Superior Hydrogen Sensing Property of Porous NiO/SnO2 Nanofibers Synthesized via Carbonization
Abstract
:1. Introduction
2. Experimental Procedure
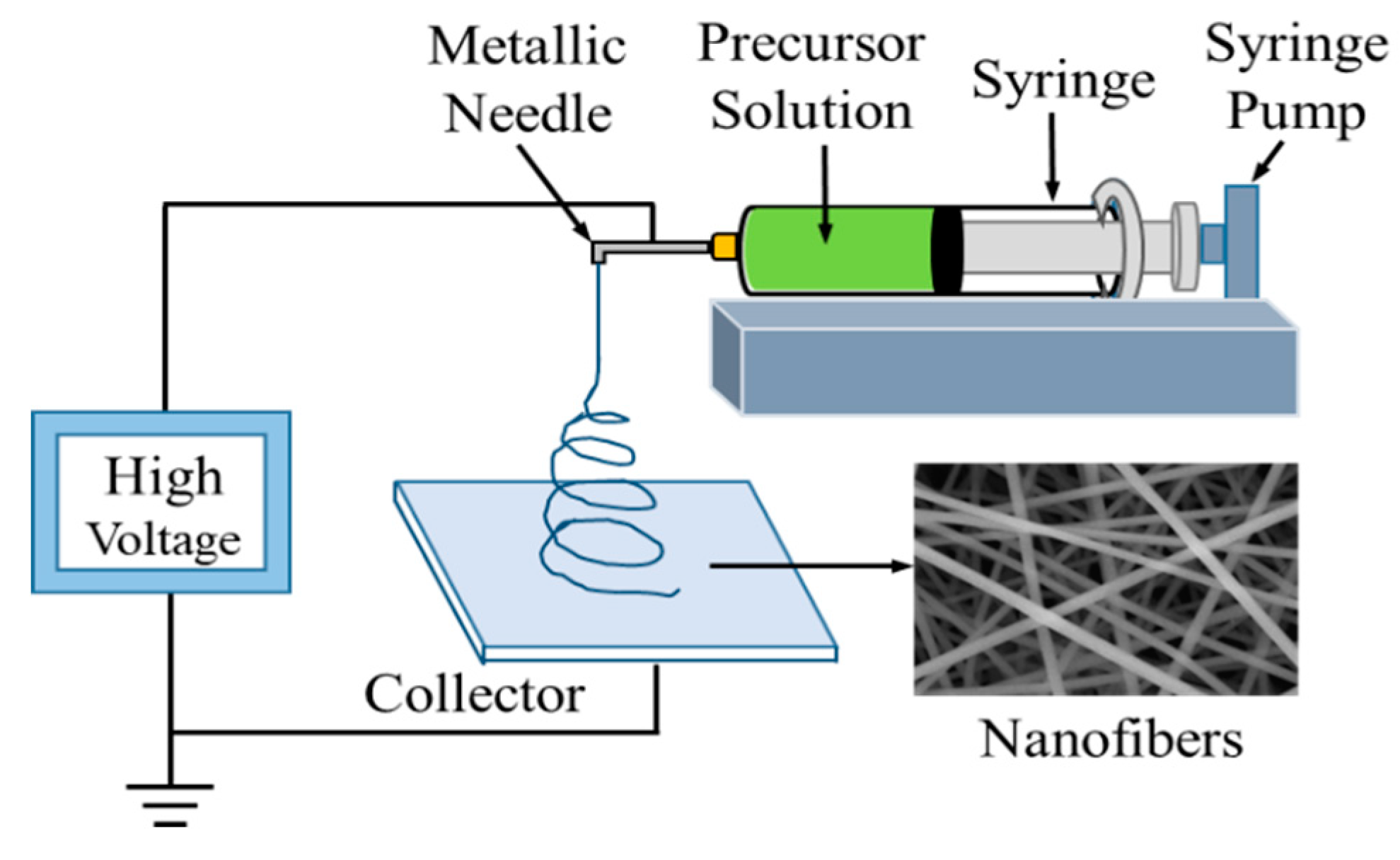
2.1. Preparation
2.2. Carbonization
2.3. Characterization
2.4. Fabrication and Measurement
3. Results and Discussion
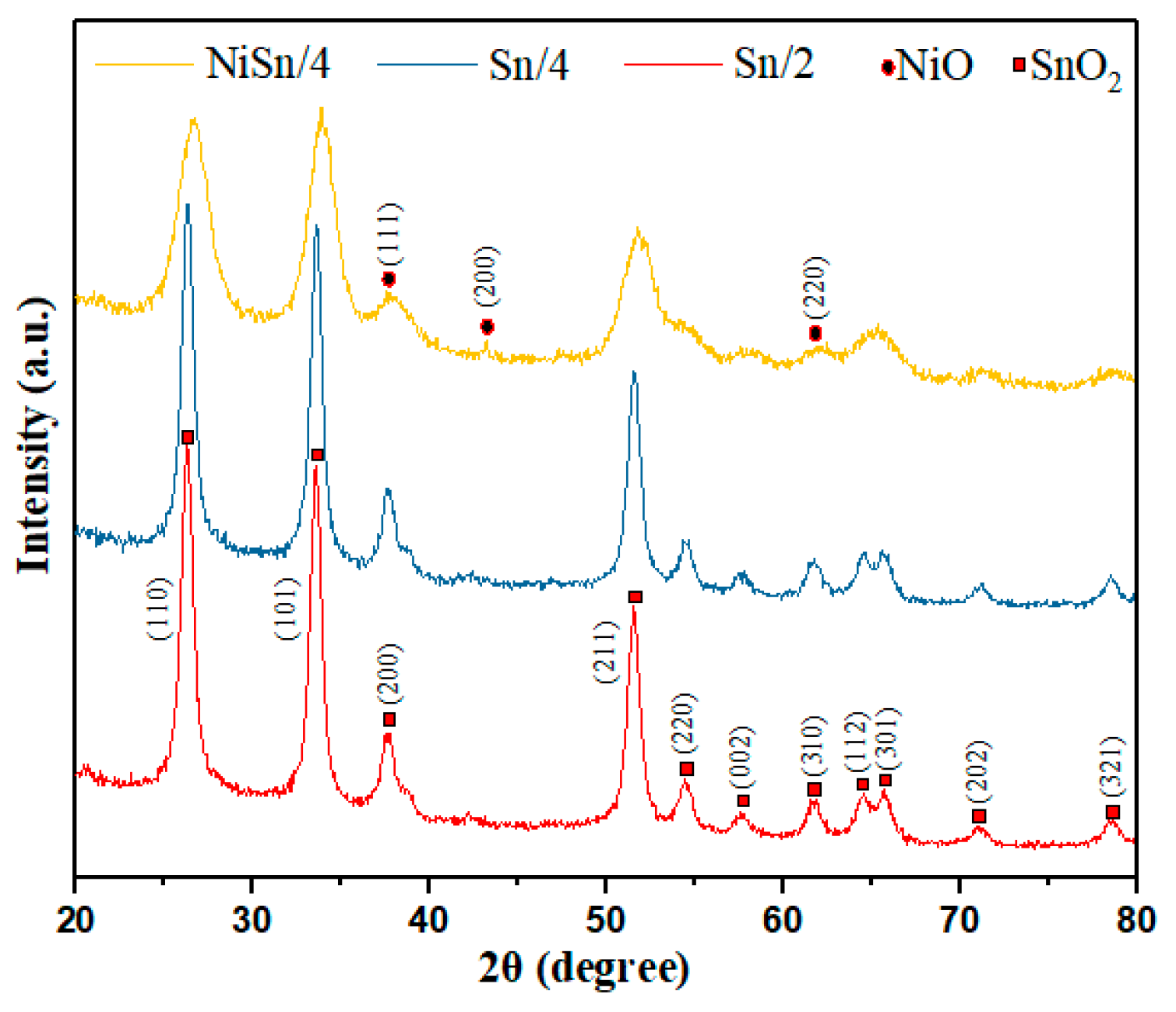
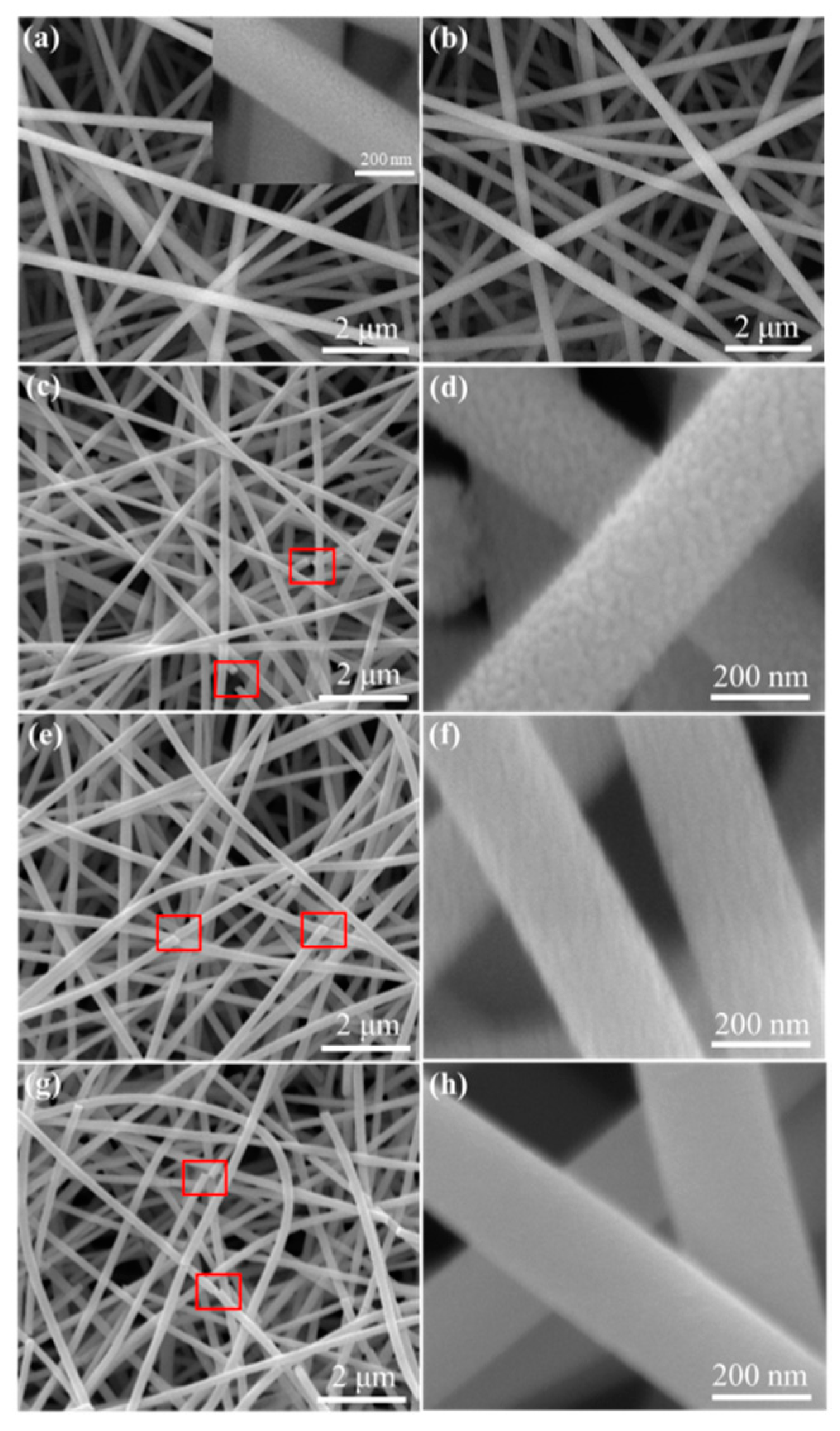
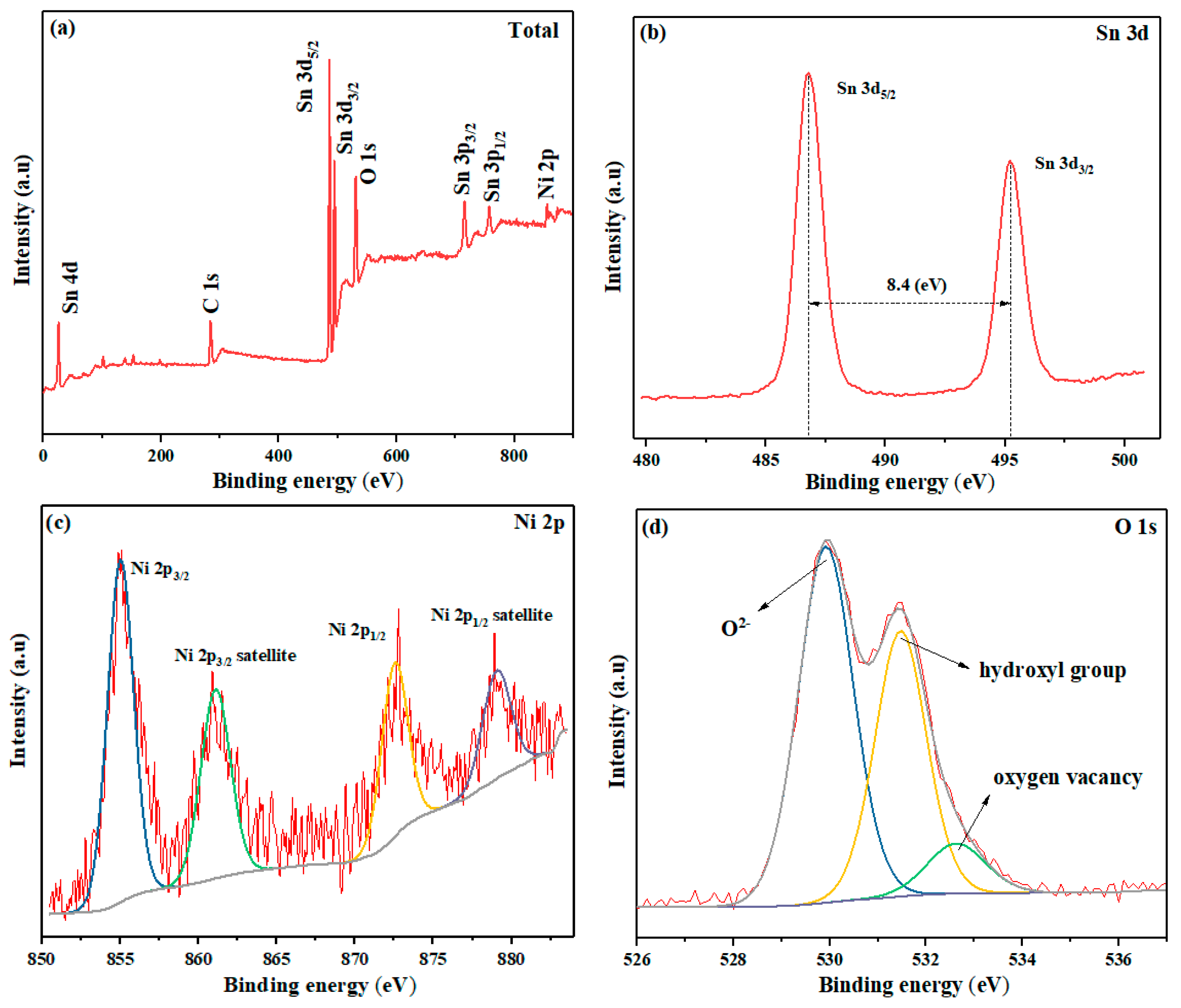
3.1. Structural and Morphological Characterizations
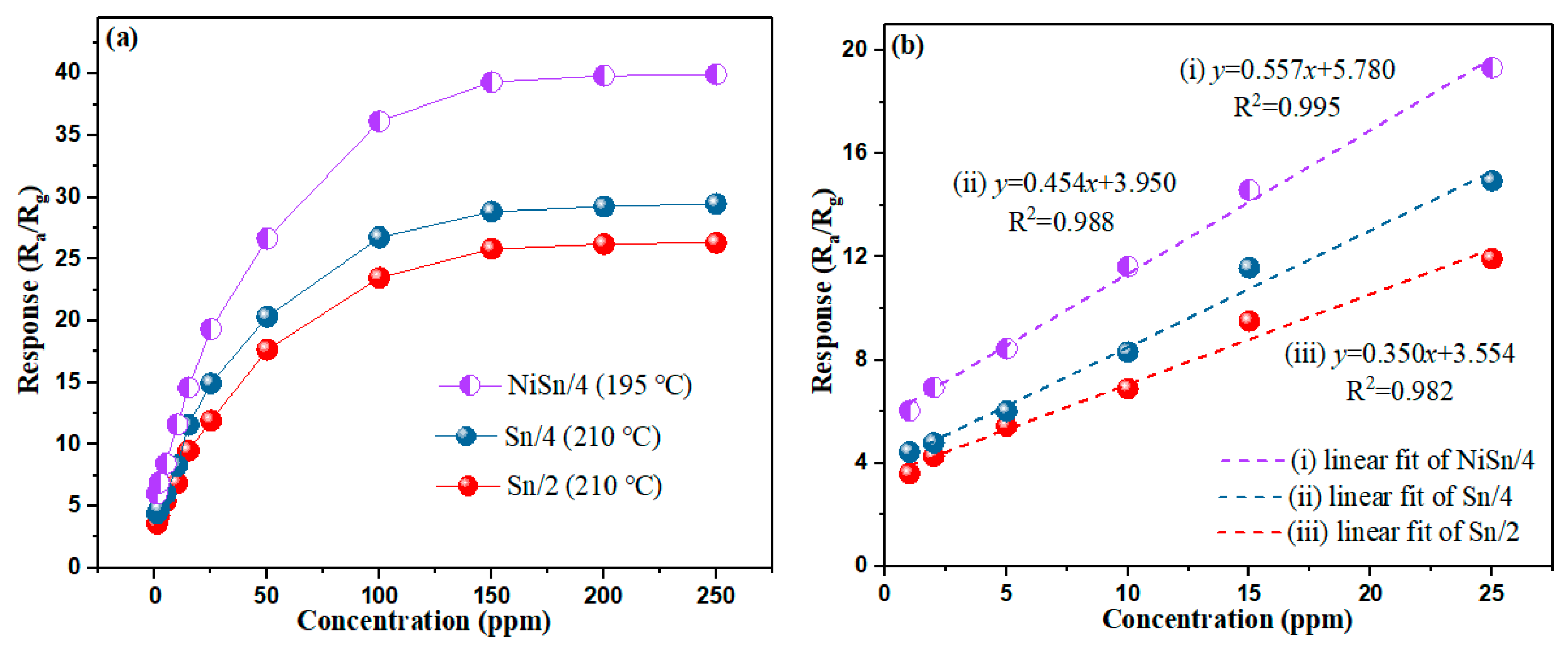
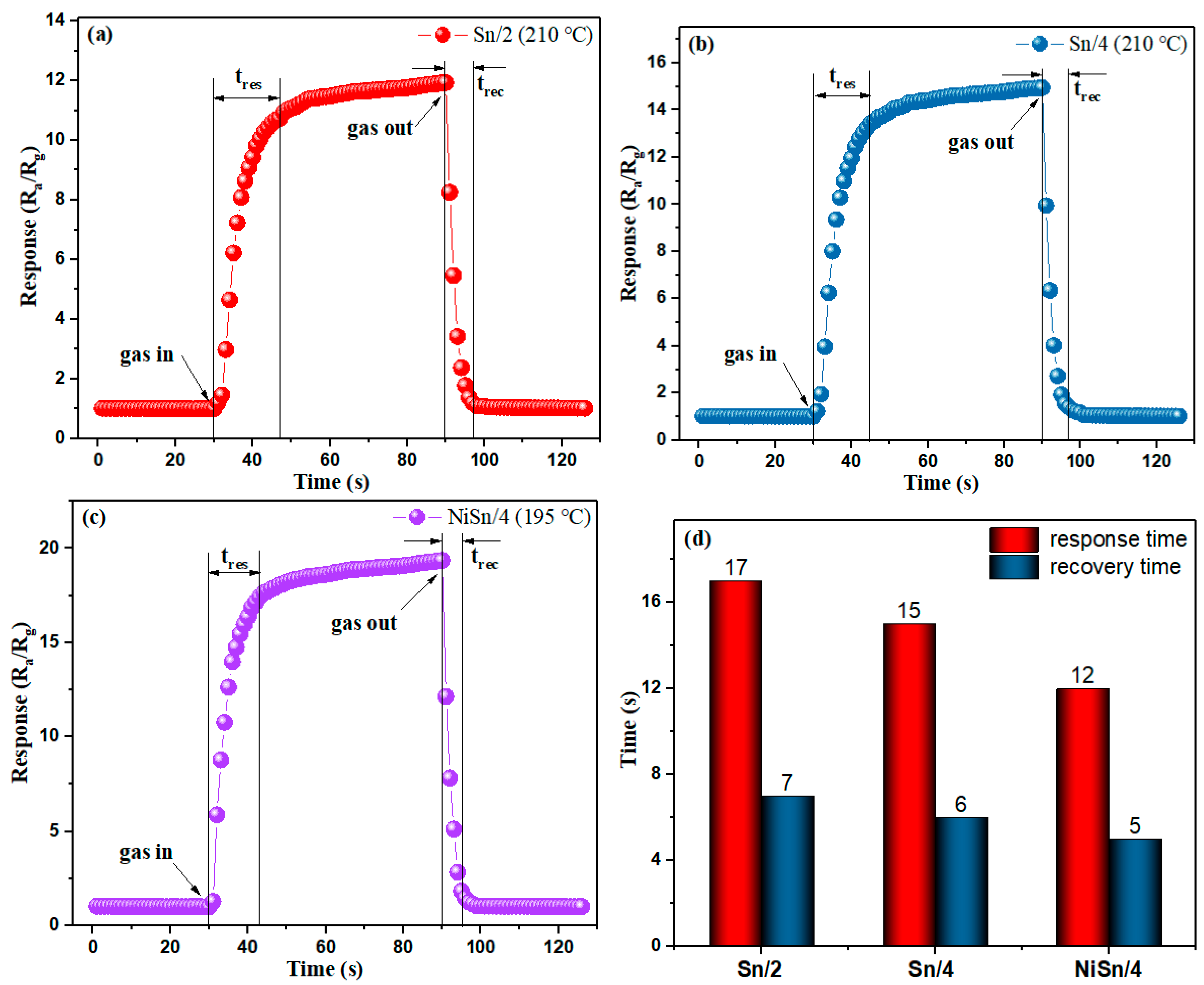
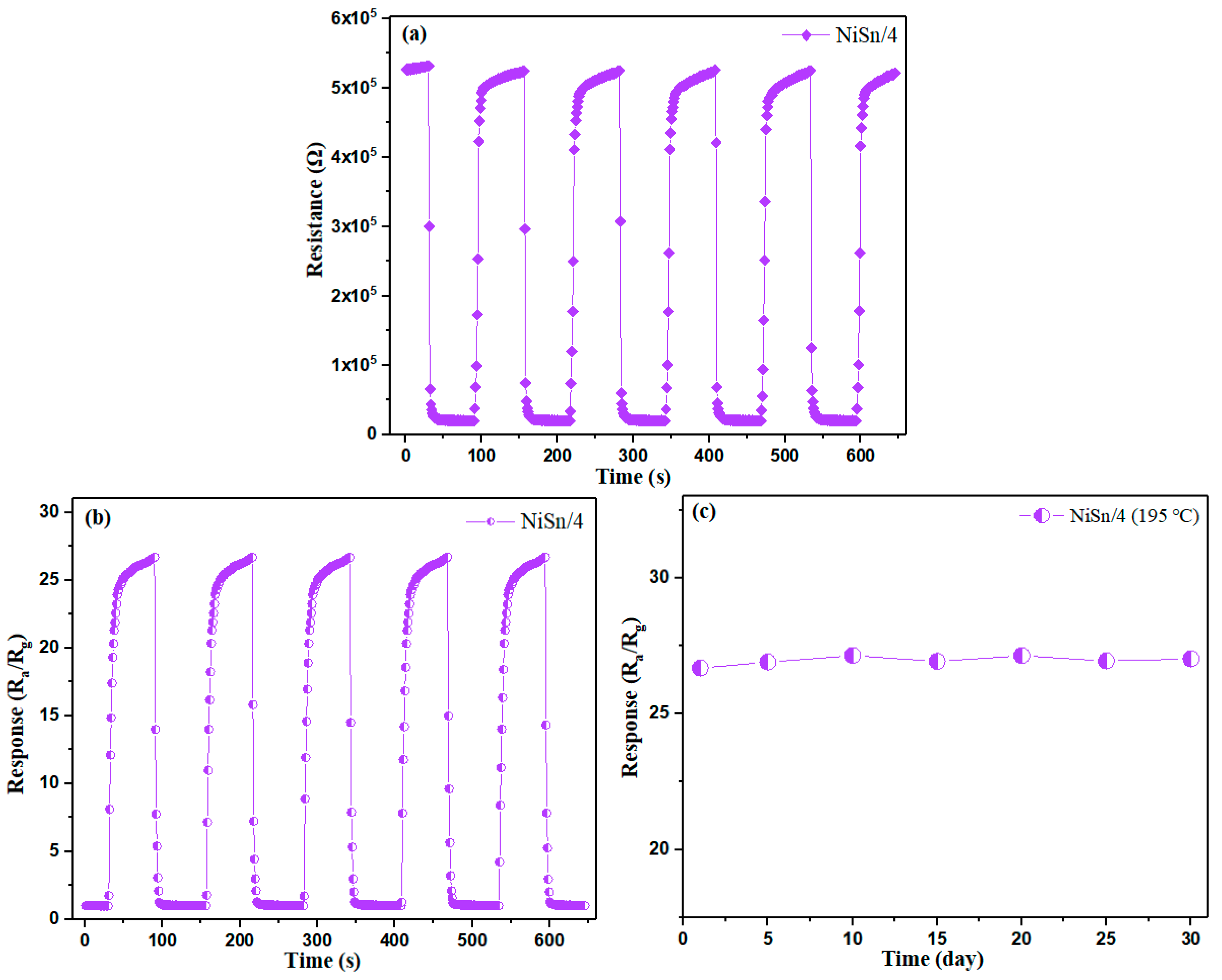
3.2. Gas-Sensing Properties
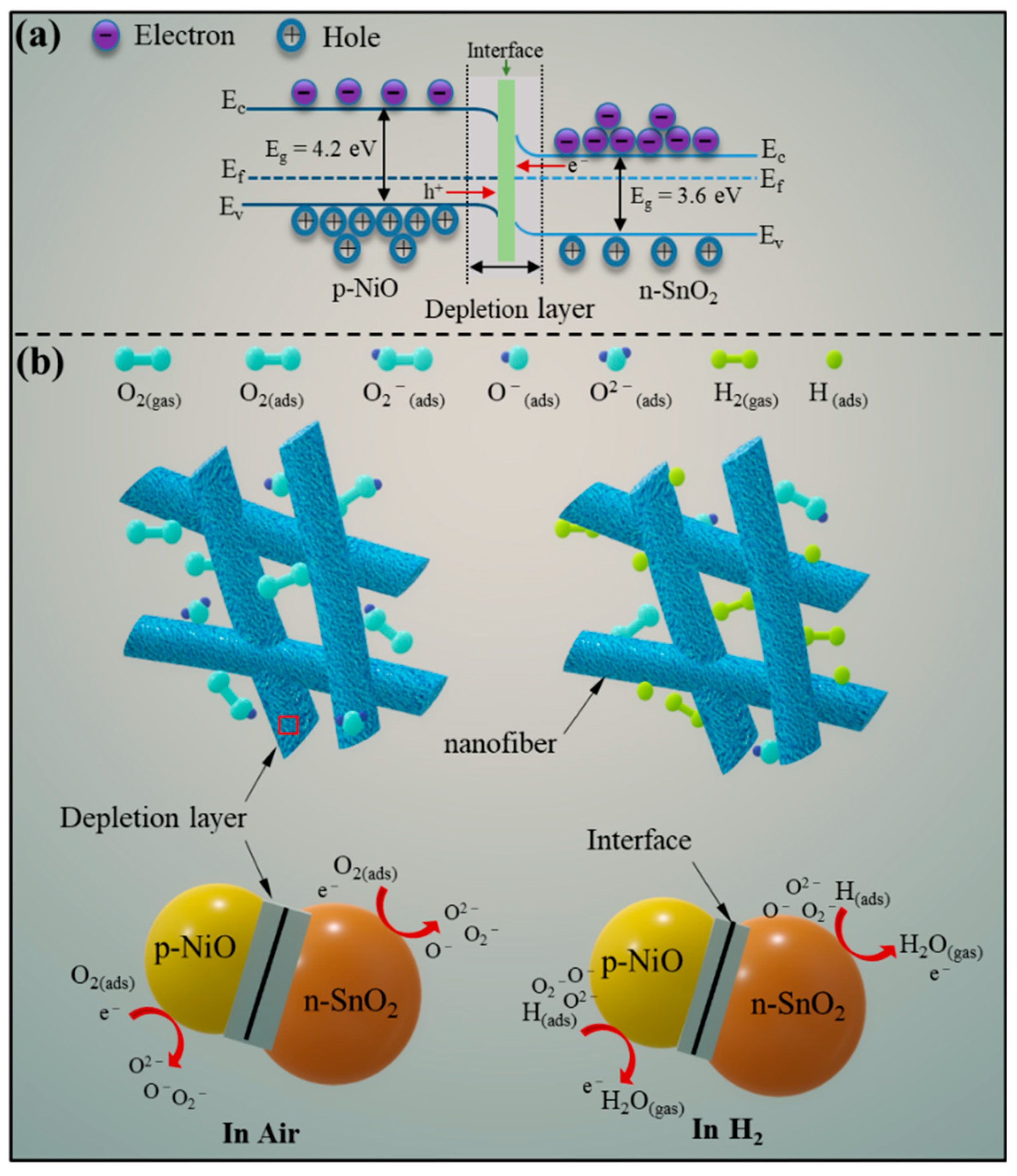
3.3. Sensing Mechanism
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jang, B.; Kim, M.H.; Baek, J.; Kim, W.; Lee, W. Highly sensitive hydrogen sensors: Pd-coated Si nanowire arrays for detection of dissolved hydrogen in oil. Sens. Actuators B Chem. 2018, 273, 809–814. [Google Scholar] [CrossRef]
- Xu, L.N.; Chen, W.G.; Jin, L.F.; Zeng, W. A novel SnO2 nanostructures and their gas-sensing properties for CO. J. Mater. Sci. Mater. Electron. 2016, 27, 4826–4832. [Google Scholar] [CrossRef]
- Yoo, R.; Park, Y.; Jung, H.; Rim, H.J.; Cho, S.; Lee, H.S.; Lee, W. Acetone-sensing properties of doped ZnO nanoparticles for breath-analyzer applications. J. Alloys Compd. 2019, 803, 135–144. [Google Scholar] [CrossRef]
- Zhao, G.D.; Xuan, J.Y.; Liu, X.L.; Jia, F.C.; Sun, Y.P.; Sun, M.L.; Yin, G.C.; Liu, B. Low-cost and high-performance ZnO nanoclusters gas sensor based on new-type FTO electrode for the low-concentration H2S gas detection. Nanomaterials 2019, 9, 435. [Google Scholar] [CrossRef] [PubMed]
- Haviar, S.; Capek, J.; Batkova, S.; Kumar, N.; Dvorak, F.; Duchon, T.; Fialova, M.; Zeman, P. Hydrogen gas sensing properties of WO3 sputter-deposited thin films enhanced by on-top deposited CuO nanoclusters. Int. J. Hydrogen Energy 2018, 43, 22756–22764. [Google Scholar] [CrossRef]
