An Organic Electrochemical Transistor-Based Sensor for IgG Levels Detection of Relevance in SARS-CoV-2 Infections
Abstract
:1. Introduction
2. Device Description
2.1. Organic Electrochemical Transistors (OECTs)
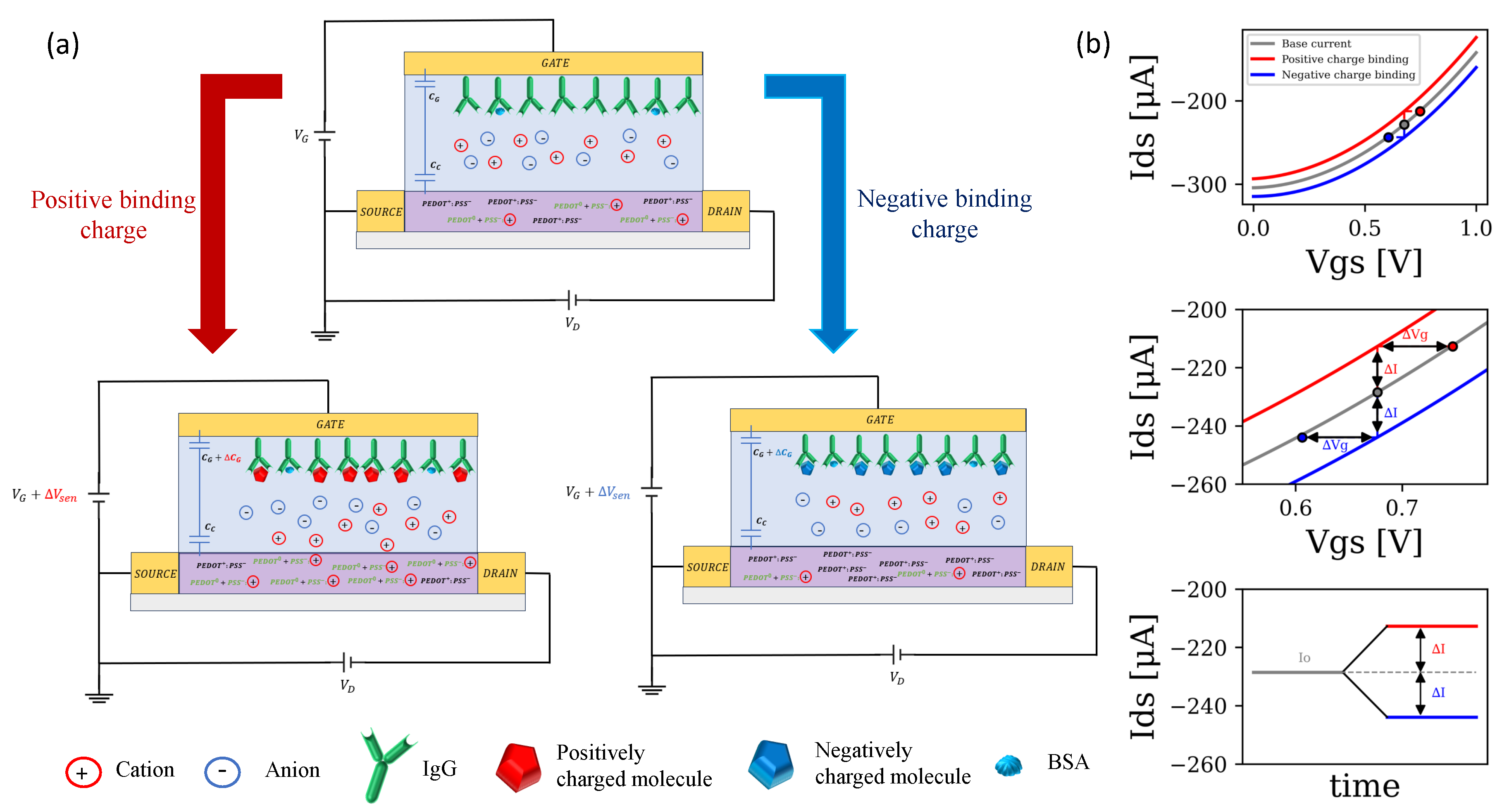
2.2. Operating Principle
2.3. Sensing Principle
3. Materials and Methods
3.1. Reagents and Chemicals
3.2. Characterization Set-Up
3.3. Fabrication of the OECTs
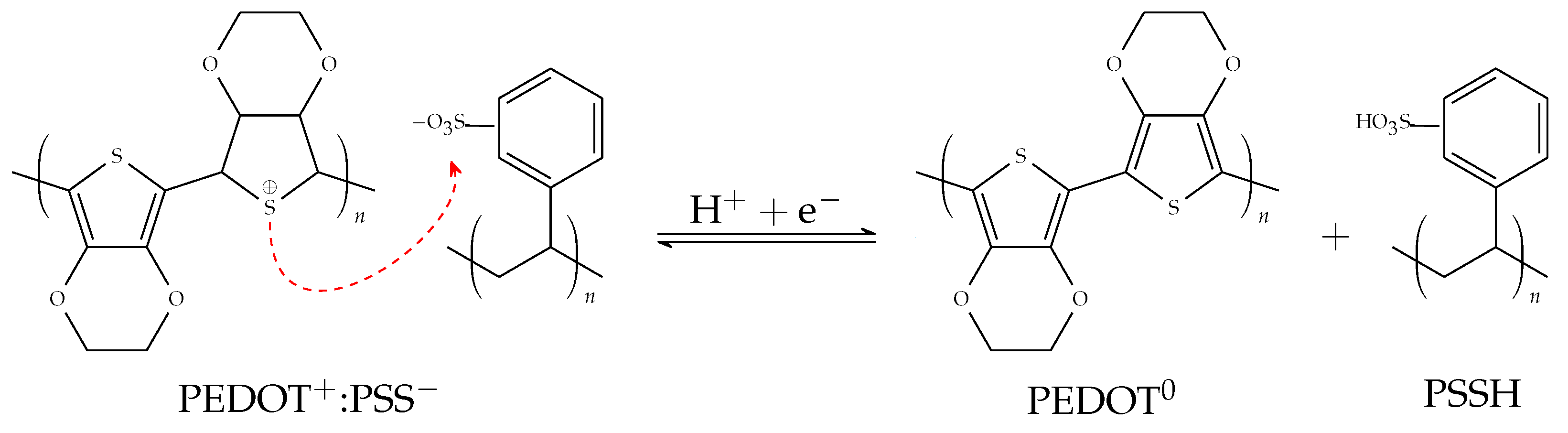
