Carbon Nanotube-Quicklime Nanocomposites Prepared Using a Nickel Catalyst Supported on Calcium Oxide Derived from Carbonate Stones
Abstract
:1. Introduction
2. Materials and Methods
2.1. Catalyst Preparation
2.2. Synthesis of Carbon Nanotube Quicklime Nanocomposites
2.3. Material Characterizations
2.4. Modification of Screen-Printed Carbon Electrodes and Electrochemical Measurement
2.4.1. Modification of Screen-Printed Electrodes Using Nanomaterials
2.4.2. Cyclic Voltammetry Studies of the Modified SPCE
3. Results and Discussions
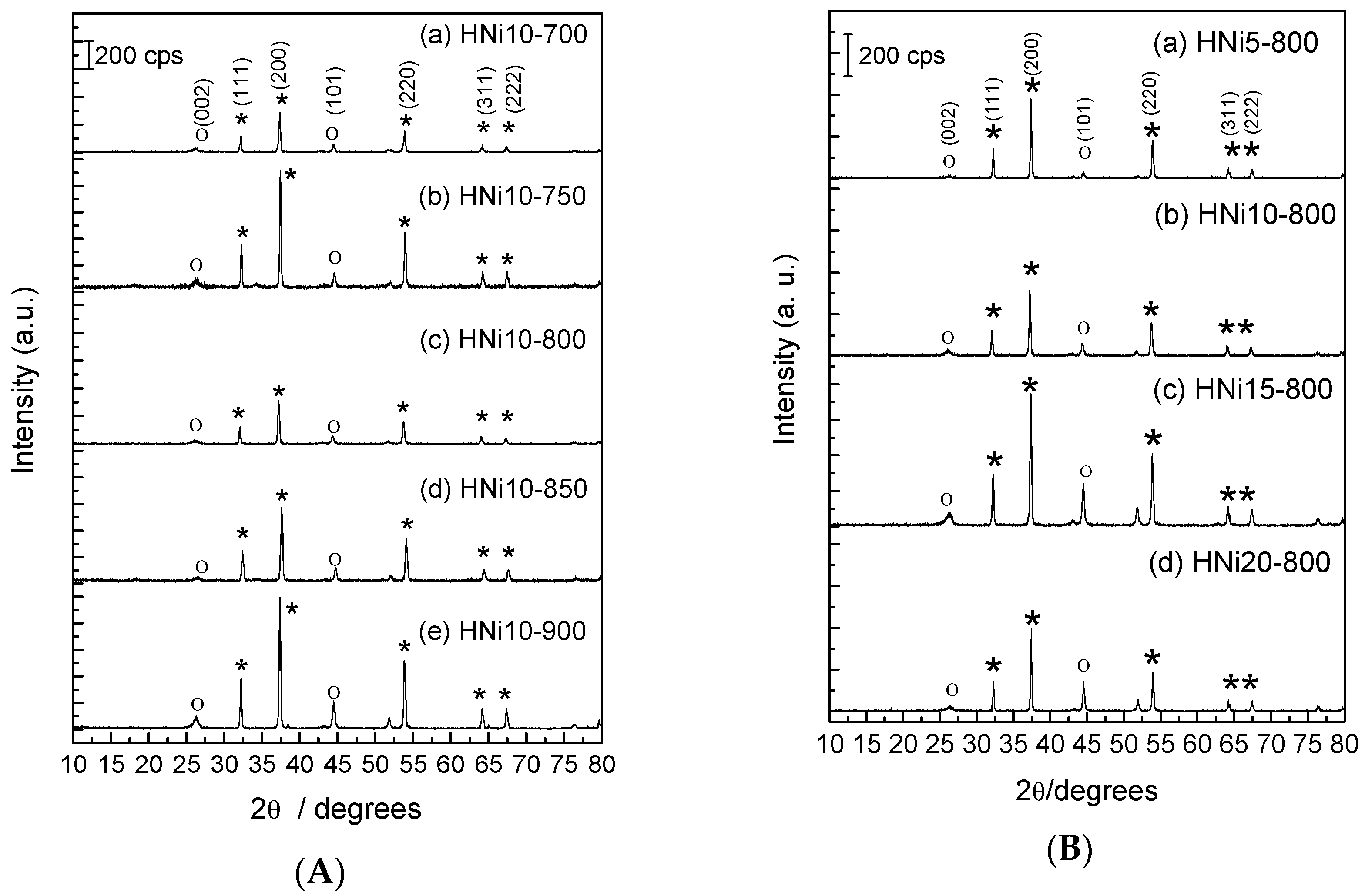
3.1. X-Ray Diffraction Results
3.2. Raman Spectroscopy
3.3. BET Surface Area and Porosity of Ni-Catalyzed CQNs
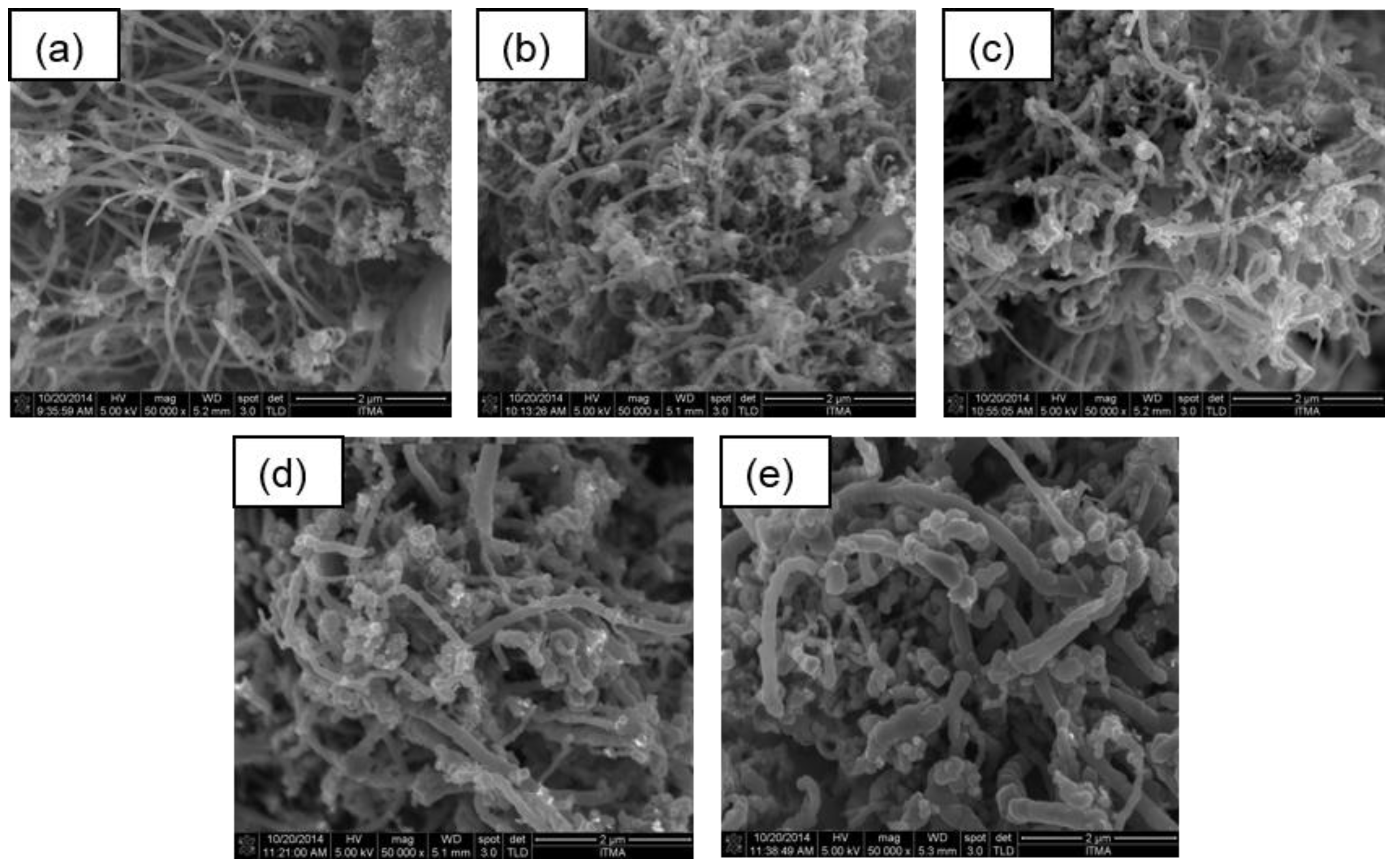
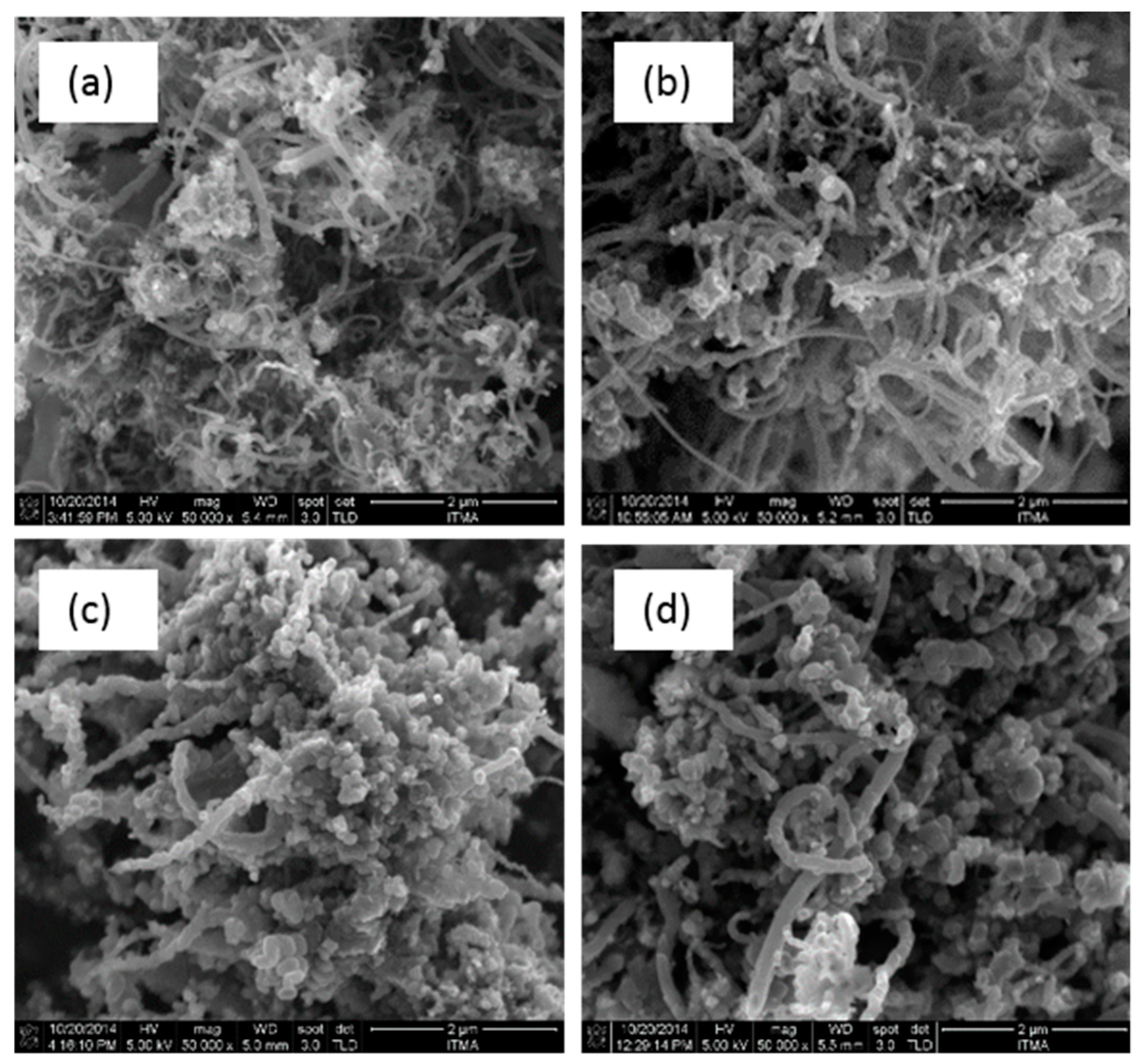
3.4. Field Emission Scanning Electron Microscopy
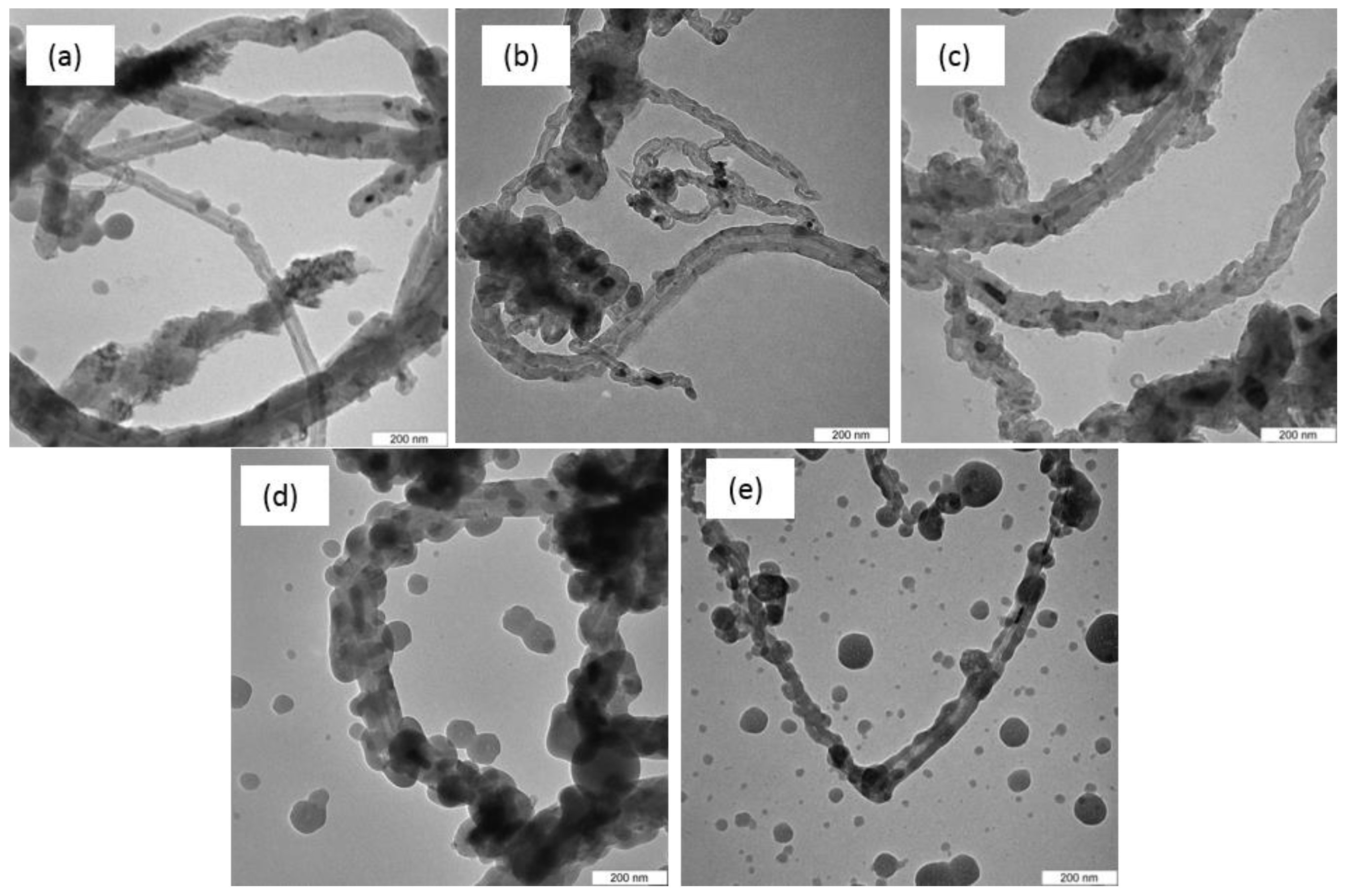
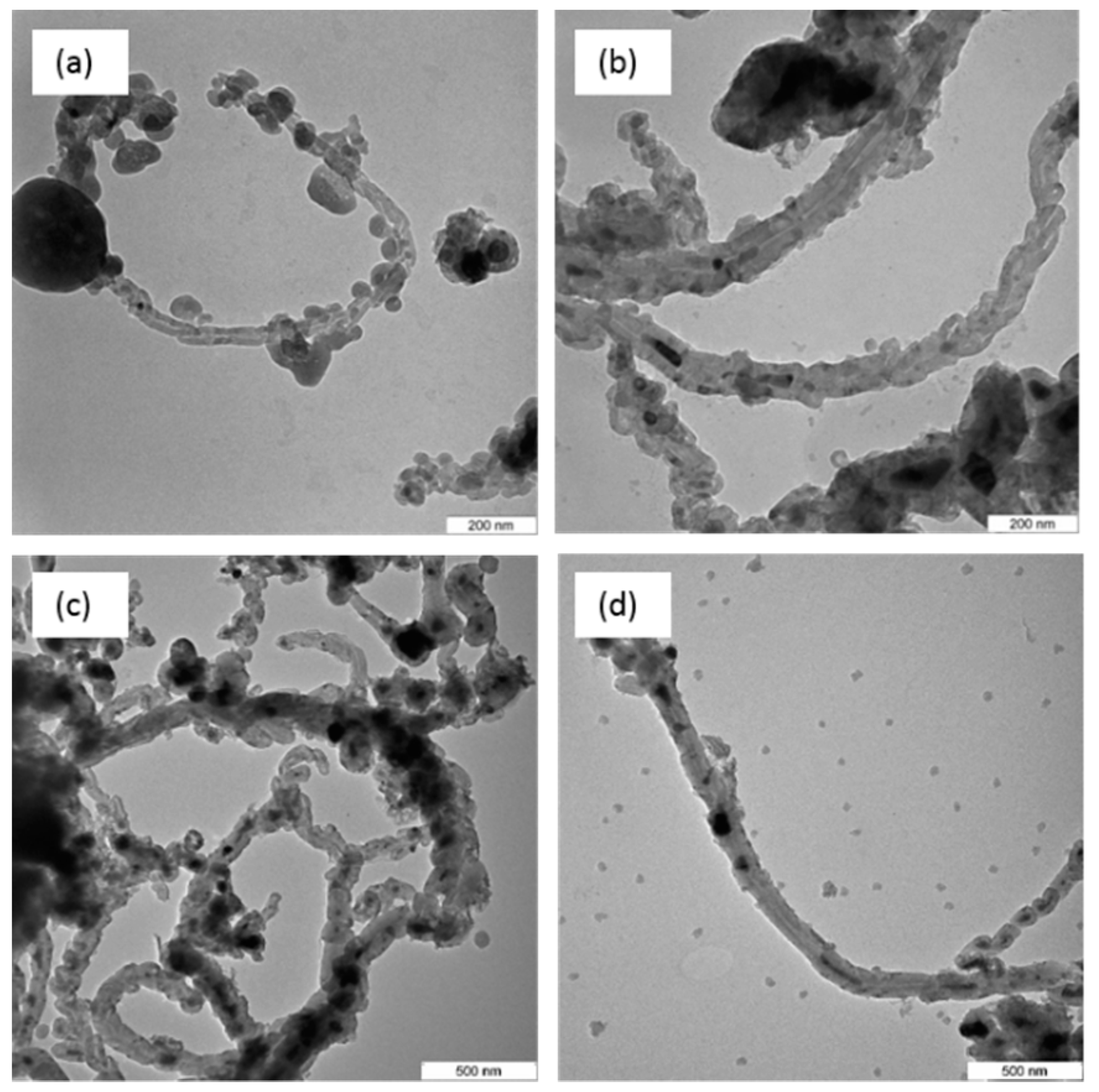
3.5. Transmission Electron Microscopy
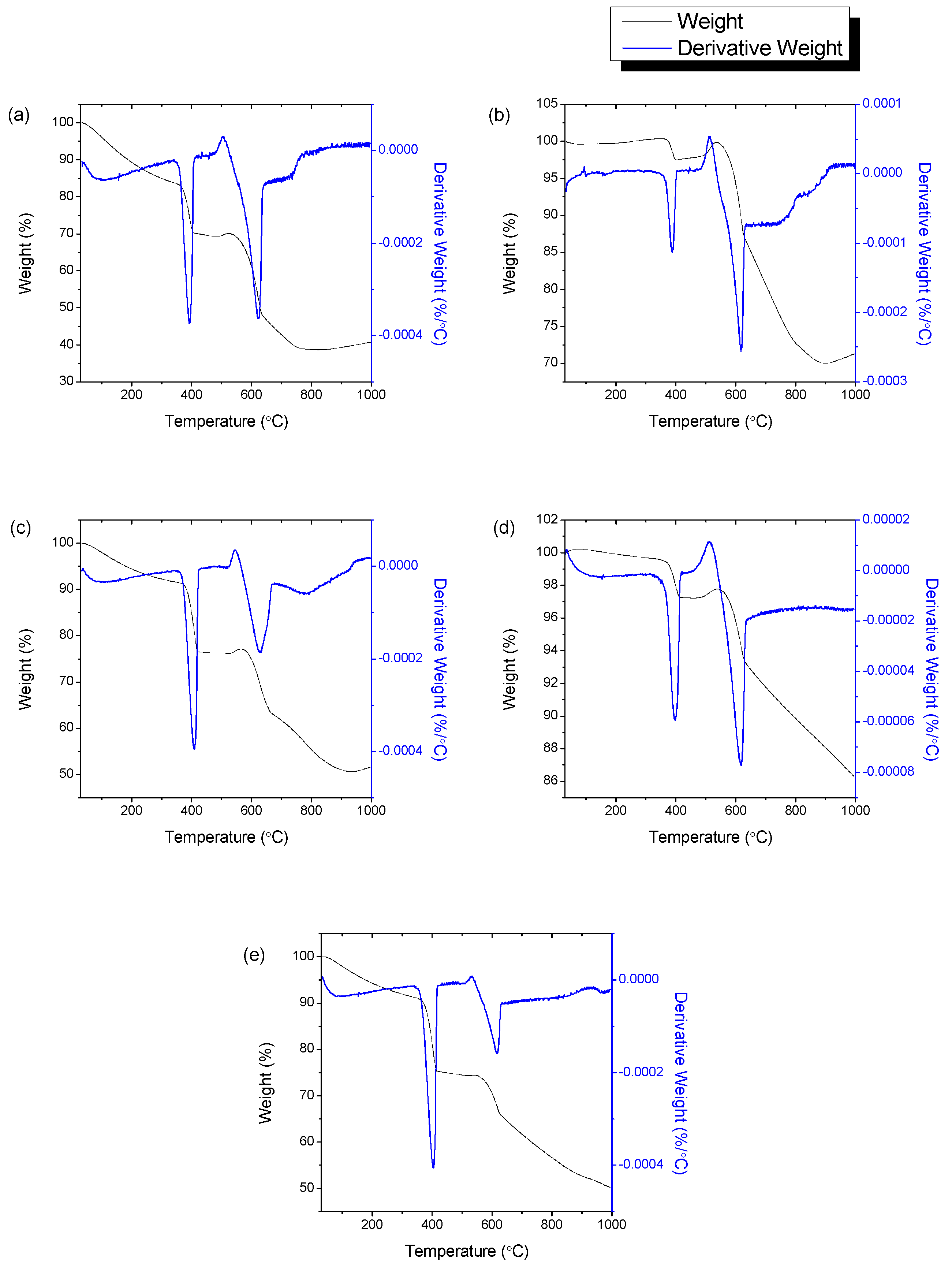
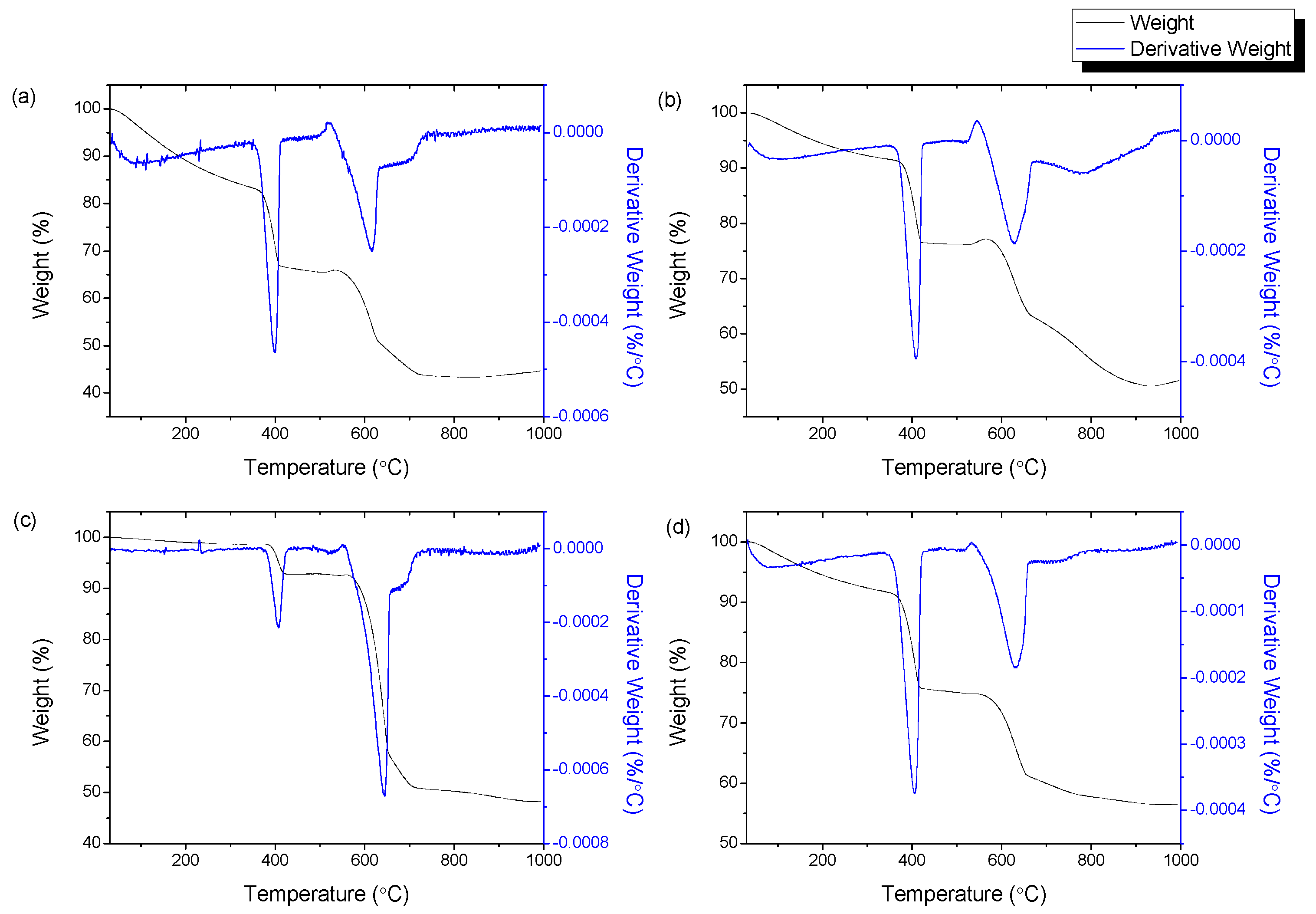
3.6. Thermogravimetric Analysis
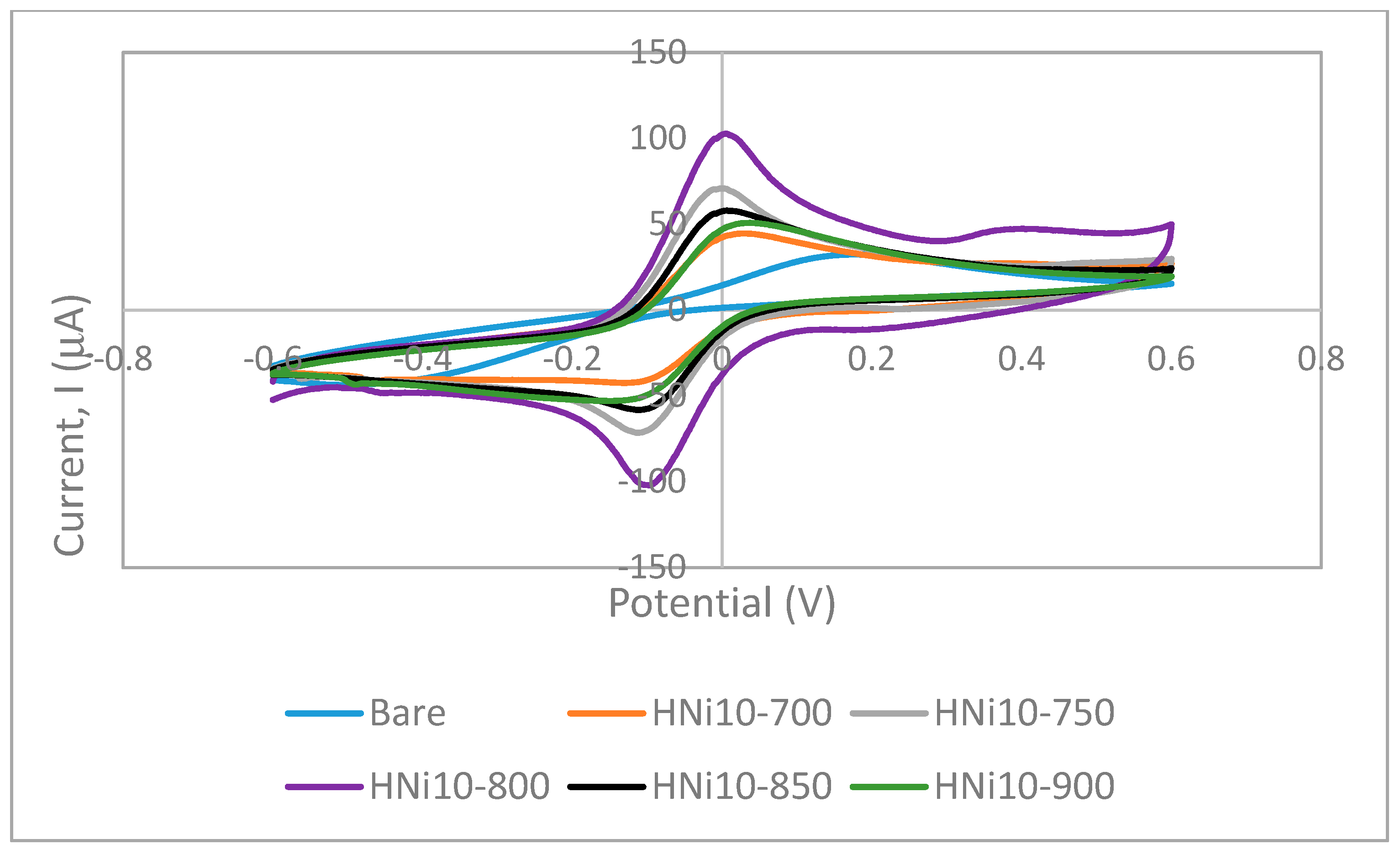
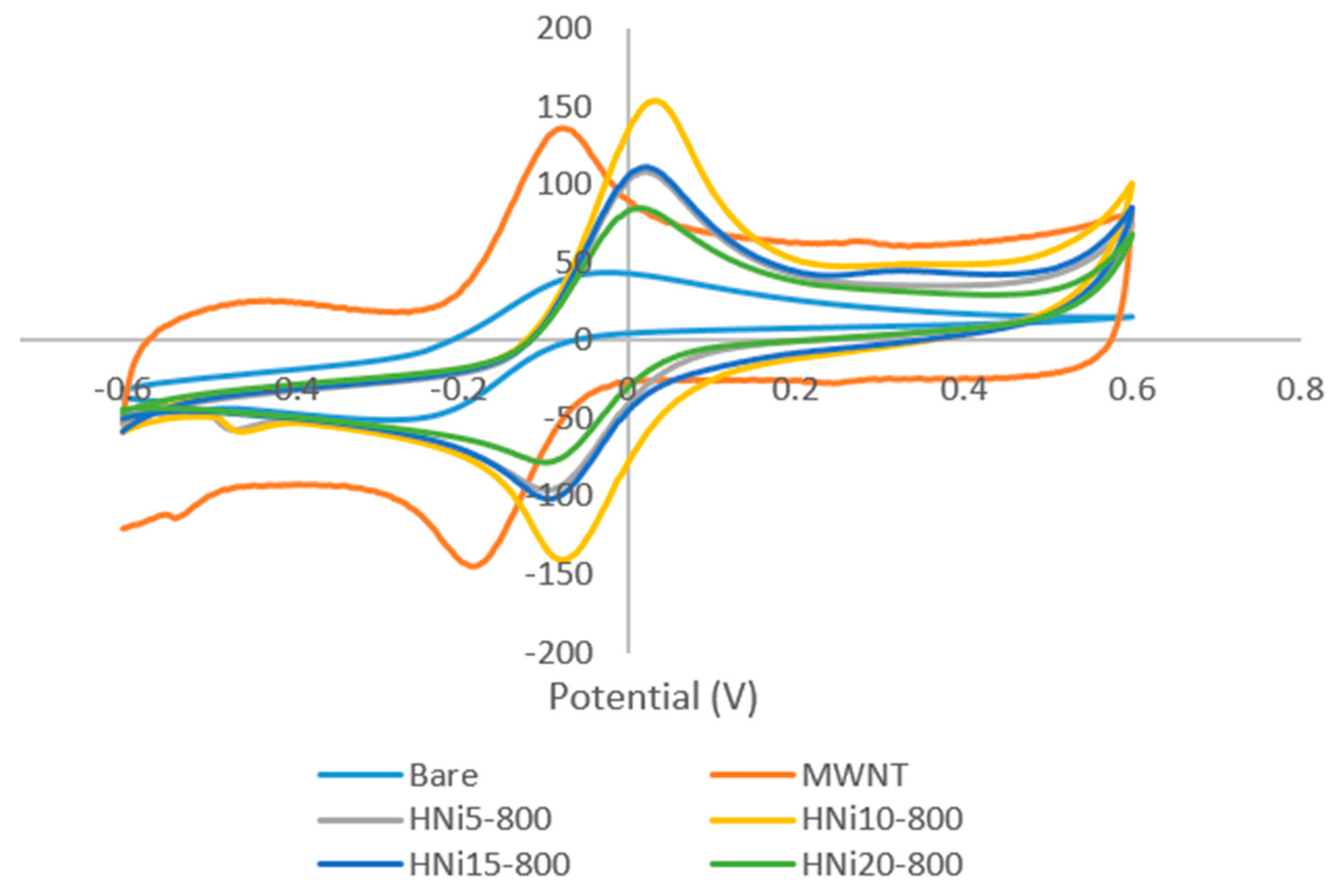
3.7. Cyclic Voltammetry of Nanocomposite-Modified Screen-Printed Electrodes
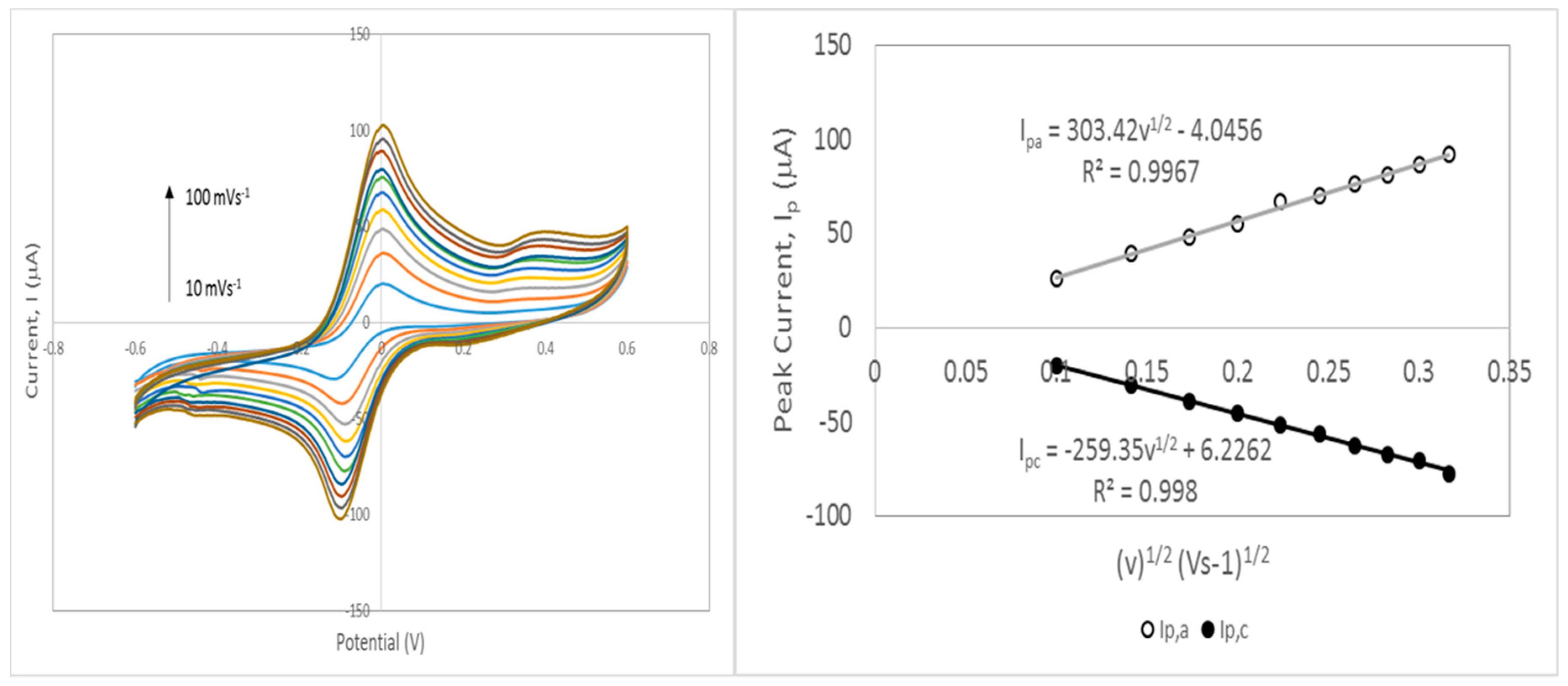
3.7.1. Electroactive Surface Area of HNi Nanocomposite-Modified Screen-Printed Electrode
3.7.2. Estimation of Kinetic Parameter: Heterogeneous Electron Transfer Rate Constant (k0)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Gao, C.; Guo, Z.; Liu, J.H.; Huang, X.J. The new age of carbon nanotubes: An updated review of functionalized carbon nanotubes in electrochemical sensors. Nanoscale 2012, 4, 1948–1963. [Google Scholar] [CrossRef] [PubMed]