- Zhou, Q.; Chen, W.G.; Xu, L.N.; Kumar, R.; Gui, Y.G.; Zhao, Z.Y.; Tang, C.; Zhu, S.P. Highly sensitive carbon monoxide (CO) gas sensors based on Ni and Zn doped SnO2 nanomaterials. Ceram. Int. 2018, 44, 4392–4399. [Google Scholar] [CrossRef]
- Deepa, S.; Kumari, K.P.; Thomas, B. Contribution of oxygen-vacancy defect-types in enhanced CO2 sensing of nanoparticulate Zn-doped SnO2 films. Ceram. Int. 2017, 43, 17128–17141. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Yan, Y. Synthesis of biomorphic tube-like CuO using pomelo white flesh as biotemplate and its sensing properties over H2S at room temperature. J. Mater. Sci. 2017, 52, 13711–13718. [Google Scholar] [CrossRef]
- Zhou, Q.; Umar, A.; Sodki, E.M.; Amine, A.; Xu, L.N.; Gui, Y.G.; Ibrahim, A.A.; Kumar, R.; Baskoutas, S. Fabrication and characterization of highly sensitive and selective sensors based on porous NiO nanodisks. Sens. Actuators B Chem. 2018, 259, 604–615. [Google Scholar] [CrossRef]
- Choi, P.G.; Izu, N.; Shirahata, N.; Masuda, Y. Improvement of sensing properties for SnO2 gas sensor by tuning of exposed crystal face. Sens. Actuators B Chem. 2019, 296, 126655. [Google Scholar] [CrossRef]
- Li, H.K.; Zhu, D.C.; Yang, Z.Y.; Lu, W.R.; Pu, Y. The ethanol-sensitive property of hierarchical MoO3-mixed SnO2 aerogels via facile ambient pressure drying. Appl. Surf. Sci. 2019, 489, 384–391. [Google Scholar] [CrossRef]
- Luan, V.H.; Tien, H.N.; Hur, S.H.; Han, J.H. Three-Dimensional Porous Nitrogen-Doped NiO Nanostructures as Highly Sensitive NO2 Sensors. Nanomaterials 2017, 7, 313. [Google Scholar] [CrossRef] [PubMed]
- Colak, H.; Karakose, E. Synthesis and characterization of different dopant (Ge, Nd, W)-doped ZnO nanorods and their CO2 gas sensing applications. Sens. Actuators B-Chem. 2019, 296, 126629. [Google Scholar] [CrossRef]
- Ganbavle, V.V.; Inamdar, S.I.; Agawane, G.L.; Kim, J.H.; Rajpure, K.Y. Synthesis of fast response, highly sensitive and selective Ni:ZnO based NO2 sensor. Chem. Eng. J. 2016, 286, 36–47. [Google Scholar] [CrossRef]
- Tomer, V.K.; Duhan, S. Ordered mesoporous Ag-doped TiO2/SnO2 nanocomposite based highly sensitive and selective VOCs sensor. J. Mater. Chem. A 2016, 4, 1033–1043. [Google Scholar] [CrossRef]
