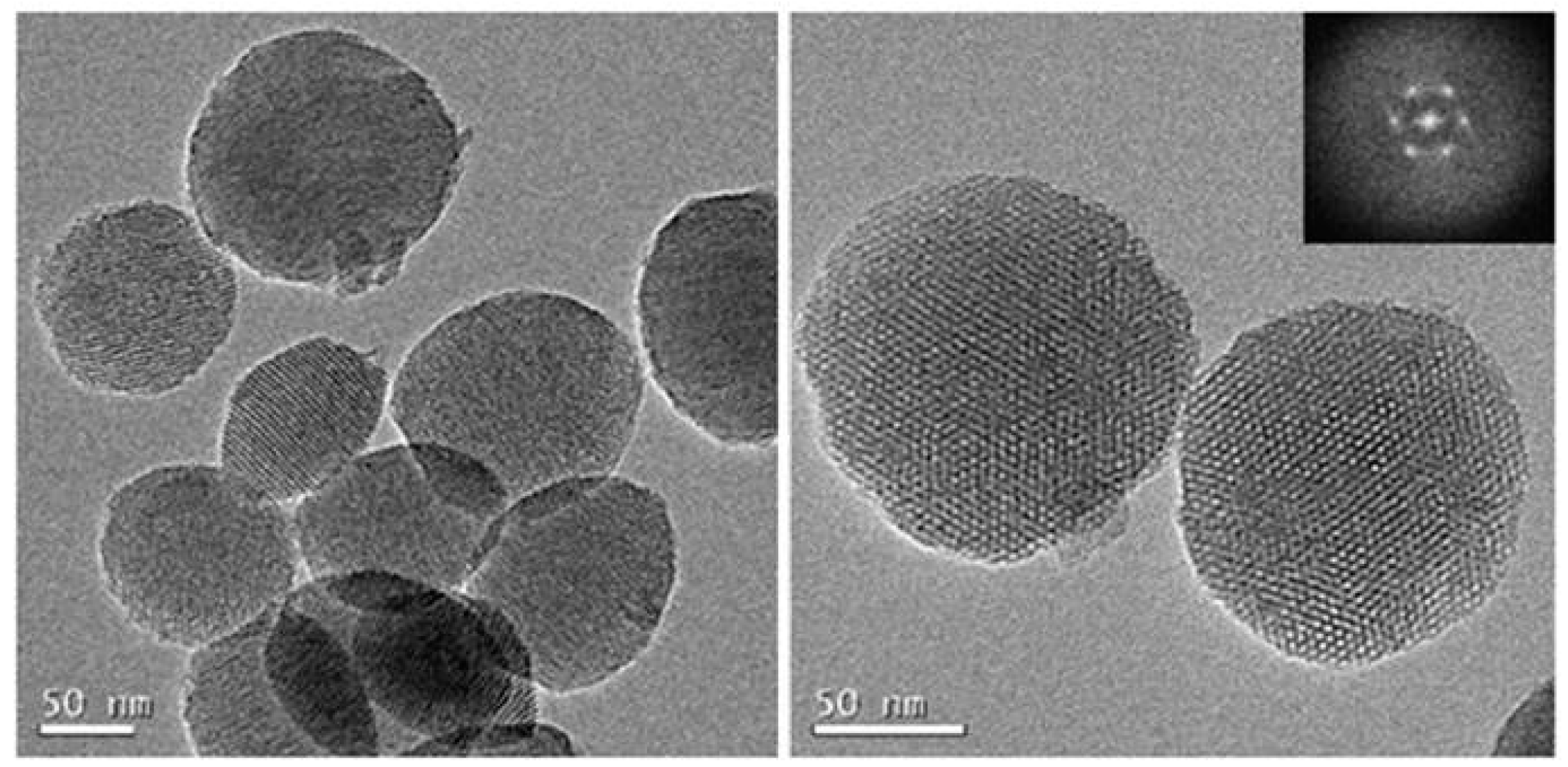
3.1. Synthesis and Characterization of Isothiocyanate MSNs (MSN–(NCS))
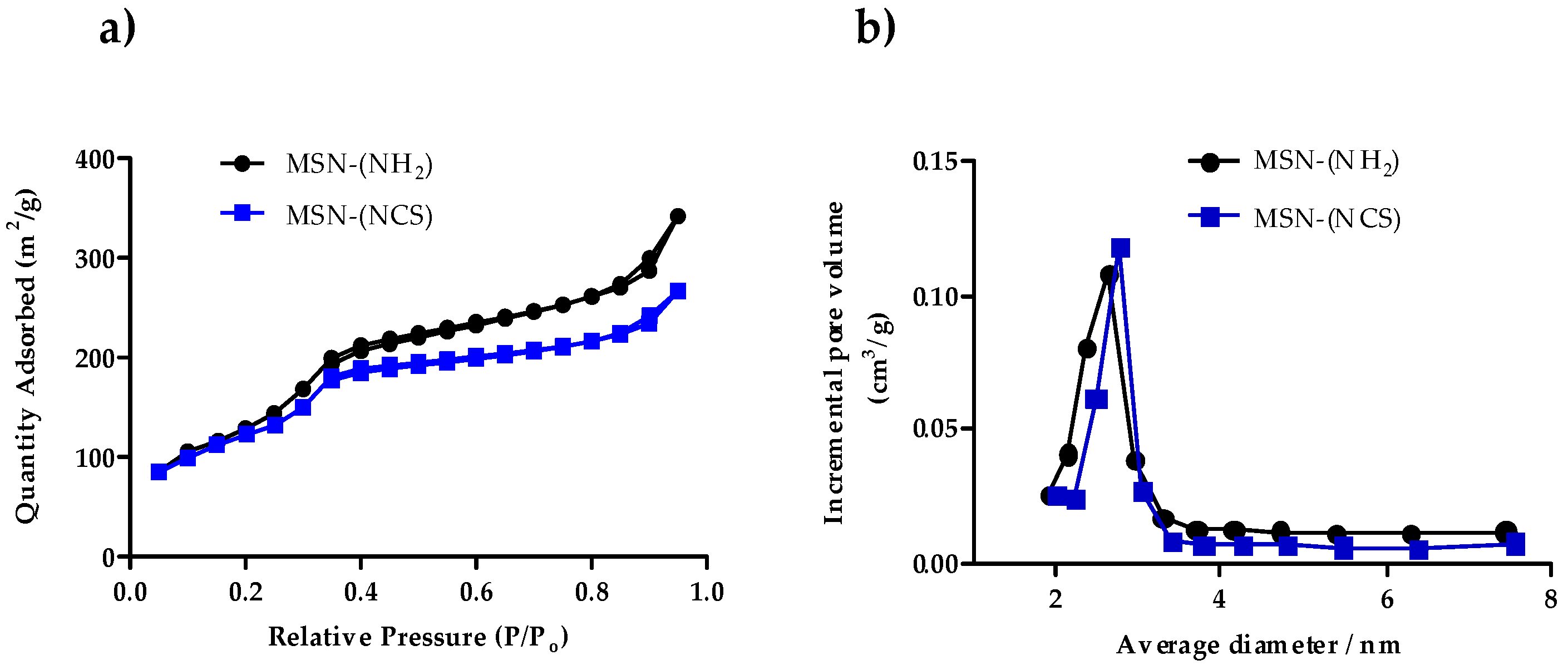
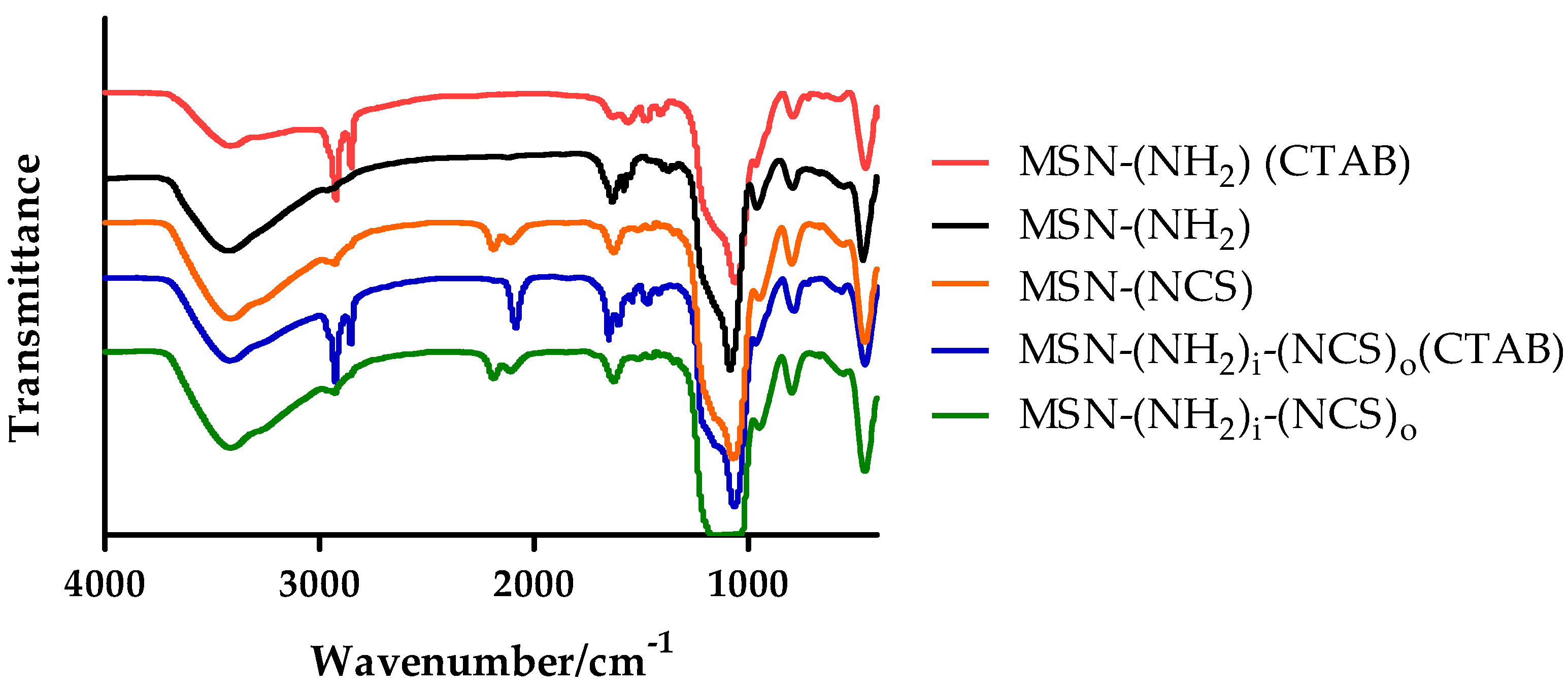
3.2. Assessment of the Functionalization of MSN–(NCS)
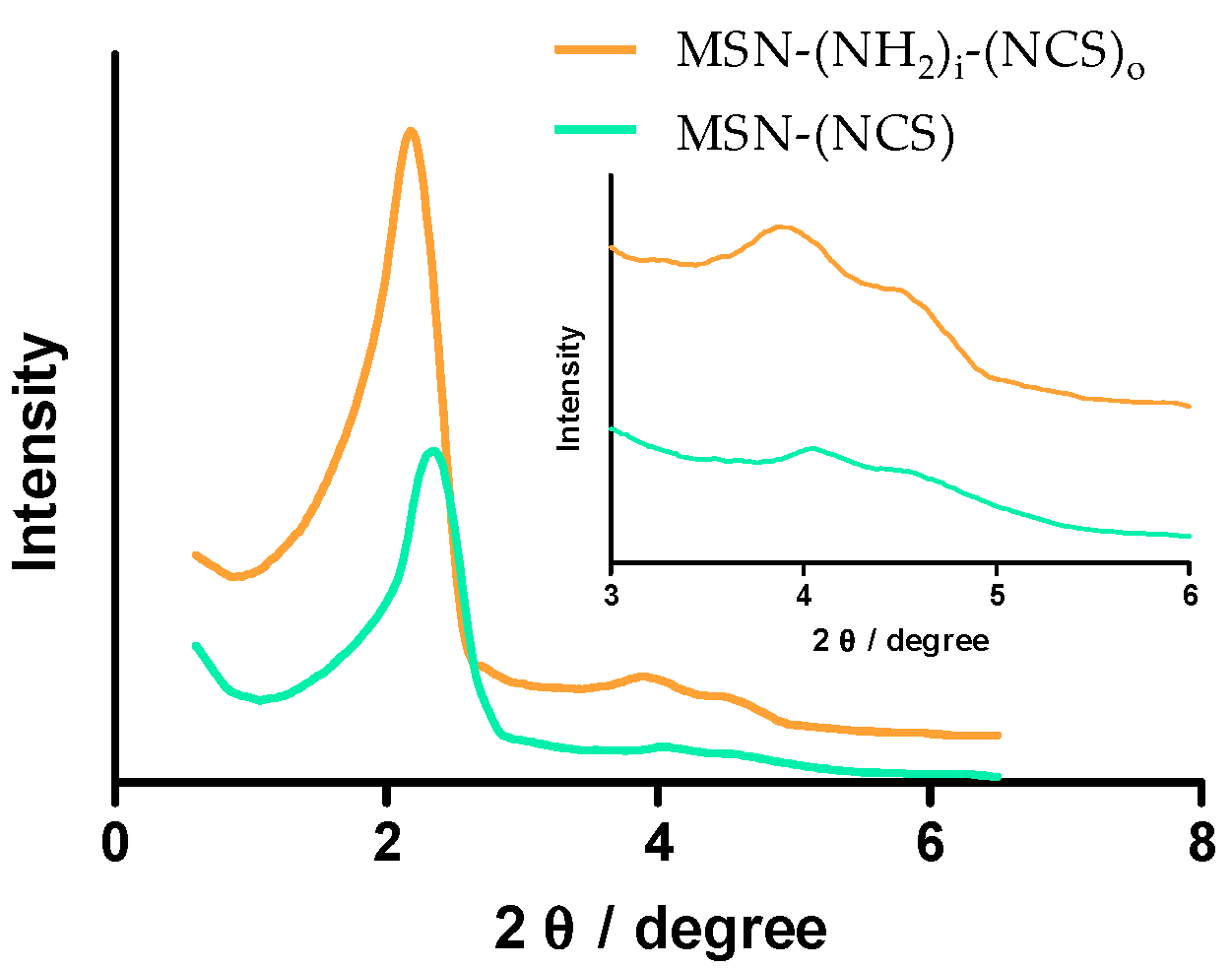
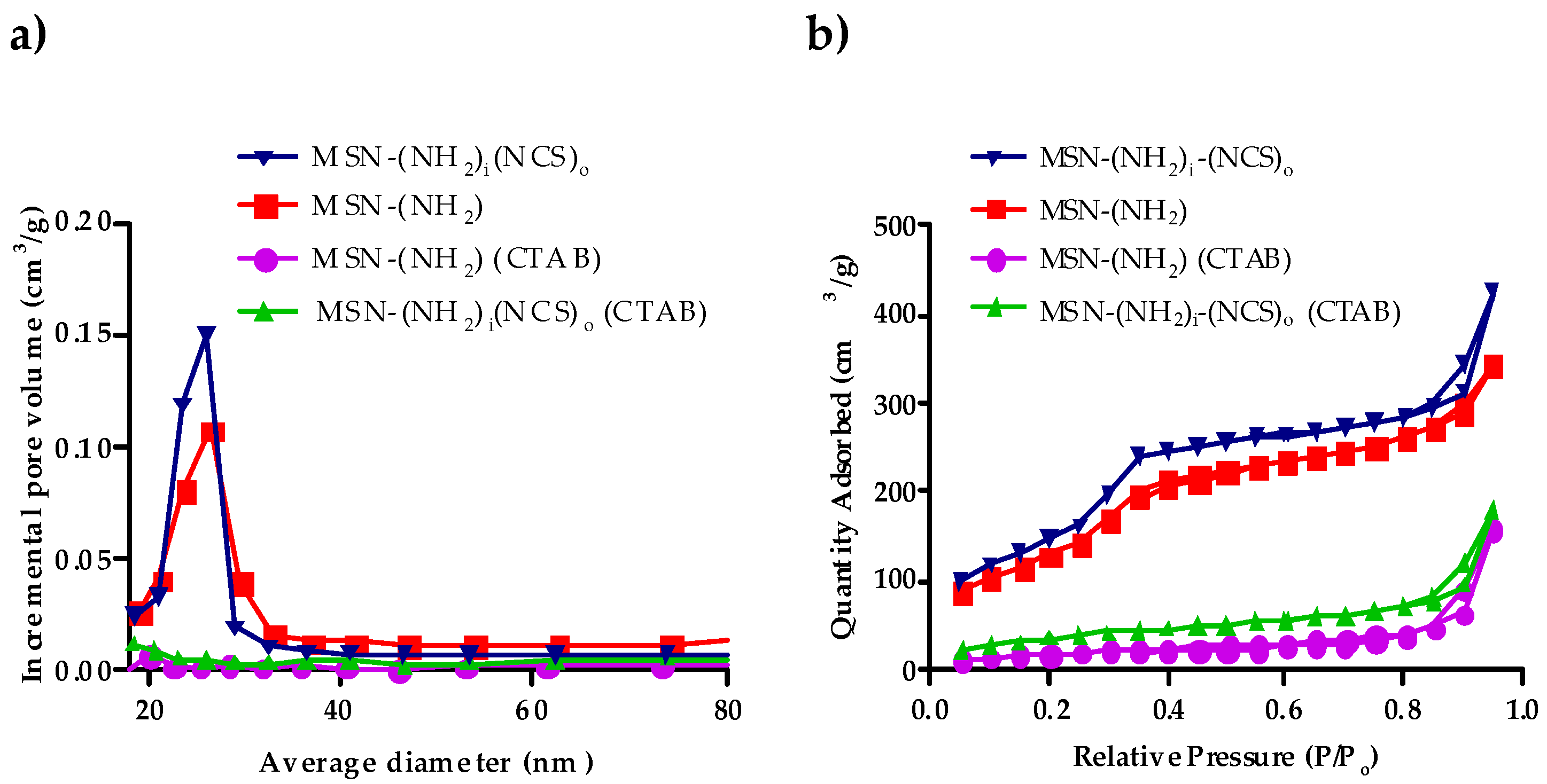
3.3. Synthesis and Characterization of Bifunctionalized Amino-Isothiocianate MSNs (MSN–(NH2)i(NCS)o)
3.4. Application of MSN–(NH2)i(NCS)o for the Preparation of a Nanocarrier for Ataluren Release
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vallet-Regí, M. Mesoporous Silica Nanoparticles: Their Projection in Nanomedicine. ISRN Mater. Sci. 2012, 2012, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Angelos, S.; Liong, M.; Choi, E.; Zink, J.I. Mesoporous silicate materials as substrates for molecular machines and drug delivery. Chem. Eng. J. 2008, 137, 4–13. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Q.; Han, N.; Bai, L.; Li, J.; Liu, J.; Che, E.; Hu, L.; Zhang, Q.; Jiang, T.; et al. Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Mamaeva, V.; Sahlgren, C.; Lindén, M. Mesoporous silica nanoparticles in medicine—Recent advances. Adv. Drug Deliv. Rev. 2013, 65, 689–702. [Google Scholar] [CrossRef] [PubMed]
- Vivero-Escoto, J.L.; Slowing, I.I.; Trewyn, B.G.; Lin, V.S.-Y. Mesoporous Silica Nanoparticles for Intracellular Controlled Drug Delivery. Small 2010, 6, 1952–1967. [Google Scholar] [CrossRef]
- Ai, H.-W. Fluorescent Sensors for Biological Applications. Sensors 2014, 14, 17829–17831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolfbeis, O.S. An overview of nanoparticles commonly used in fluorescent bioimaging. Chem. Soc. Rev. 2015, 44, 4743–4768. [Google Scholar] [CrossRef] [Green Version]