- Dresselhaus, A.M.S.; Dresselhaus, G.; Hofmann, M.; Fundamentals, C.E.; Jan, D.A.; Hofmann, M. Raman Spectroscopy as a Probe of Graphene and Carbon Nanotubes. Philos. Trans. Math. Phys. Eng. Sci. 2013, 366, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Tsoufis, T.; Xidas, P.; Jankovic, L.; Gournis, D.; Saranti, A.; Bakas, T.; Karakassides, M.A. Catalytic production of carbon nanotubes over Fe-Ni bimetallic catalysts supported on MgO. Diam. Relat. Mater. 2007, 16, 155–160. [Google Scholar] [CrossRef]
- Maruyama, S.; Kojima, R.; Miyauchi, Y.; Chiashi, S.; Kohno, M. Low-temperature synthesis of high-purity single-walled carbon nanotubes from alcohol. Chem. Phys. Lett. 2002, 360, 229–234. [Google Scholar] [CrossRef]
- Mendoza, D.; Santiago, P.; Mendozaa, D.; P’erez, E.R. Carbon nanotubes produced from hexane and ethanol. Rev. Mex. F´IsicaCarta. 2006, 52, 1–5. [Google Scholar]
- Li, Z.; Dervishi, E.; Xu, Y.; Saini, V.; Mahmood, M.; Oshin, O.D.; Biris, A.R.; Biris, A.S. Carbon nanotube growth on calcium carbonate supported molybdenum-transition bimetal catalysts. Catal. Letters 2009, 131, 356–363. [Google Scholar] [CrossRef]
- Buasri, A.; Rochanakit, K.; Wongvitvichot, W. The Application of Calcium Oxide and Magnesium Oxide from Natural Dolomitic Rock for Biodiesel Synthesis; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Radfarnia, H.R.; Iliuta, M.C. Developmentof Zirconium-Stabilized Calcium Oxide Absorbent for Cyclic High-Temperature CO2 Capture. Ind. Eng. Chem. Res. 2012, 51, 10390–10398. [Google Scholar] [CrossRef]
- Sultana, K.S.; Tran, D.T.; Walmsley, J.C.; Ronning, M.; Chen, D. CaO Nanoparticles Coated by ZrO2 Layers for Enhanced CO2 Capture Stability. Ind. Eng. Chem. Res. 2015, 54, 8929–8939. [Google Scholar] [CrossRef]