- Malik, R.; Tomer, V.K.; Dankwort, T.; Mishra, Y.K.; Kienle, L. Cubic mesoporous Pd–WO3 loaded graphitic carbon nitride (g-CN) nanohybrids: Highly sensitive and temperature dependent VOC sensors. J. Mater. Chem. A 2018, 6, 10718–10730. [Google Scholar] [CrossRef]
- Malik, R.; Tomer, V.K.; Joshi, N.; Dankwort, T.; Lin, L.; Kienle, L. Au–TiO2-Loaded Cubic g-C3N4 Nanohybrids for Photocatalytic and Volatile Organic Amine Sensing Applications. ACS Appl. Mater. Interfaces 2018, 10, 34087–34097. [Google Scholar] [CrossRef] [PubMed]
- Tomer, V.K.; Malik, R.; Chaudhary, V.; Mishra, Y.K.; Kienle, L.; Ahuja, R.; Lin, L. Superior visible light photocatalysis and low-operating temperature VOCs sensor using cubic Ag(0)-MoS2 loaded g-CN 3D porous hybrid. Appl. Mater. Today 2019, 16, 193–203. [Google Scholar] [CrossRef]
- Katoch, A.; Abideen, Z.U.; Kim, J.H.; Kim, S.S. Influence of hollowness variation on the gas-sensing properties of ZnO hollow nanofibers. Sens. Actuators B Chem. 2016, 232, 698–704. [Google Scholar] [CrossRef]
- Xue, D.P.; Wang, Y.; Cao, J.L.; Zhang, Z.Y. Hydrothermal Synthesis of CeO2-SnO2 Nanoflowers for Improving Triethylamine Gas Sensing Property. Nanomaterials 2018, 8, 1025. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, H.; Sun, X.H. Electrospun nanowebs of NiO/SnO2 p-n heterojunctions for enhanced gas sensing. Appl. Surf. Sci. 2016, 389, 514–520. [Google Scholar] [CrossRef]
- Gao, H.Y.; Zhao, L.P.; Wang, L.W.; Sun, P.; Lu, H.Y.; Liu, F.M.; Chuai, X.H.; Lu, G.Y. Ultrasensitive and low detection limit of toluene gas sensor based on SnO2-decorated NiO nanostructure. Sens. Actuators B-Chem. 2018, 255, 3505–3515. [Google Scholar] [CrossRef]
- Jayababu, N.; Poloju, M.; Shruthi, J.; Reddy, M.V.R. Semi shield driven p-n heterostructures and their role in enhancing the room temperature ethanol gas sensing performance of NiO/SnO2 nanocomposites. Ceram. Int. 2019, 45, 15134–15142. [Google Scholar] [CrossRef]
