A Facile Method of Preparing the Asymmetric Supercapacitor with Two Electrodes Assembled on a Sheet of Filter Paper
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Flexible AS on One-Sheet of FP
2.3. Characterization and Electrochemical Measurements
3. Results and Discussion
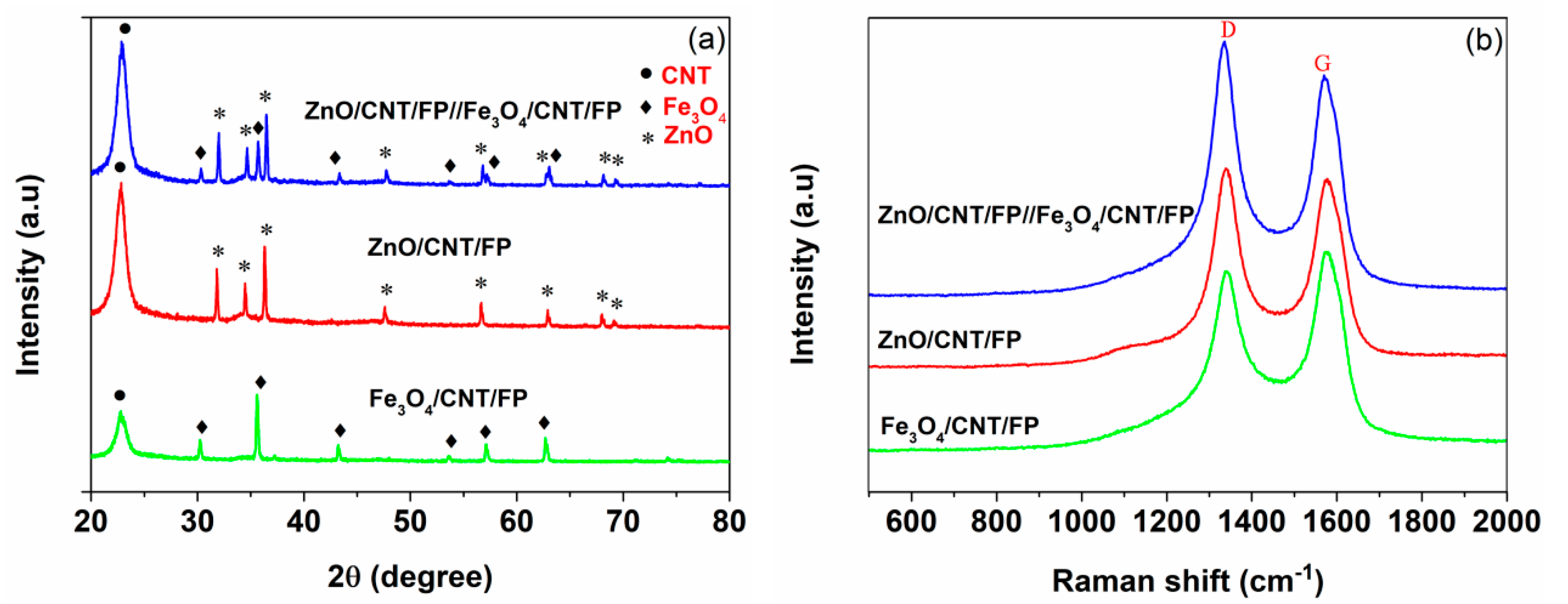
3.1. Structure and Morphology
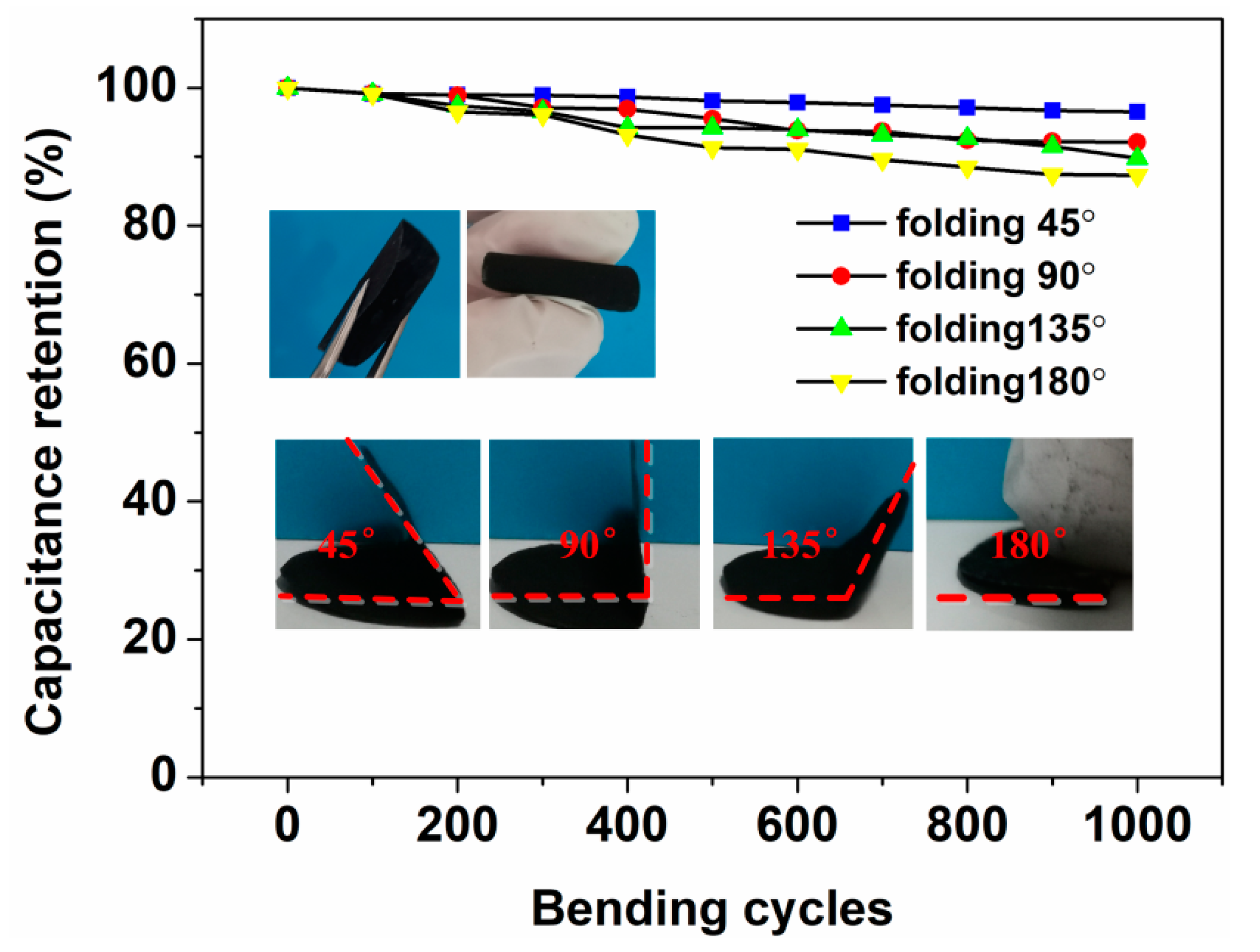
3.2. Electrochemical Tests of the As-Prepared Electrodes and AS
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Borenstein, A.; Hanna, O. Carbon-based composite materials for supercapacitor electrodes: A review. J. Mater. Chem. A 2017, 5, 12653–12672. [Google Scholar] [CrossRef]
- Zuo, W.; Li, R. Battery-Supercapacitor Hybrid Devices: Recent progress and future prospects. Adv. Sci. 2017, 4, 1600539. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Yu, M. Iron-based supercapacitor electrodes: Advances and Challenges. Adv. Energy Mater. 2016, 6, 1601053. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, Y. Removal of rhodamine B with Fe-supported bentonite as heterogeneous photo-fenton catalyst under visible irradiation. Appl. Catal. B Environ. 2015, 178, 29–36. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, Q. Waterproof, ultrahigh areal-capacitance, wearable supercapacitor fabrics. Adv. Mater. 2017, 29, 1606679. [Google Scholar] [CrossRef] [PubMed]
- Libich, J.; Máca, J. Supercapacitors: Properties and applications. J. Energy Storage 2018, 17, 224–227. [Google Scholar] [CrossRef]
- Redondo, E.; Carretero-González, J. Effect of pore texture on performance of activated carbon supercapacitor electrodes derived from olive pits. Electrochim. Acta 2015, 160, 178–184. [Google Scholar] [CrossRef]
- Cao, J.; He, P. Supercapacitor electrodes from the in situ reaction between two-dimensional sheets of black phosphorus and graphene oxide. ACS Appl. Mater. Interfaces 2018, 10, 10330–10338. [Google Scholar] [CrossRef]
- Ma, Y.; Chang, H. Graphene-based materials for lithium-ion hybrid supercapacitors. Adv. Mater. 2015, 27, 5296–5308. [Google Scholar] [CrossRef]