- Zhuiykov, S. Electrochemistry of Zirconia Gas Sensors; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Wang, Z.L. Nanowires and Nanobelts:Materials, Properties and Devices: Volume 2: Nanowires and Nanobelts of Functional Materials; Springer Science and Business Media: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Kobun, R.; Siddiquee, S.; Shaarani, S.M. Sensitive Determination of Tartrazine (E 102) Based on Chitosan/Nanoparticles/MWCNTs Modified Gold Electrode in Food and Beverage Products. Trans. Sci. Technol. 2016, 3, 176–180. [Google Scholar]
- Kathyayini, H.; Nagaraju, B.; Fonseca, A.; Nagy, J.B. Catalytic activity of Fe, Co and Fe/Co supported on Ca and Mg oxides, hydroxides and carbonates in the synthesis of carbon nanotubes. Mol. Catal. 2004, 223, 129–136. [Google Scholar] [CrossRef]
- Couteau, E.; Hernadi, K.; Seo, J.W.; Thiên-Nga, L.; Mikó, C.; Gaál, R.; Forró, L. CVD synthesis of high-purity multiwalled carbon nanotubes using CaCO3 catalyst support for large-scale production. Chem. Phys. Lett. 2003, 378, 9–17. [Google Scholar] [CrossRef]
- See, C.H.; Harris, A.T. CaCO3 Supported Co-Fe Catalysts for Carbon Nanotube Synthesis in Fluidized Bed Reactors. AIChE J. 2008, 54, 657–664. [Google Scholar] [CrossRef]
- Dikio, E.D.; Shooto, N.D. Raman and TGA Study of Carbon Nanotubes Synthesized Over Mo / Fe Catalyst on Aluminium Oxide, Calcium Carbonate and Magnesium Oxide Support. Chem. Sci. Trans. 2011, 2, 1160–1173. [Google Scholar]
- Wang, B.; Han, Y.; Liu, S. Effect of highly dispersed carbon nanotubes on the flexural toughness of cement-based composites. Constr. Build. Mater. 2013, 46, 8–12. [Google Scholar] [CrossRef]
- Morsy, M.S.; Alsayed, S.H.; Aqel, M. Hybrid effect of carbon nanotube and nano-clay on physico-mechanical properties of cement mortar. Constr. Build. Mater. 2011, 25, 145–149. [Google Scholar] [CrossRef]
- Konsta-Gdoutos, M.S.; Metaxa, Z.S.; Shah, S.P. Highly dispersed carbon nanotube reinforced cement based materials. Cem. Concr. Res. 2010, 40, 1052–1059. [Google Scholar] [CrossRef]