- Kempen, P.J.; Greasley, S.; Parker, K.A.; Campbell, J.C.; Chang, H.-Y.; Jones, J.R.; Sinclair, R.; Gambhir, S.S.; Jokerst, J.V. Theranostic Mesoporous Silica Nanoparticles Biodegrade after Pro-Survival Drug Delivery and Ultrasound/Magnetic Resonance Imaging of Stem Cells. Theranostics 2015, 5, 631–642. [Google Scholar] [CrossRef] [Green Version]
- Knezevic, N.Z.; Mauriello Jimenez, C.; Albino, M.; Vukadinovic, A.; Mrakovic, A.; Illes, E.; Janackovic, D.; Durand, J.-O.; Sangregorio, C.; Peddis, D. Synthesis and Characterization of Core-Shell Magnetic Mesoporous Silica and Organosilica Nanostructures. MRS Adv. 2017, 2, 1037–1045. [Google Scholar] [CrossRef] [Green Version]
- Song, N.; Yang, Y.-W. Molecular and supramolecular switches on mesoporous silica nanoparticles. Chem. Soc. Rev. 2015, 44, 3474–3504. [Google Scholar] [CrossRef]
- Stein, A.; Melde, B.J.; Schroden, R.C. Hybrid Inorganic-Organic Mesoporous Silicates—Nanoscopic Reactors Coming of Age. Adv. Mater. 2000, 12, 1403–1419. [Google Scholar] [CrossRef]
- Popat, A.; Hartono, S.B.; Stahr, F.; Liu, J.; Zhang Qiao, S.; Qing, G.; Lu, M. Mesoporous silica nanoparticles for bioadsorption, enzyme immobilisation, and delivery carriers. Nanoscale 2011, 3, 2801–2818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muhammad, F.; Guo, M.; Qi, W.; Sun, F.; Wang, A.; Guo, Y.; Zhu, G. pH-Triggered Controlled Drug Release from Mesoporous Silica Nanoparticles via Intracelluar Dissolution of ZnO Nanolids. J. Am. Chem. Soc. 2011, 133, 8778–8781. [Google Scholar] [CrossRef] [PubMed]
- Kamarudin, N.H.N.; Jalil, A.A.; Triwahyono, S.; Salleh, N.F.M.; Karim, A.H.; Mukti, R.R.; Hameed, B.H.; Ahmad, A. Role of 3-aminopropyltriethoxysilane in the preparation of mesoporous silica nanoparticles for ibuprofen delivery: Effect on physicochemical properties. Microporous Mesoporous Mater. 2013, 180, 235–241. [Google Scholar] [CrossRef]
- Natarajan, S.K.; Selvaraj, S. Mesoporous silica nanoparticles: Importance of surface modifications and its role in drug delivery. RSC Adv. 2014, 4, 14328. [Google Scholar] [CrossRef]