- Meng, D.; Liu, D.Y.; Wang, G.S.; Shen, Y.B.; San, X.G.; Li, M.; Meng, F.L. Low-temperature formaldehyde gas sensors based on NiO-SnO2 heterojunction microflowers assembled by thin porous nanosheets. Sens. Actuators B Chem. 2018, 173, 418–428. [Google Scholar] [CrossRef]
- Kou, X.Y.; Xie, N.; Chen, F.; Wang, T.S.; Guo, L.L.; Wang, C.; Wang, Q.J.; Ma, J.; Sun, Y.F.; Zhang, H.; et al. Superior acetone gas sensor based on electrospun SnO2 nanofibers by Rh doping. Sens. Actuators B Chem. 2018, 256, 861–869. [Google Scholar] [CrossRef]
- Katoch, A.; Choi, S.W.; Kim, H.W.; Kim, S.S. Highly sensitive and selective H2 sensing by ZnO nanofibers and the underlying sensing mechanism. J. Hazard. Mater. 2015, 186, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Gao, X.; Wang, R.; Zhang, T.; Lu, G.Y. Study on TiO2-SnO2 core-shell heterostructure nanofibers with different work function and its application in gas sensor. Sens. Actuators B Chem. 2017, 248, 812–819. [Google Scholar] [CrossRef]
- Feng, C.H.; Kou, X.Y.; Chen, B.; Qian, G.B.; Sun, Y.F.; Lu, G.Y. One-pot synthesis of In doped NiO nanofibers and their gas sensing properties. Sens. Actuators B Chem. 2017, 253, 584–591. [Google Scholar] [CrossRef]
- Lu, Z.R.; Zhou, Q.; Xu, L.N.; Gui, Y.G.; Zhao, Z.Y.; Tang, C.; Chen, W.G. Synthesis and Characterization of Highly Sensitive Hydrogen (H-2) Sensing Device Based on Ag Doped SnO2 Nanospheres. Materials 2018, 11, 492. [Google Scholar] [CrossRef]
- Wei, Z.J.; Zhou, Q.; Wang, J.X.; Gui, Y.G.; Zeng, W. A novel porous NiO nanosheet and its H2 sensing performance. Mater. Lett. 2019, 245, 166–169. [Google Scholar] [CrossRef]
- Xiao, X.Y.; Zhou, X.R.; Ma, J.H.; Zhu, Y.H.; Cheng, X.W.; Luo, W.; Deng, Y.H. Rational synthesis and gas sensing performance of ordered mesoporous semiconducting WO3/NiO composites. ACS Appl. Mater. Interfaces 2019, 11, 26268–26276. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.C.; Zhou, Q.; Zhang, Q.Y.; Hong, C.X.; Xu, L.N.; Jin, L.F.; Chen, W.G. Synthesis, characterization and enhanced sensing properties of a NiO/ZnO p-n junctions sensor for the SF6 decomposition byproducts SO2, SO2F2, and SOF2. Sensors 2017, 17, 4. [Google Scholar]