- Nien, Y.; Huang, C. The mechanical study of acrylic bone cement reinforced with carbon nanotube. Mater. Sci. Eng. B 2010, 169, 134–137. [Google Scholar] [CrossRef]
- Sobolkina, A.; Mechtcherine, V.; Khavrus, V.; Maier, D.; Mende, M.; Ritschel, M.; Leonhardt, A. Dispersion of carbon nanotubes and its influence on the mechanical properties of the cement matrix. Cem. Concr. Compos. 2012, 34, 1104–1113. [Google Scholar] [CrossRef]
- Boey, P.L.; Maniam, G.P.; Hamid, S.A. Performance of calcium oxide as a heterogeneous catalyst in biodiesel production: A review. Chem. Eng. J. 2011, 168, 15–22. [Google Scholar] [CrossRef] [Green Version]
- Marinkovic, D.M.; Stankovic, M.V.; Velickovic, A.V.; Avramovic, J.M.; Miladinovic, M.R.; Stamenkovic, O.O.; Veljkovic, V.B.; Jovanovic, D.M. Calcium oxide as a promising heterogeneous catalyst for biodiesel production: Current state and perspectives. Renew. Sustain. Energy Rev. 2016, 56, 1387–1408. [Google Scholar] [CrossRef]
- Guan, Q.; Li, Y.; Chen, Y.; Shi, Y.; Gu, J.; Li, B.; Miao, R.; Chen, Q.; Ning, P. Sulfonated multi-walled carbon nanotubes for biodiesel production through triglycerides transesterification. RSC Adv. 2017, 7, 7250–7258. [Google Scholar] [CrossRef] [Green Version]
- Zu, Y.; Tang, J.; Zhu, W.; Zhang, M.; Liu, G.; Liu, Y.; Zhang, W.; Jia, M. Graphite oxide-supported CaO catalysts for transesterification of soybean oil with methanol. Bioresour. Technol. 2011, 102, 8939–8944. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, X.; Pan, L.; Li, H.; Sun, Z.; Sun, C.; Tay, K. Carbon nanotube – zinc oxide electrode and gel polymer electrolyte for electrochemical supercapacitors. J. Alloys Compd. 2009, 480, 17–19. [Google Scholar] [CrossRef]