- Wang, G.; Otuonye, A.N.; Blair, E.A.; Denton, K.; Tao, Z.; Asefa, T. Functionalized mesoporous materials for adsorption and release of different drug molecules: A comparative study. J. Solid State Chem. 2009, 182, 1649–1660. [Google Scholar] [CrossRef]
- Lee, C.-H.; Cheng, S.-H.; Huang, I.-P.; Souris, J.S.; Yang, C.-S.; Mou, C.-Y.; Lo, L.-W. Intracellular pH-Responsive Mesoporous Silica Nanoparticles for the Controlled Release of Anticancer Chemotherapeutics. Angew. Chem. Int. Ed. 2010, 49, 8214–8219. [Google Scholar] [CrossRef]
- Li, Z.; Barnes, J.C.; Bosoy, A.; Stoddart, J.F.; Zink, J.I. Mesoporous silica nanoparticles in biomedical applications. Chem. Soc. Rev. 2012, 41, 2590. [Google Scholar] [CrossRef]
- Rosenholm, J.; Sahlgren, C.; Lindén, M. Cancer-cell targeting and cell-specific delivery by mesoporous silica nanoparticles. J. Mater. Chem. 2010, 20, 2707. [Google Scholar] [CrossRef]
- Ferris, D.P.; McGonigal, P.R.; Witus, L.S.; Kawaji, T.; Algaradah, M.M.; Alnajadah, A.R.; Nassar, M.S.; Stoddart, J.F. Oxime Ligation on the Surface of Mesoporous Silica Nanoparticles. Org. Lett. 2015, 17, 2146–2149. [Google Scholar] [CrossRef]
- Thanh, N.T.K.; Green, L.A.W. Author’s personal copy Functionalisation of nanoparticles for biomedical applications. Nano Today 2010, 5, 213–230. [Google Scholar] [CrossRef]
- Wan, X.; Yao, S.; Liu, H.; Yao, Y. Selective fluorescence sensing of Hg2+ and Zn2+ ions through dual independent channels based on the site-specific functionalization of mesoporous silica nanoparticles. J. Mater. Chem. A 2013, 1, 10505. [Google Scholar] [CrossRef]
- de Juan, F.; Ruiz-Hitzky, E. Selective Functionalization of Mesoporous Silica. Adv. Mater. 2000, 12, 430–432. [Google Scholar] [CrossRef]
- Gao, J.; Wu, S.; Tan, F.; Tian, H.; Liu, J.; Lu, G.Q.M. Nanoengineering of amino—functionalized mesoporous silica nanospheres as nanoreactors. Prog. Nat. Sci. Mater. Int. 2018, 28, 242–245. [Google Scholar] [CrossRef]