- Kalidoss, R.; Umapathy, S.; Anandan, R.; Ganesh, V.; Sivalingam, Y. Comparative study on the preparation and gas sensing properties of reduced graphene Oxide/SnO2 Binary nanocomposite for detection of acetone in exhaled breath. Anal. Chem. 2019, 91, 5116–5124. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, Y.; Wang, G.G.; Li, S.C.; Wang, L.Y.; Han, Y.; Jiang, X.X.; Wei, A.G. High toluene sensing properties of NiO-SnO2 composite nanofiber sensors operating at 330 degrees C. Sens. Actuators B Chem. 2011, 160, 448–454. [Google Scholar] [CrossRef]
- Wang, D.; Wan, K.C.; Zhang, M.L.; Li, H.J.; Wang, P.; Wang, X.Y.; Yang, J.H. Constructing hierarchical SnO2 nanofiber/nanosheets for efficient formaldehyde detection. Sens. Actuators B Chem. 2019, 283, 714–723. [Google Scholar] [CrossRef]
- Park, J.Y.; Asokan, K.; Choi, S.W.; Kim, S.S. Growth kinetics of nanograins in SnO2 fibers and size dependent sensing properties. Sens. Actuators B Chem. 2011, 152, 254–260. [Google Scholar] [CrossRef]
- Bai, S.L.; Fu, H.; Zhao, Y.Y.; Tian, K.; Luo, R.X.; Li, D.Q.; Chen, A.F. On the construction of hollow nanofibers of ZnO-SnO2 heterojunctions to enhance the NO2 sensing properties. Sens. Actuators B Chem. 2018, 266, 692–702. [Google Scholar] [CrossRef]
- Lian, X.X.; Li, Y.; Zhu, J.W.; Zou, Y.L.; An, D.M.; Wang, Q. Fabrication of Au-decorated SnO2 nanoparticles with enhanced n-buthanol gas sensing properties. Mater. Sci. Semicon. Proc. 2019, 101, 198–205. [Google Scholar] [CrossRef]
- Gao, H.Y.; Yu, Q.; Zhang, S.F.; Wang, T.S.; Sun, P.; Lu, H.Y.; Liu, F.M.; Yan, X.; Liu, F.M.; Liang, X.S.; et al. Nanosheet-assembled NiO microspheres modified by Sn2+ ions isovalent interstitial doping for xylene gas sensors. Sens. Actuators B Chem. 2018, 269, 210–222. [Google Scholar] [CrossRef]
- Yang, X.H.; Fu, H.T.; Tian, Y.; Xie, Q.; Xiong, S.X.; Han, D.Z.; Zhang, H.; An, X.Z. Au decorated In2O3 hollow nanospheres: A novel sensing material toward amine. Sens. Actuators B Chem. 2019, 296, 126696. [Google Scholar] [CrossRef]