- Xiong, C.; Li, B. The recent progress on three-dimensional porous graphene-based hybrid structure for supercapacitor. Compos. Part B Eng. 2019, 165, 10–46. [Google Scholar] [CrossRef]
- Choi, C.; Sim, H.J. Elastomeric and dynamic MnO2/CNT core-Shell structure coiled yarn supercapacitor. Adv. Energy Mater. 2016, 6, 1502119. [Google Scholar] [CrossRef]
- Xue, M.; Xie, Z. Microfluidic etching for fabrication of flexible and all-solid-state micro supercapacitor based on MnO2 nanoparticles. Nanoscale 2011, 3, 2703–2708. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, N.; Li, C. Asymmetric supercapacitor electrodes and devices. Adv. Mater. 2017, 29, 1605336. [Google Scholar] [CrossRef] [PubMed]
- Shown, I.; Ganguly, A. Conducting polymer-based flexible supercapacitor. Energy Sci. Eng. 2015, 3, 2–26. [Google Scholar] [CrossRef]
- Jiang, L.; Sheng, L. Densely packed graphene nanomesh-carbon nanotube hybrid film for ultra-high volumetric performance supercapacitors. Nano Energy 2015, 11, 471–480. [Google Scholar] [CrossRef]
- Liu, Z.; Yu, X.-Y. Etching-in-a-Box: A novel strategy to synthesize unique yolk-shelled Fe3O4@carbon with an ultralong cycling life for lithium storage. Adv. Energy Mater. 2016, 6, 1502318. [Google Scholar] [CrossRef]
- Godillot, G.; Taberna, P.L. High power density aqueous hybrid supercapacitor combining activated carbon and highly conductive spinel cobalt oxide. J. Power Sources 2016, 331, 277–284. [Google Scholar] [CrossRef] [Green Version]
- Xiong, C.; Li, T.; Zhao, T.; Dang, A.; Ji, X.; Li, H.; Etesami, M. Three-dimensional graphene/MnO2 nanowalls hybrid for high-efficiency electrochemical supercapacitors. Nano 2018, 13, 1850013. [Google Scholar] [CrossRef]
- Zhi, M.; Xiang, C. Nanostructured carbon-metal oxide composite electrodes for supercapacitors: A review. Nanoscale 2013, 5, 72–88. [Google Scholar] [CrossRef]
- Huang, G.; Zhang, Y. Fiber-based MnO2/carbon nanotube/polyimide asymmetric supercapacitor. Carbon 2017, 125, 595–604. [Google Scholar] [CrossRef]
- Salunkhe, R.R.; Zakaria, M.B. Fabrication of asymmetric supercapacitors based on coordination polymer derived nanoporous materials. Electrochim. Acta 2015, 183, 94–99. [Google Scholar] [CrossRef]