- Khung, Y.L.; Narducci, D. Surface modification strategies on mesoporous silica nanoparticles for anti-biofouling zwitterionic film grafting. Adv. Colloid Interface Sci. 2015, 226, 166–186. [Google Scholar] [CrossRef]
- Miranda, F.S.; Ronconi, C.M.; Sousa, M.O.B.; Silveira, G.Q.; Vargas, M.D. 6-Aminocoumarin-Naphthoquinone Conjugates: Design, Synthesis, Photophysical and Electrochemical Properties and DFT Calculations. J. Braz. Chem. Soc. 2013, 25, 133–142. [Google Scholar] [CrossRef]
- Huh, S.; Wiench, J.W.; Yoo, J.C.; Pruski, M.; Lin, V.S.Y. Organic Functionalization and Morphology Control of Mesoporous Silicas via a Co-Condensation Synthesis Method. Chem. Mater. 2003, 15, 4247–4256. [Google Scholar] [CrossRef]
- Suteewong, T.; Sai, H.; Cohen, R.; Wang, S.; Bradbury, M.; Baird, B.; Gruner, S.M.; Wiesner, U. Highly Aminated Mesoporous Silica Nanoparticles with Cubic Pore Structure. J. Am. Chem. Soc. 2011, 133, 172–175. [Google Scholar] [CrossRef]
- Kecht, J.; Schlossbauer, A.; Bein, T. Selective Functionalization of the Outer and Inner Surfaces in Mesoporous Silica Nanoparticles. Chem. Mater. 2008, 20, 7207–7214. [Google Scholar] [CrossRef]
- Lallana, E.; Sousa-Herves, A.; Fernandez-Trillo, F.; Riguera, R.; Fernandez-Megia, E. Click Chemistry for Drug Delivery Nanosystems. Pharm. Res. 2012, 29, 1–34. [Google Scholar] [CrossRef]
- Li, N.; Binder, W.H. Click-chemistry for nanoparticle-modification. J. Mater. Chem. 2011, 21, 16717. [Google Scholar] [CrossRef]
- Pan, L.; He, Q.; Liu, J.; Chen, Y.; Ma, M.; Zhang, L.; Shi, J. Nuclear-Targeted Drug Delivery of TAT Peptide-Conjugated Monodisperse Mesoporous Silica Nanoparticles. J. Am. Chem. Soc. 2012, 134, 5722–5725. [Google Scholar] [CrossRef]