- Fu, H.T.; Yang, X.H.; Zhang, Z.K.; Wang, W.W.; An, X.Z.; Dong, Y.; Li, X. Preparation of plasmonic porous Au@AgVO3 belt-like nanocomposites with enhanced visible light photocatalytic activity. Nanotechnology 2018, 29, 295706. [Google Scholar] [CrossRef]
- Cheng, J.P.; Wang, B.B.; Zhao, M.G.; Liu, F.; Zhang, X.B. Nickel-doped tin oxide hollow nanofibers prepared by electrospinning for acetone sensing. Sens. Actuators B Chem. 2014, 190, 78–85. [Google Scholar] [CrossRef]
- George, G.; Anandhan, S. Synthesis and characterisation of nickel oxide nanofibre webs with alcohol sensing characteristics. RSC Adv. 2014, 4, 62009–62020. [Google Scholar] [CrossRef]
- Zhang, Q.P.; Chen, C.X.; Liu, Y.T.; Pan, H.; Du, H.F.; Su, Y.J.; Tai, H.L.; Xie, G.Z.; Xu, M.; Du, X.S. Improved response/recovery speeds of ZnO nanoparticle-based sensor toward NO2 gas under UV irradiation induced by surface oxygen vacancies. J. Mater. Sci.-Mater. El. 2019, 30, 11395–11403. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, Y.H.; Wang, M.X.; Ma, S.Y.; Wang, P.Y.; Zhang, G.H.; Chen, W.J.; Jiao, H.Y.; Liu, L.W.; Xu, X.L. Enhanced acetone sensor based on Au functionalized In-doped ZnSnO3 nanofibers synthesized by electrospinning method. J. Colloid Interface Sci. 2019, 543, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Anand, K.; Singh, O.; Singh, M.P.; Kaur, J.; Singh, R.C. Hydrogen sensor based on graphene/ZnO nanocomposite. Sens. Actuators B Chem. 2014, 195, 409–415. [Google Scholar] [CrossRef]
- Toan, N.V.; Chien, N.V.; Duy, N.V.; Hong, H.S.; Nguyen, H.; Hoa, N.D.; Hieu, N.V. Fabrication of highly sensitive and selective H2 gas sensor based on SnO2 thin film sensitized with microsized Pd islands. J. Hazard. Mater. 2016, 301, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, A.; Sun, G.J.; Lee, J.K.; Lee, C.; Choi, S.; Kim, H.W. Hydrogen sensing properties and mechanism of NiO-Nb2O5 composite nanoparticle-based electrical gas sensors. Ceram. Int. 2017, 43, 5247–5254. [Google Scholar] [CrossRef]