- Yang, G.; Park, S.-J. Facile hydrothermal synthesis of NiCo2O4-decorated filter carbon as electrodes for high performance asymmetric supercapacitors. Electrochim. Acta 2018, 285, 405–414. [Google Scholar] [CrossRef]
- Afzal, A.; Abuilaiwi, F.A. Polypyrrole/carbon nanotube supercapacitors: Technological advances and challenges. J. Power Sources 2017, 352, 174–186. [Google Scholar] [CrossRef]
- Cao, X.; Zheng, B. Reduced graphene oxide-wrapped MoO3 composites prepared by using metal-organic frameworks as precursor for all-solid-state flexible supercapacitors. Adv. Mater. 2015, 27, 4695–4701. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, S. Double polymer sheathed carbon nanotube supercapacitors show enhanced cycling stability. Nanoscale 2016, 8, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Lang, A.W.; Ponder, J.F. Flexible, aqueous-electrolyte supercapacitors based on water-processable dioxythiophene polymer/carbon nanotube textile electrodes. J. Mater. Chem. A 2017, 5, 23887–23897. [Google Scholar] [CrossRef]
- Lee, K.M.; Lai, C.W. Recent developments of zinc oxide based photocatalyst in water treatment technology: A review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef]
- Bhuyan, T.; Mishra, K.; Khanuja, M.; Prasad, R.; Varma, A. Biosynthesis of zinc oxide nanoparticles from Azadirachta indica for antibacterial and photocatalytic applications. Mater. Sci. Semicond. Proc. 2015, 32, 55–61. [Google Scholar] [CrossRef]
- Kumar, S.; Ahlawat, W. Graphene, carbon nanotubes, zinc oxide and gold as elite nanomaterials for fabrication of biosensors for healthcare. Biosens. Bioelectron. 2015, 70, 498–503. [Google Scholar] [CrossRef]
- Lo, I.H.; Wang, J.-Y. Synthesis of Ni(OH)2 nanoflakes on ZnO nanowires by pulse electrodeposition for high-performance supercapacitors. J. Power Sources 2016, 308, 29–36. [Google Scholar] [CrossRef]
- Li, T.; Bai, X. Fe3O4 nanoparticles decorated on the biochar derived from pomelo pericarp as excellent anode materials for Li-ion batteries. Electrochim. Acta 2016, 222, 1562–1568. [Google Scholar] [CrossRef]