- Doussineau, T.; Trupp, S.; Mohr, G.J. Ratiometric pH-nanosensors based on rhodamine-doped silica nanoparticles functionalized with a naphthalimide derivative. J. Colloid Interface Sci. 2009, 339, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Kotsuchibashi, Y.; Ebara, M.; Aoyagi, T.; Narain, R. Fabrication of doubly responsive polymer functionalized silica nanoparticles via a simple thiol–ene click chemistry. Polym. Chem. 2012, 3, 2545. [Google Scholar] [CrossRef]
- Tian, Y.-Q.; Cai, C.-X.; Ren, X.-M.; Duan, C.-Y.; Xu, Y.; Gao, S.; You, X.-Z. The Silica-Like Extended Polymorphism of Cobalt(II) Imidazolate Three-Dimensional Frameworks: X-ray Single-Crystal Structures and Magnetic Properties. Chem. A Eur. J. 2003, 9, 5673–5685. [Google Scholar] [CrossRef]
- Bolley, J.; Guenin, E.; Lievre, N.; Lecouvey, M.; Soussan, M.; Lalatonne, Y.; Motte, L. Carbodiimide versus Click Chemistry for Nanoparticle Surface Functionalization: A Comparative Study for the Elaboration of Multimodal Superparamagnetic Nanoparticles Targeting α v β 3 Integrins. Langmuir 2013, 29, 14639–14647. [Google Scholar] [CrossRef] [PubMed]
- Malvi, B.; Sarkar, B.R.; Pati, D.; Mathew, R.; Ajithkumar, T.G.; Sen Gupta, S. “Clickable” SBA-15 mesoporous materials: Synthesis, characterization and their reaction with alkynes. J. Mater. Chem. 2009, 19, 1409. [Google Scholar] [CrossRef]
- Radu, D.R.; Lai, C.Y.; Jeftinija, K.; Rowe, E.W.; Jeftinija, S.; Lin, V.S.Y. A Polyamidoamine Dendrimer-Capped Mesoporous Silica Nanosphere-Based Gene Transfection Reagent. J. Am. Chem. Soc. 2004. [Google Scholar] [CrossRef]
- Zhang, Q.; Neoh, K.G.; Xu, L.; Lu, S.; Kang, E.T.; Mahendran, R.; Chiong, E. Functionalized Mesoporous Silica Nanoparticles with Mucoadhesive and Sustained Drug Release Properties for Potential Bladder Cancer Therapy. Langmuir 2014, 30, 6151–6161. [Google Scholar] [CrossRef]
- El Malti, W.; Mongin, O.; Blanchard-Desce, M.; Raehm, L.; Durand, J.-O. Functionalisation of mesoporous silica nanoparticles with 3-isocynatopropyltrichlorosilane. Comptes Rendus Chim. 2011, 14, 1055–1058. [Google Scholar] [CrossRef]