- Park, S. Enhancement of hydrogen sensing response of ZnO nanowires for the decoration of WO3 nanoparticles. Mater. Lett. 2019, 234, 315–318. [Google Scholar] [CrossRef]
- Zhao, C.H.; Huang, B.Y.; Xie, E.Q.; Zhou, J.Y.; Zhang, Z.X. Improving gas-sensing properties of electrospun In2O3 nanotubes by Mg acceptor doping. Sens. Actuators B Chem. 2015, 207, 313–320. [Google Scholar] [CrossRef]
- Mirzaei, A.; Kang, S.Y.; Choi, S.W.; Kwon, Y.J.; Choi, M.S.; Bang, J.H.; Kim, S.S.; Kim, H.W. Fabrication and gas sensing properties of vertically aligned Si nanowires. Appl. Surf. Sci. 2018, 427, 215–226. [Google Scholar] [CrossRef]
- Lin, B.Z.; Jia, F.C.; Lv, B.J.; Qin, Z.L.; Liu, P.D.; Chen, Y.L. Facile synthesis and remarkable hydrogen sensing performance of Pt-loaded SnO2 hollow microspheres. Mater. Res. Bull. 2018, 106, 403–408. [Google Scholar]
- Wang, Z.J.; Li, Z.Y.; Sun, J.H.; Zhang, H.N.; Wang, W.; Zheng, W.; Wang, C. Improved hydrogen monitoring properties based on p-NiO/n-SnO2 heterojunction composite nanofibers. J. Phys. Chem. C. 2010, 114, 6100–6105. [Google Scholar] [CrossRef]
- Wei, C.; Bo, B.; Tao, F.B.; Lu, Y.C.; Peng, S.D.; Song, W.; Zhou, Q. Hydrothermal synthesis and structural characterization of NiO/SnO2 composites and hydrogen sensing properties. J. Spectrosc. 2015, 2015, 450485. [Google Scholar] [CrossRef]
- Soltabayev, B.; Yildirim, M.A.; Ates, A.; Acar, S. The effect of indium doping concentration on structural, morphological and gas sensing properties of IZO thin films deposited SILAR method. Mater. Sci. Semicon. Proc. 2019, 101, 28–36. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, L.L.; Fei, T.; Zhang, T. Enhanced ethanol sensing properties of NiO-doped SnO2 polyhedra. New J. Chem. 2012, 36, 1003–1007. [Google Scholar] [CrossRef]
- Wei, Z.J.; Zhou, Q.; Wang, J.X.; Lu, Z.R.; Xu, L.N.; Zeng, W. Hydrothermal synthesis of SnO2 nanoneedle-anchored NiO microsphere and its gas sensing performances. Nanomaterials 2019, 9, 1015. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.H.; Kim, J.Y.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Enhancement of CO and NO2 sensing in n-SnO2-p-Cu2O core-shell nanofibers by shell optimization. J. Hazard. Mater. 2019, 376, 68–82. [Google Scholar] [CrossRef]