- Ye, Y.; Kong, T. Enhanced nonenzymatic hydrogen peroxide sensing with reduced graphene oxide/ferroferric oxide nanocomposites. Talanta 2012, 89, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Xu, X. Facile complex-coprecipitation synthesis of mesoporous Fe3O4 nanocages and their high lithium storage capacity as anode material for lithium-ion batteries. Electrochim. Acta 2015, 160, 114–122. [Google Scholar] [CrossRef]
- Sun, J.; Zan, P. Room-temperature synthesis of Fe3O4/Fe-carbon nanocomposites with Fe-carbon double conductive network as supercapacitor. Electrochim. Acta 2016, 215, 483–491. [Google Scholar] [CrossRef]
- Chang, B.; Guo, Y. Hierarchical porous carbon derived from recycled waste filter paper as high-performance supercapacitor electrodes. RSC Adv. 2015, 5, 72019–72027. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, P. Flexible Asymmetrical Solid-State Supercapacitors based on laboratory filter paper. ACS Nano 2016, 10, 1273–1282. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, D.; Tang, Z.; Liang, G.; Yang, Q.; Li, H.; Ma, L.; Mo, F.; Zhi, C. A mechanically durable and device-level tough Zn-MnO2 battery with high flexibility. Energy Storage Mater. 2019. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, F.; Liu, Z.; Tang, Z.; Yang, Q.; Zhao, Y.; Du, S.; Chen, Q.; Zhi, C. A Highly Elastic and Reversibly Stretchable All-polymer Supercapacitor. Angew. Chem. Int. Ed. 2019. [Google Scholar] [CrossRef]
- Liu, A.; Kovacik, P. Monolithic flexible supercapacitors integrated into single sheets of paper and membrane via vapor printing. Adv. Mater. 2017, 29, 1606091. [Google Scholar] [CrossRef]
- Xia, C.; Jiang, Q. Asymmetric supercapacitors with metal-like ternary selenides and porous graphene electrodes. Nano Energy 2016, 24, 78–86. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.; Yang, W. Flexible all-solid-state hierarchical NiCo2O4/porous graphene paper asymmetric supercapacitors with an exceptional combination of electrochemical properties. Nano Energy 2015, 13, 306–317. [Google Scholar] [CrossRef]