- Wiberg, K.B.; Wang, Y.; Miller, S.J.; Puchlopek, A.L.A.; Bailey, W.F.; Fair, J.D. Disparate Behavior of Carbonyl and Thiocarbonyl Compounds: Acyl Chlorides vs. Thiocarbonyl Chlorides and Isocyanates vs Isothiocyanates. J. Org. Chem. 2009, 74, 3659–3664. [Google Scholar] [CrossRef] [PubMed]
- Hermanson, G.T. Bioconjugate Techniques; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Li, C.; Liu, J.; Alonso, S.; Li, F.; Zhang, Y. Upconversion nanoparticles for sensitive and in-depth detection of Cu2+ ions. Nanoscale 2012, 4, 6065. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.-H.; Lee, C.-H.; Yang, C.-S.; Tseng, F.-G.; Mou, C.-Y.; Lo, L.-W. Mesoporous silica nanoparticles functionalized with an oxygen-sensing probe for cell photodynamic therapy: Potential cancer theranostics. J. Mater. Chem. 2009, 19, 1252. [Google Scholar] [CrossRef]
- Grabchev, I.; Moneva, I.; Bojinov, V.; Guittonneau, S. Synthesis and properties of fluorescent 1,8-naphthalimide dyes for application in liquid crystal displays. J. Mater. Chem. 2000, 10, 1291–1296. [Google Scholar] [CrossRef]
- Grandjean, C.; Boutonnier, A.; Guerreiro, C.; Fournier, J.-M.; Mulard, L.A. On the Preparation of Carbohydrate−Protein Conjugates Using the Traceless Staudinger Ligation. J. Org. Chem. 2005, 70, 7123–7132. [Google Scholar] [CrossRef] [PubMed]
- Yum, J.-H.; Baranoff, E.; Wenger, S.; Nazeeruddin, M.K.; Gr, M. Panchromatic engineering for dye-sensitized solar cells. Energy Environ. 2011, 4, 842–857. [Google Scholar] [CrossRef]
- Suga, Y.; Sunayama, H.; Ooya, T.; Takeuchi, T. Molecularly imprinted polymers prepared using protein-conjugated cleavable monomers followed by site-specific post-imprinting introduction of fluorescent reporter molecules. Chem. Commun. 2013, 49, 8450. [Google Scholar] [CrossRef]
