TiO2 Coated ZnO Nanorods by Mist Chemical Vapor Deposition for Application as Photoanodes for Dye-Sensitized Solar Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of ZnO/TiO2 Core–Shell Nanorods
2.2. Characterization
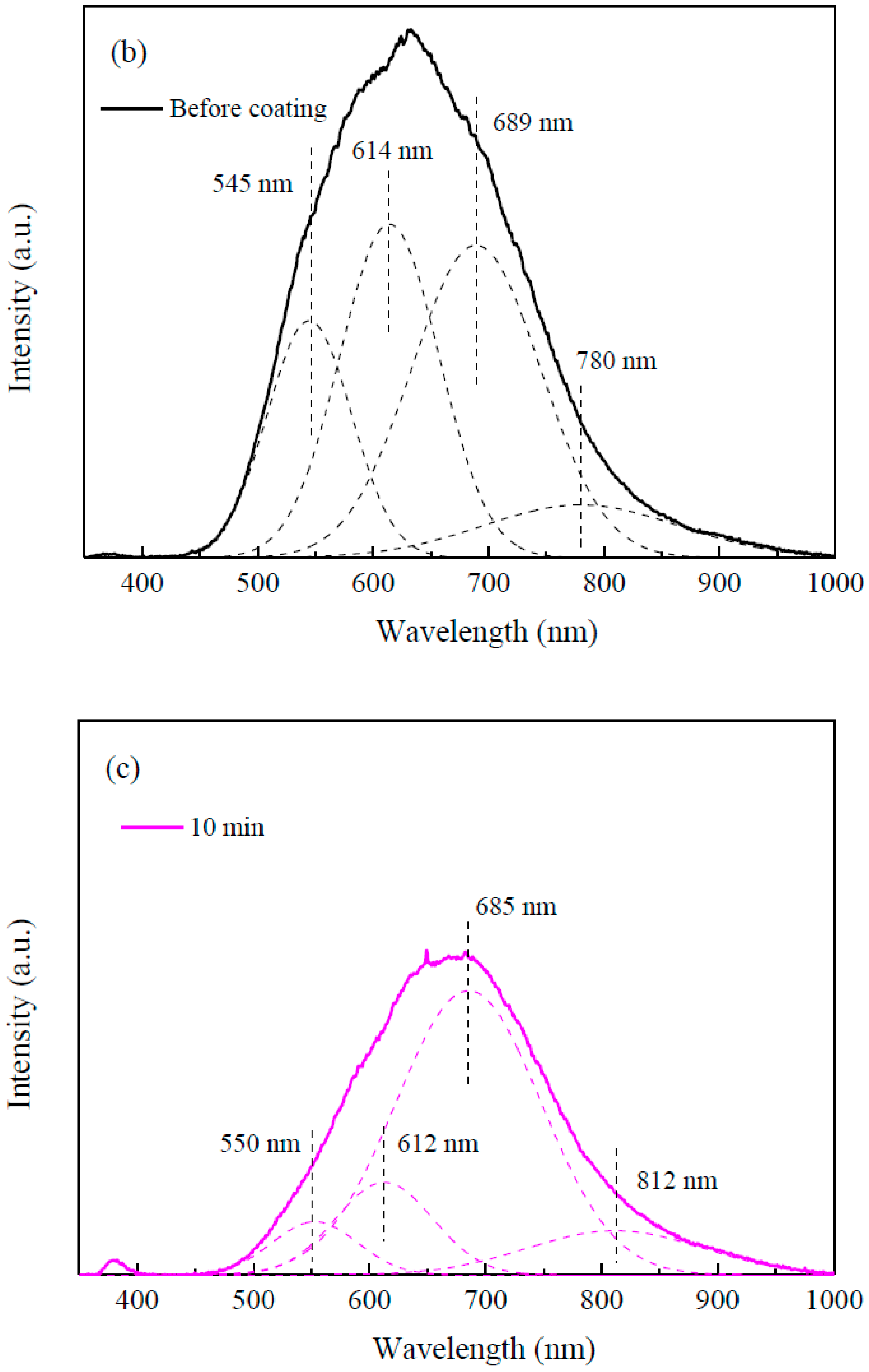
3. Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Regan, B.O.; Gratzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Yang, L.; Leung, W.W.F. Application of a bilayer TiO2 nanofiber photoanode for optimization of dye-sensitized solar cells. Adv. Mater. 2011, 23, 4559–4562. [Google Scholar] [CrossRef] [PubMed]
- Graetzel, M.; Janssen, R.A.J.; Mitzi, D.B.; Sargent, E.H. Materials interface engineering for solution-processed photovoltaics. Nature 2012, 488, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Kakiage, K.; Aoyama, Y.; Yano, T.; Oya, K.; Fujisawa, J.I.; Hanaya, M. Highly-efficient dye-sensitized solar cells with collaborative sensitization by silyl-anchor and carboxy-anchor dyes. Chem. Commun. 2015, 51, 15894–15897. [Google Scholar] [CrossRef] [PubMed]
- Law, M.; Greene, L.E.; Johnson, J.C.; Saykally, R.; Yang, P. Nanowire dye-sensitized solar cells. Nat. Mater. 2005, 4, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Dandeneau, C.S.; Zhou, X.; Cao, G. ZnO nanostructures for dye-sensitized solar cells. Adv. Mater. 2009, 21, 4087–4108. [Google Scholar] [CrossRef]
- Kolodziejczak-Radzimska, A.; Jesionowski, T. Zinc oxide-from synthesis to application: A review. Materials 2014, 7, 2833–2881. [Google Scholar] [CrossRef]
- Tiwana, P.; Docampo, P.; Johnston, M.B.; Snaith, H.J.; Herz, L.M. Electron mobility and injection dynamics in mesoporous ZnO, SnO2, and TiO2 films used in dye-sensitized solar cells. ACS Nano 2011, 5, 5158–5166. [Google Scholar] [CrossRef]
- Gonzalez-Valls, I.; Lira-Cantu, M. Vertically-aligned nanostructures of ZnO for excitonic solar cells: A review. Energy Environ. Sci. 2009, 2, 19–34. [Google Scholar] [CrossRef]
- Vittal, R.; Ho, K.C. Zinc oxide based dye-sensitized solar cells: A review. Renew. Sustain. Energy Rev. 2017, 70, 920–935. [Google Scholar] [CrossRef]
- Memarian, N.; Concina, I.; Braga, A.; Rozati, S.M.; Vomiero, A.; Sberveglieri, G. Hierarchically assembled ZnO nanocrystallites for high-efficiency dye-sensitized solar cells. Angew. Chem. Int. Ed. 2011, 50, 12321–12325. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Li, R.; Fan, K.; Peng, T. Effects of annealing conditions on the photoelectrochemical properties of dye-sensitized solar cells made with ZnO nanoparticles. Sol. Energy 2010, 84, 844–853. [Google Scholar] [CrossRef]
- Ambade, S.B.; Mane, R.S.; Ghule, A.V.; Takwale, M.G.; Abhyankar, A.; Cho, B.W.; Han, S.H. Contact angle measurement: A preliminary diagnostic method for evaluating the performance of ZnO platelet-based dye-sensitized solar cells. Scr. Mater. 2009, 61, 12–15. [Google Scholar] [CrossRef]