- Xiong, C.; Li, T. Two-step approach of fabrication of three-dimensional MnO2-graphene-carbon nanotube hybrid as a binder-free supercapacitor electrode. J. Power Sources 2016, 306, 602–610. [Google Scholar] [CrossRef]
- Arod, P.; Shivashankar, S.A. Single-step synthesis of carbon nanotubes/iron/iron oxide composite films through inert-ambient CVD using ferric acetylacetonate as a precursor. RSC Adv. 2015, 5, 59463–59471. [Google Scholar] [CrossRef]
- Kim, C.H.; Kim, B.-H. Zinc oxide/activated carbon nanofiber composites for high-performance supercapacitor electrodes. J. Power Sources 2015, 274, 512–520. [Google Scholar] [CrossRef]
- Sreejesh, M.; Dhanush, S. Microwave assisted synthesis of rGO/ZnO composites for non-enzymatic glucose sensing and supercapacitor applications. Ceram. Int. 2017, 43, 4895–4903. [Google Scholar] [CrossRef]
- Li, J.; Sun, Y. Novel ternary composites reduced-graphene oxide/zine oxide/poly(p-phenylenediamine) for supercapacitor: Synthesis and properties. J. Alloys Compd. 2017, 708, 787–795. [Google Scholar] [CrossRef]



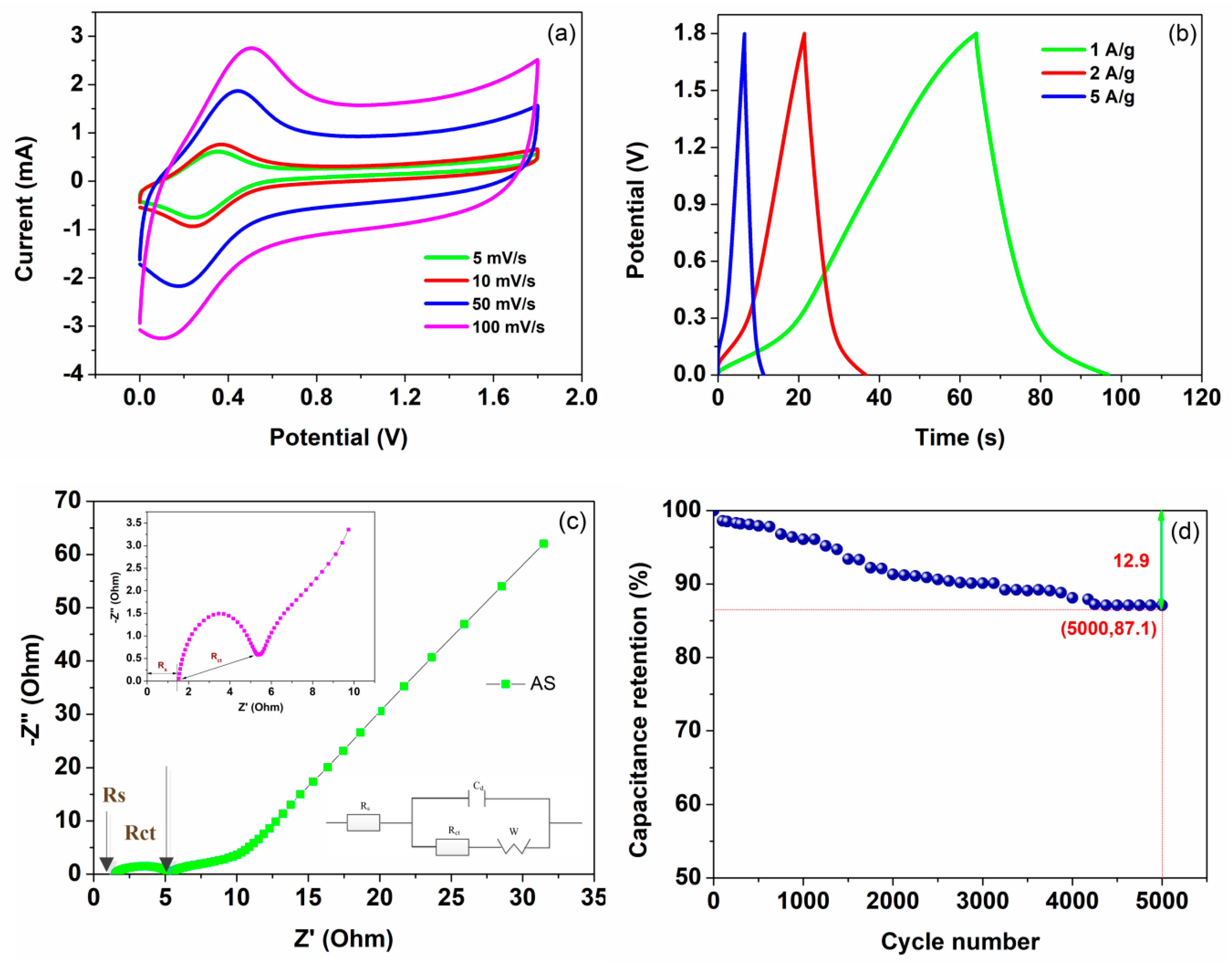


| Current Density (A/g) | Specific Capacitance (F/g) | Energy Density (W h/kg) | Power Density (kW/kg) |
|---|---|---|---|
| 1 | 139.12 | 125.21 | 13.74 |
| 2 | 129.00 | 116.11 | 27.48 |
| 5 | 100.19 | 90.17 | 68.70 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, S.; Li, T.; Xiong, C.; Tang, C.; Dang, A.; Li, H.; Zhao, T. A Facile Method of Preparing the Asymmetric Supercapacitor with Two Electrodes Assembled on a Sheet of Filter Paper. Nanomaterials 2019, 9, 1338. https://doi.org/10.3390/nano9091338
Jiao S, Li T, Xiong C, Tang C, Dang A, Li H, Zhao T. A Facile Method of Preparing the Asymmetric Supercapacitor with Two Electrodes Assembled on a Sheet of Filter Paper. Nanomaterials. 2019; 9(9):1338. https://doi.org/10.3390/nano9091338
Chicago/Turabian StyleJiao, Shasha, Tiehu Li, Chuanyin Xiong, Chen Tang, Alei Dang, Hao Li, and Tingkai Zhao. 2019. "A Facile Method of Preparing the Asymmetric Supercapacitor with Two Electrodes Assembled on a Sheet of Filter Paper" Nanomaterials 9, no. 9: 1338. https://doi.org/10.3390/nano9091338