- Pei, J.; Wang, J.L.; Cao, X.Y.; Zhou, X.H.; Zhang, W.B. Star-Shaped Polycyclic Aromatics Based on Oligothiophene-Functionalized Truxene: Synthesis, Properties, and Facile Emissive Wavelength Tuning. J. Am. Chem. Soc. 2003. [Google Scholar] [CrossRef]
- Li, G.; Bhosale, S.V.; Wang, T.; Hackbarth, S.; Roeder, B.; Siggel, U.; Fuhrhop, J.-H. Nanowells on Silica Particles in Water Containing Long-Distance Porphyrin Heterodimers. J. Am. Chem. Soc. 2003, 125, 10693–10702. [Google Scholar] [CrossRef]
- Faure, A.-C.; Hoffmann, C.; Bazzi, R.; Goubard, F.; Pauthe, E.; Marquette, C.A.; Blum, L.J.; Perriat, P.; Roux, S.; Tillement., O. Functionalization of Luminescent Aminated Particles for Facile Bioconjugation. ACS Nano 2008, 2, 2273–2282. [Google Scholar] [CrossRef]
- Wong, R.; Dolman, S.J. Isothiocyanates from Tosyl Chloride Mediated Decomposition of in Situ Generated Dithiocarbamic Acid Salts. J. Org. Chem. 2007, 72, 3969–3971. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Li, B.; Shao, J.; Mo, W.; Hu, B.; Shen, Z.; Hu, X. A general and facile one-pot process of isothiocyanates from amines under aqueous conditions. Beilstein J. Org. Chem. 2012, 8, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Harpp, D.N.; MacDonald, J.G.; Larsen, C. Utilization of thiocarbonyl transfer reagents. Preparation of thioamides, mercapto esters, and thiapyran derivatives. Can. J. Chem. 1985, 63, 951–957. [Google Scholar] [CrossRef]
- Kim, S.; Yi, K.Y. 1,1′-Thiocarbonyldi-2,2′-pyridone. A new useful reagent for functional group conversions under essentially neutral conditions. J. Org. Chem. 1986, 51, 2613–2615. [Google Scholar] [CrossRef]
- Ohno, K.; Akashi, T.; Tsujii, Y.; Yamamoto, M.; Tabata, Y. Blood Clearance and Biodistribution of Polymer Brush-Afforded Silica Particles Prepared by Surface-Initiated Living Radical Polymerization. Biomacromolecules 2012, 13, 927–936. [Google Scholar] [CrossRef]
- Department of Reproductive Health World Health Organization. Medical Eligibility Criteria for Contraceptive Use, 4th ed.; WHO: Geneva, Switzerland, 2010; pp. 1–130. [Google Scholar]