- Sakellariou, K.G.; Karagiannakis, G.; Criado, Y.A.; Konstandopoulos, A.G. Calcium oxide based materials for thermochemical heat storage in concentrated solar power plants. Sol. Energy. 2015, 122, 215–230. [Google Scholar]
- Yen, M.; Hsiao, M.; Liao, S.; Liu, P.; Tsai, H. Preparation of graphene/multi-walled carbon nanotube hybrid and its use as photoanodes of dye-sensitized solar cells. Carbon 2011, 49, 3597–3606. [Google Scholar] [CrossRef]
- Ansaldo, A.; Haluška, M.; Čech, J.; Meyer, J.C.; Ricci, D.; Gatti, F.; di Zitti, E.; Cincotti, S.; Roth, S. A study of the effect of different catalysts for the efficient CVD growth of carbon nanotubes on silicon substrates. Phys. E Low-dimensional Syst. Nanostructures 2007, 37, 6–10. [Google Scholar] [CrossRef]
- Belin, T.; Epron, F. Characterization methods of carbon nanotubes: A review. Mater. Sci. Eng. B 2005, 119, 105–118. [Google Scholar] [CrossRef]
- Kang, J.; Li, J.; Zhao, N.; Du, X.; Shi, C.; Nash, P. The effect of catalyst evolution at various temperatures on carbon nanostructures formed by chemical vapor deposition. J. Mater. Sci. 2009, 44, 2471–2476. [Google Scholar] [CrossRef]
- Kamalakar, G.; Hwang, D.W.; Hwang, L.P. Synthesis and characterization of multiwalled carbon nanotubes produced using zeolite Co-beta. J. Mater. Chem. 2002, 12, 1819–1823. [Google Scholar] [CrossRef]
- Andrews, R.; Jaqques, D.; Qian, D.; Rantell, T. Multiwall carbon nanotubes: Synthesis and application. J. Acc. Chem. Res. 2002, 35, 1008–1017. [Google Scholar] [CrossRef]
- Costa, S.; Borowiak-palen, E.; Bachmatiuk, A.; Rümmeli, M.H.; Gemming, T.; Kalenczuk, R.J. Iron filled carbon nanostructures from different precursors. Energy Convers. Manag. 2008, 49, 2483–2486. [Google Scholar] [CrossRef]