- Yan, F.; Huang, L.; Zheng, J.; Huang, J.; Lin, Z.; Huang, F.; Wei, M. Effect of surface etching on the efficiency of ZnO-based dye-sensitized solar cells. Langmuir 2010, 26, 7153–7156. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, H.; Katoh, R.; Hara, K.; Yanagida, M.; Murata, S.; Arakawa, H.; Tachiya, M. Electron injection efficiency from excited N3 into nanocrystalline ZnO films: Effect of (N3-Zn2+) aggregate formation. J. Phys. Chem. B 2003, 107, 2570–2574. [Google Scholar] [CrossRef]
- Law, M.; Greene, L.E.; Radenovic, A.; Kuykendall, T.; Liphardt, J.; Yang, P. ZnO-Al2O3 and ZnO-TiO2 core-shell nanowire dye-sensitized solar cells. J. Phys. Chem. B 2006, 110, 22652–22663. [Google Scholar] [CrossRef] [PubMed]
- Chandiran, A.K.; Abdi-Jalebi, M.; Nazeeruddin, M.K.; Grätzel, M. Analysis of electron transfer properties of ZnO and TiO2 photoanodes for dye-sensitized solar cells. ACS Nano 2014, 8, 2261–2268. [Google Scholar] [CrossRef] [PubMed]
- Atienzar, P.; Ishwara, T.; Illy, B.N.; Ryan, M.P.; O’Regan, B.C.; Durrant, J.R.; Nelson, J. Control of photocurrent generation in polymer/ZnO nanorod solar cells by using a solution-processed TiO2 overlayer. J. Phys. Chem. Lett. 2010, 1, 708–713. [Google Scholar] [CrossRef]
- Feng, Y.; Ji, X.; Duan, J.; Zhu, J.; Jiang, J.; Ding, H.; Meng, G.; Ding, R.; Liu, J.; Hu, A.; et al. Synthesis of ZnO@TiO2 core-shell long nanowire arrays and their application on dye-sensitized solar cells. J. Solid State Chem. 2012, 190, 303–308. [Google Scholar] [CrossRef]
- Prabakar, K.; Son, M.; Kim, W.Y.; Kim, H. TiO2 thin film encapsulated ZnO nanorod and nanoflower dye sensitized solar cells. Mater. Chem. Phys. 2011, 125, 12–14. [Google Scholar] [CrossRef]
- Paola, A.D.; Bellardita, M.; Palmisano, L. Brookite, the Least Known TiO2 Photocatalyst. Catalysts 2013, 3, 36–73. [Google Scholar] [CrossRef]
- Moret, M.P.; Zallen, R.; Vijay, D.P.; Desu, S.B. Broo Kite-Rich Titania Films Made by Pulsed Laser Deposition. Thin Solid Films 2000, 366, 8–10. [Google Scholar] [CrossRef]
- Sennik, E.; Kilinc, N.; Ozturk, Z.Z. Electrical and VOC sensing properties of anatase and rutile TiO2 nanotubes. J. Alloy. Compd. 2014, 616, 89–96. [Google Scholar] [CrossRef]
- Zou, C.W.; Wang, J.; Xie, W. Synthesis and enhanced NO2 gas sensing properties of ZnO nanorods/TiO2 nanoparticles heterojunction composites. J. Colloid Interface Sci. 2016, 478, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Zhu, L.; Cai, F.; Yang, Z.; Gu, X.; Huang, J.; Cao, L. ZnO/TiO2 core-shell nanowire arrays for enhanced dye-sensitized solar cell efficiency. Appl. Phys. A Mater. Sci. Process. 2013, 113, 67–73. [Google Scholar] [CrossRef]
- Goh, G.K.L.; Le, H.Q.; Huang, T.J.; Hui, B.T.T. Low temperature grown ZnO@TiO2 core shell nanorod arrays for dye sensitized solar cell application. J. Solid State Chem. 2014, 214, 17–23. [Google Scholar] [CrossRef]