- Bibee, K.P.; Cheng, Y.-J.; Ching, J.K.; Marsh, J.N.; Li, A.J.; Keeling, R.M.; Connolly, A.M.; Golumbek, P.T.; Myerson, J.W.; Hu, G.; et al. Rapamycin nanoparticles target defective autophagy in muscular dystrophy to enhance both strength and cardiac function. FASEB J. 2014, 28, 2047–2061. [Google Scholar] [CrossRef] [Green Version]
- Vallet-Regí, M.; Balas, F.; Arcos, D. Mesoporous Materials for Drug Delivery. Angew. Chemie Int. Ed. 2007, 46, 7548–7558. [Google Scholar] [CrossRef]





| MSNs | C (%) | H (%) | N (%) |
|---|---|---|---|
| MSN–(NCS) | 9.78 | 1.87 | 1.92 |
| MSN–(UNaph) | 14.59 | 2.59 | 2.32 |
| MSN–(N3) | 7.22 | 2.56 | 2.64 |
| MSN–(TNaph) | 11.79 | 2.56 | 3.08 |
| Property | MSN–(NH2) (CTAB) | MSN–(NH2) | MSN–(NH2)i(NCS)o |
|---|---|---|---|
| BET surface area (m2/g) | 17.3 | 1120.90 | 1000.70 |
| BJH pore volume (cm3/g) | 0.03 | 0.72 | 0.63 |
| Pore size (nm) | – | 2.20 | 2.20 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Edo, G.; Llinàs, M.C.; Borrós, S.; Sánchez-García, D. Isothiocyanate-Functionalized Mesoporous Silica Nanoparticles as Building Blocks for the Design of Nanovehicles with Optimized Drug Release Profile. Nanomaterials 2019, 9, 1219. https://doi.org/10.3390/nano9091219
Martínez-Edo G, Llinàs MC, Borrós S, Sánchez-García D. Isothiocyanate-Functionalized Mesoporous Silica Nanoparticles as Building Blocks for the Design of Nanovehicles with Optimized Drug Release Profile. Nanomaterials. 2019; 9(9):1219. https://doi.org/10.3390/nano9091219
Chicago/Turabian StyleMartínez-Edo, Gabriel, Maria C. Llinàs, Salvador Borrós, and David Sánchez-García. 2019. "Isothiocyanate-Functionalized Mesoporous Silica Nanoparticles as Building Blocks for the Design of Nanovehicles with Optimized Drug Release Profile" Nanomaterials 9, no. 9: 1219. https://doi.org/10.3390/nano9091219