- Shi, Q.; Yang, D.; Su, Y.; Li, J.; Jiang, Z.; Jiang, Y.; Yuan, W. Covalent functionalization of multi-walled carbon nanotubes by lipase. J. Nanoparticle Res. 2007, 9, 1205–1210. [Google Scholar] [CrossRef]
- Li, W.; Wang, H.; Ren, Z.; Wang, G.; Bai, J. Co-production of hydrogen and multi-wall carbon nanotubes from ethanol decomposition over Fe/Al2O3 catalysts. Appl. Catal. B Environ. 2008, 84, 433–439. [Google Scholar] [CrossRef]
- Birch, M.E.; Ruda-eberenz, T.A.; Chai, M.; Andrews, R.; Hatfield, R.L. Properties that Influence the Specific Surface Areas of Carbon Nanotubes and Nanofibers. Ann. Occup. Hyg. 2013, 57, 1148–1166. [Google Scholar] [Green Version]
- Alexiadis, V.I.; Boukos, N.; Verykios, X.E. Influence of the composition of Fe2O3/Al2O3 catalysts on the rate of production and quality of carbon nanotubes. Mater. Chem. Phys. 2011, 128, 96–108. [Google Scholar] [CrossRef]
- Alves, J.O.; Zhuo, C.; Levendis, Y.A.; Tenório, J.A.S. Catalytic conversion of wastes from the bioethanol production into carbon nanomaterials. Appl. Catal. B Environ. 2011, 106, 433–444. [Google Scholar] [CrossRef]
- Hoyos-Palacio, L.M.; García, A.G.; Pérez-Robles, J.F.; González, J.; Martínez-Tejada, H.V. Catalytic effect of Fe, Ni, Co and Mo on the CNTs production. IOP Conf. Ser. Mater. Sci. Eng. 2014, 59, 1–8. [Google Scholar] [CrossRef]
- Mizoguti, E.; Nihey, F.; Yudasaka, M.; Iijima, S.; Ichihashi, T.; Nakamura, K. Purification of single-wall carbon nanotubes by using ultrafine gold particles. Chem. Phys. Lett. 2000, 321, 297–301. [Google Scholar] [CrossRef]
- Pumera, M.; Ambrosi, A.; Chng, E.L.K. Impurities in graphenes and carbon nanotubes and their influence on the redox properties. Chem. Sci. 2012, 3, 3347. [Google Scholar] [CrossRef]