- Greene, L.E.; Law, M.; Yuhas, B.D.; Yang, P. ZnO-TiO2 core-shell nanorod/P3HT solar cells. J. Phys. Chem. C 2007, 111, 18451–18456. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, C. Pure anatase phase titanium dioxide films prepared by mist chemical vapor deposition. Nanomaterials 2018, 8, 827. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, C. Effect of substrates on structural properties of pure anatase phase titanium dioxide thin films prepared by mist chemical vapor deposition. ECS J. Solid State Sci. Technol. 2018, 7, P654–P659. [Google Scholar] [CrossRef]
- Kawaharamura, T.; Mori, K.; Orita, H.; Shirahata, T.; Fujita, S.; Hirao, T. Effect of O3 and aqueous ammonia on crystallization of MgO thin film grown by mist chemical vapor deposition. Jpn. J. Appl. Phys. 2013, 52, 035501. [Google Scholar] [CrossRef]
- Li, X.; Li, C.; Kawaharamura, T.; Wang, D.; Nitta, N.; Furuta, M.; Furuta, H.; Hatta, A. Influence of substrates on formation of zinc oxide nanostructures by a novel reducing annealing method. Nanosci. Nanotechnol. Lett. 2014, 6, 174–180. [Google Scholar] [CrossRef]
- Li, X.; Li, C.; Hou, S.; Hatta, A.; Yu, J.; Jiang, N. Thickness of ITO thin film influences on fabricating ZnO nanorods applying for dye-sensitized solar cell. Compos. Part B Eng. 2015, 74, 147–152. [Google Scholar] [CrossRef]
- Hou, S.; Li, C. Aluminum-doped zinc oxide thin film as seeds layer effects on the alignment of zinc oxide nanorods synthesized in the chemical bath deposition. Thin Solid Films 2016, 605, 37–43. [Google Scholar] [CrossRef]
- Ansari, S.A.; Cho, M.H. Facile and sustainable synthesis of carbon-doped ZnO nanostructures towards the superior visible light photocatalytic performance. New J. Chem. 2017, 41, 9314–9320. [Google Scholar] [CrossRef]
- Ansari, S.A.; Cho, M.H. Simple and large scale construction of MoS2-g-C3N4 heterostructures using mechanochemistry for high performance electrochemical supercapacitor and visible light photocatalytic applications. Sci. Rep. 2017, 7, 43055. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.Z.; Ansari, S.A.; Parveen, N.; Cho, M.H.; Song, T. Lithium ion storage ability, supercapacitor electrode performance, and photocatalytic performance of tungsten disulfide nanosheets. New J. Chem. 2018, 42, 5859–5867. [Google Scholar] [CrossRef]
- Ansari, S.A.; Cho, M.H. Growth of three-dimensional flower-like SnS2 on g-C3N4 sheets as an efficient visible-light photocatalyst, photoelectrode, and electrochemical supercapacitance material. Sustain. Energy Fuels 2017, 1, 510–519. [Google Scholar] [CrossRef]
- Lee, C.T. Fabrication methods and luminescent properties of ZnO materials for light-emitting diodes. Materials 2010, 3, 2218–2259. [Google Scholar] [CrossRef]