- Qin, C.; Wang, Y.; Gong, Y.X.; Zhang, Z.Y.; Cao, J.L. CuO-ZnO hetero-junctions decorated graphitic carbon nitride hybrid nanocomposite: Hydrothermal synthesis and ethanol gas sensing application. J. Alloys Compd. 2019, 770, 972–980. [Google Scholar] [CrossRef]
- Zhou, Q.; Zeng, W.; Chen, W.G.; Xu, L.N.; Kumarc, R.; Umar, A. High sensitive and low-concentration sulfur dioxide (SO2) gas sensor application of heterostructure NiO-ZnO nanodisks. Sens. Actuators B Chem. 2019, 298, 126870. [Google Scholar] [CrossRef]


| Materials | H2 (ppm) | Optimal Temperature (°C) | Response (Ra/Rg) | Year | Reference |
|---|---|---|---|---|---|
| rGO/ZnO composite | 200 | 150 | 3.5 | 2014 | [46] |
| Pd/SnO2 thin film | 250 | 300 | 28.0 | 2016 | [47] |
| Nb2O5-NiO nanocomposite | 500 | R.T. | 1.68 | 2017 | [48] |
| WO3-ZnO nanowire | 2000 | 200 | 12.6 | 2019 | [49] |
| Mg-In2O3 nanotubes | 100 | 150 | 1.55 | 2015 | [50] |
| Si nanowires | 50 | 100 | 17.1 | 2018 | [51] |
| Pt-SnO2 hollow microspheres | 200 | 50 | 21.0 | 2018 | [52] |
| NiO/SnO2 nanocomposite | 100 | 320 | 13.6 | 2010 | [53] |
| NiO/SnO2 nanospheres | 50 | 325 | 27.84 | 2015 | [54] |
| NiO/SnO2 nanofibers | 100 | 195 | 37.15 | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Wang, F.; Hu, K.; Zhang, B.; He, L.; Zhou, Q. Superior Hydrogen Sensing Property of Porous NiO/SnO2 Nanofibers Synthesized via Carbonization. Nanomaterials 2019, 9, 1250. https://doi.org/10.3390/nano9091250
Liu H, Wang F, Hu K, Zhang B, He L, Zhou Q. Superior Hydrogen Sensing Property of Porous NiO/SnO2 Nanofibers Synthesized via Carbonization. Nanomaterials. 2019; 9(9):1250. https://doi.org/10.3390/nano9091250
Chicago/Turabian StyleLiu, Hongcheng, Feipeng Wang, Kelin Hu, Bin Zhang, Li He, and Qu Zhou. 2019. "Superior Hydrogen Sensing Property of Porous NiO/SnO2 Nanofibers Synthesized via Carbonization" Nanomaterials 9, no. 9: 1250. https://doi.org/10.3390/nano9091250