- Purwidyantri, A.; Chen, C.H.; Chen, L.Y.; Chen, C.C.; Luo, J.D.; Chiou, C.C.; Tian, Y.C.; Lin, C.Y.; Yang, C.M.; Lai, H.C.; et al. Speckled ZnO Nanograss Electrochemical Sensor for Staphylococcus epidermidis Detection. J. Electrochem. Soc. 2017, 164, B205–B211. [Google Scholar] [CrossRef]
- Silva, T.A.; Zanin, H.; Saito, E.; Medeiros, R.A.; Vincentini, F.C.; Corat, E.J.; Fatibello-filho, O. Electrochemical behaviour of vertically aligned carbon nanotubes and graphene oxide nanocomposite as electrode material. Electrochim. Acta 2014, 119, 114–119. [Google Scholar] [CrossRef]
- Bollo, S.; Finger, S.; Sturm, J.C.; Squella, J.A. Cyclic voltammetry and scanning electrochemical microscopy studies of the heterogeneous electron transfer reaction of some nitrosoaromatic compounds. Electrochim. Acta 2007, 52, 4892–4898. [Google Scholar] [CrossRef]
- Mohammad, M.; Aslam, M. Heterogeneous Electron Transfer Studies on the Reduction of Some Pyridinium Cations: Substituents and the Inner-Reorganization Energy. J. Chem. Soc. Pakistan 2011, 33, 12–20. [Google Scholar]
- Lavagnini, I.; Antiochia, R.; Magno, F. An extended method for the practical evaluation of the standard rate constant from cyclic voltammetric data. Electroanalysis 2004, 16, 505–506. [Google Scholar] [CrossRef]


| No. | Sample | Catalyst Ni (wt%) | BET * Surface Area (m2/g) | Total Pore Volume (cm3/g) | BJH * Average Pore Diameter(Å) |
|---|---|---|---|---|---|
| 1 | HNi10-700 | 10 | 13 | 0.084 | 260 |
| 2 | HNi10-750 | 10 | 12 | 0.078 | 250 |
| 3 | HNi10-800 | 10 | 10 | 0.069 | 290 |
| 4 | HNi10-850 | 10 | 6 | 0.051 | 340 |
| 5 | HNi10-900 | 10 | 7 | 0.063 | 360 |
| 6 | HNi5-800 | 5 | 8 | 0.067 | 330 |
| 7 | HNi15-800 | 15 | 11 | 0.069 | 240 |
| 8 | HNi20-800 | 20 | 8 | 0.070 | 370 |
| Sample | Weight Loss 1 | Weight Loss 2 | ||
|---|---|---|---|---|
| T (°C) | wt% | T (°C) | wt% | |
| HNi10-700 | 350–373 | 13.11 | 537–576 | 14.06 |
| HNi10-750 | 330–423 | 2.73 | 537–655 | 15.26 |