- Liang, Y.; Wicker, S.; Wang, X.; Erichsen, E.S.; Fu, F. Organozinc precursor-derived crystalline ZnO nanoparticles: Synthesis, characterization and their spectroscopic properties. Nanomaterials 2018, 8, 22. [Google Scholar] [CrossRef]
- Vijayalakshmi, S.; Venkataraj, S.; Jayavel, R. Characterization of cadmium doped zinc oxide (Cd:ZnO) thin films prepared by spray pyrolysis method. J. Phys. D Appl. Phys. 2008, 41, 245403. [Google Scholar] [CrossRef]
- Saikia, L.; Bhuyan, D.; Saikia, M.; Malakar, B.; Dutta, D.K.; Sengupta, P. Photocatalytic performance of ZnO nanomaterials for self sensitized degradation of malachite green dye under solar light. Appl. Catal. A 2015, 490, 42–49. [Google Scholar] [CrossRef]
- Mi, Y.; Weng, Y. Band alignment and controllable electron migration between rutile and anatase TiO2. Sci. Rep. 2015, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lima, S.A.M.; Sigoli, F.A.; Jafelicci, M., Jr.; Davolos, M.R. Luminescent properties and lattice correlation defects on zinc oxide. Int. J. Inorg. Mater. 2001, 3, 749–754. [Google Scholar] [CrossRef]
- Reddy, R.S.; Sreedhar, A.; Reddy, A.S.; Uthanna, S. Effect of film thickness on the structural morphological and optical properties of nanocrystalline ZnO films formed by RF magnetron sputtering. Adv. Mater. Lett. 2012, 3, 239–245. [Google Scholar] [CrossRef]







| Target | AZO (2 wt.%) |
| Working distance (mm) | 60 |
| Working gas, flow rate (sccm) | Argon, 30 |
| Pressure (Pa) | 1 |
| Deposition temperature (°C) | 150 |
| RF power (W) | 60 |
| Solute | Zn(NO3)2, HMTA |
| Solvent | Ultrapure water |
| Concentration Zn(NO3)2 (mmol/L) | 15 |
| Concentration HMTA (mmol/L) | 7.5 |
| Deposition temperature (°C) | 95 |
| Deposition time (h) | 5 |
| Solute | TTIP |
| Solvent | Ethanol |
| Concentration (mol/L) | 0.10 |
| Deposition temperature (°C) | 400 |
| Carrier gas, flow rate (L/min) | Compressed air, 2.5 |
| Dilution gas, flow rate (L/min) | Compressed air, 4.5 |
| Coating time (min) | 0.5, 2, 5, 10, 15 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Li, C. TiO2 Coated ZnO Nanorods by Mist Chemical Vapor Deposition for Application as Photoanodes for Dye-Sensitized Solar Cells. Nanomaterials 2019, 9, 1339. https://doi.org/10.3390/nano9091339
Zhang Q, Li C. TiO2 Coated ZnO Nanorods by Mist Chemical Vapor Deposition for Application as Photoanodes for Dye-Sensitized Solar Cells. Nanomaterials. 2019; 9(9):1339. https://doi.org/10.3390/nano9091339
Chicago/Turabian StyleZhang, Qiang, and Chaoyang Li. 2019. "TiO2 Coated ZnO Nanorods by Mist Chemical Vapor Deposition for Application as Photoanodes for Dye-Sensitized Solar Cells" Nanomaterials 9, no. 9: 1339. https://doi.org/10.3390/nano9091339
APA StyleZhang, Q., & Li, C. (2019). TiO2 Coated ZnO Nanorods by Mist Chemical Vapor Deposition for Application as Photoanodes for Dye-Sensitized Solar Cells. Nanomaterials, 9(9), 1339. https://doi.org/10.3390/nano9091339