| HNi10-800 | 200–477 | 18.35 | 573–708 | 15.78 |
| HNi10-850 | 300–452 | 2.98 | 540–600 | 11.47 |
| HNi10-900 | 357–381 | 13.90 | 549–609 | 26.77 |
| HNi5-800 | 345–369 | 17.24 | 537–579 | 8.68 |
| HNi15-800 | 349–376 | 8.43 | 540–581 | 5.76 |
| HNi20-800 | 342–367 | 13.46 | 523–568 | 8.83 |
| Electrode | Geometrical Area (cm2) | Electroactive Area (cm2) | Roughness Factor |
|---|---|---|---|
| Bare | 0.0707 | 0.098 ± 0.010 | 1.39 |
| HNi10-700 | 0.0707 | 0.168 ± 0.031 | 2.37 |
| HNi10-750 | 0.0707 | 0.334 ± 0.053 | 4.73 |
| HNi10-800 | 0.0707 | 0.380 ± 0.051 | 5.37 |
| HNi10-850 | 0.0707 | 0.277 ± 0.050 | 3.93 |
| HNi10-900 | 0.0707 | 0.188 ± 0.026 | 2.66 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, R.; Hussein, M.Z.; Yusof, N.A.; Abu Bakar, F. Carbon Nanotube-Quicklime Nanocomposites Prepared Using a Nickel Catalyst Supported on Calcium Oxide Derived from Carbonate Stones. Nanomaterials 2019, 9, 1239. https://doi.org/10.3390/nano9091239
Ibrahim R, Hussein MZ, Yusof NA, Abu Bakar F. Carbon Nanotube-Quicklime Nanocomposites Prepared Using a Nickel Catalyst Supported on Calcium Oxide Derived from Carbonate Stones. Nanomaterials. 2019; 9(9):1239. https://doi.org/10.3390/nano9091239
Chicago/Turabian StyleIbrahim, Ruzanna, Mohd Zobir Hussein, Nor Azah Yusof, and Fatimah Abu Bakar. 2019. "Carbon Nanotube-Quicklime Nanocomposites Prepared Using a Nickel Catalyst Supported on Calcium Oxide Derived from Carbonate Stones" Nanomaterials 9, no. 9: 1239. https://doi.org/10.3390/nano9091239
APA StyleIbrahim, R., Hussein, M. Z., Yusof, N. A., & Abu Bakar, F. (2019). Carbon Nanotube-Quicklime Nanocomposites Prepared Using a Nickel Catalyst Supported on Calcium Oxide Derived from Carbonate Stones. Nanomaterials, 9(9), 1239. https://doi.org/10.3390/nano9091239