3.3.1. PEDOT:PSS
3.3.2. Derivatives of Graphene
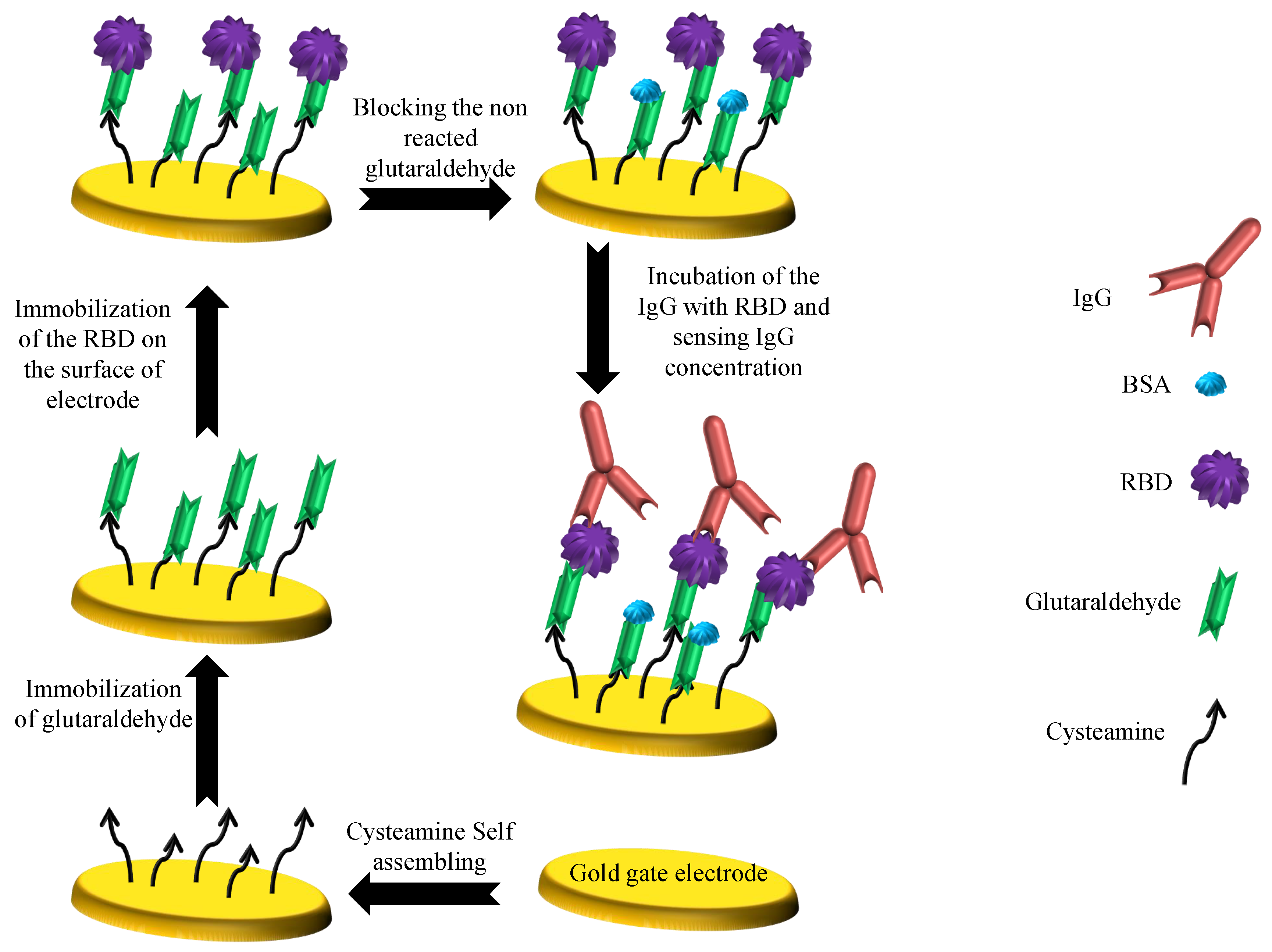
3.4. Surface Functionalization
4. Results
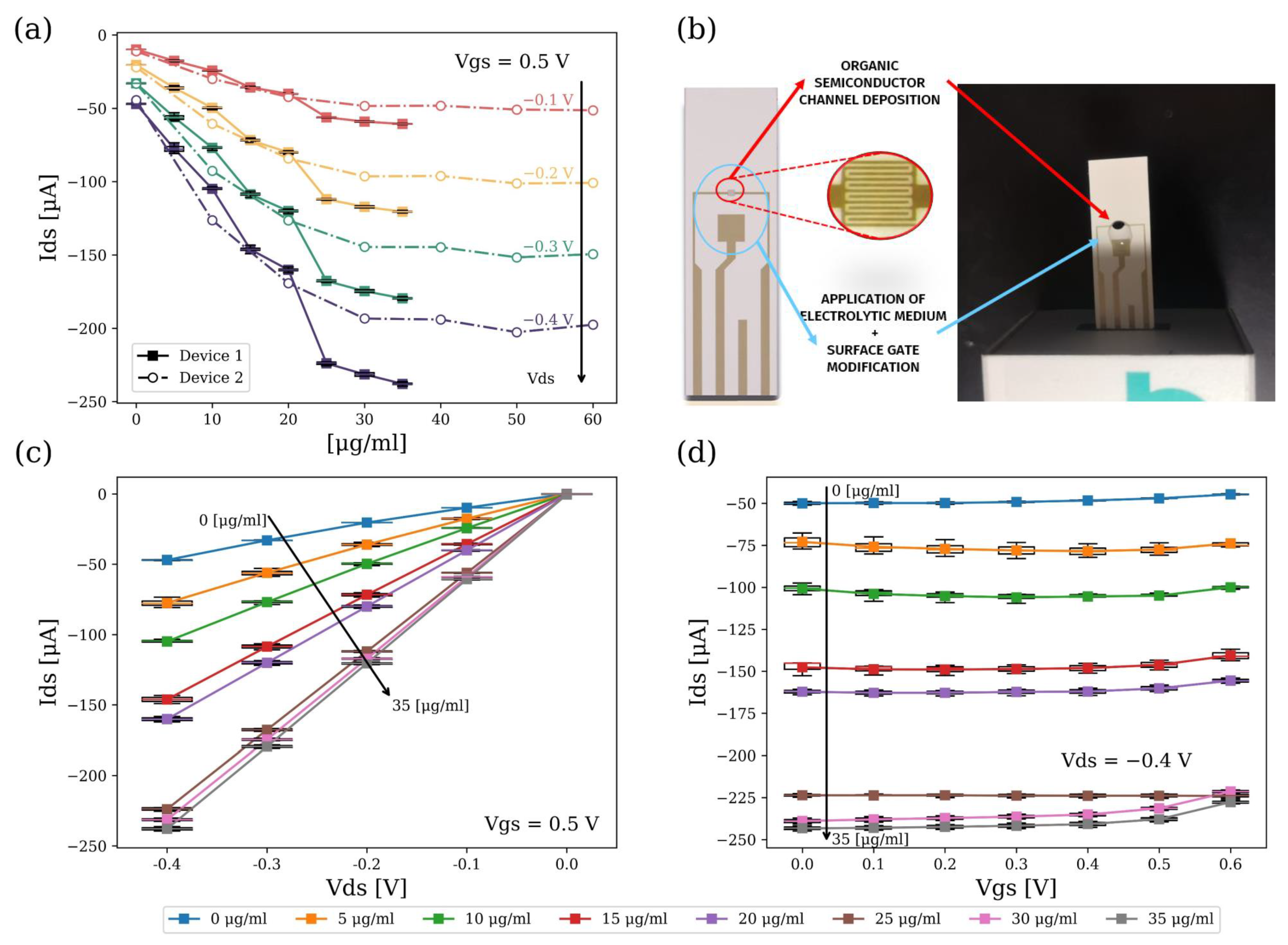
4.1. Characterization of the Static Response of the OECTs
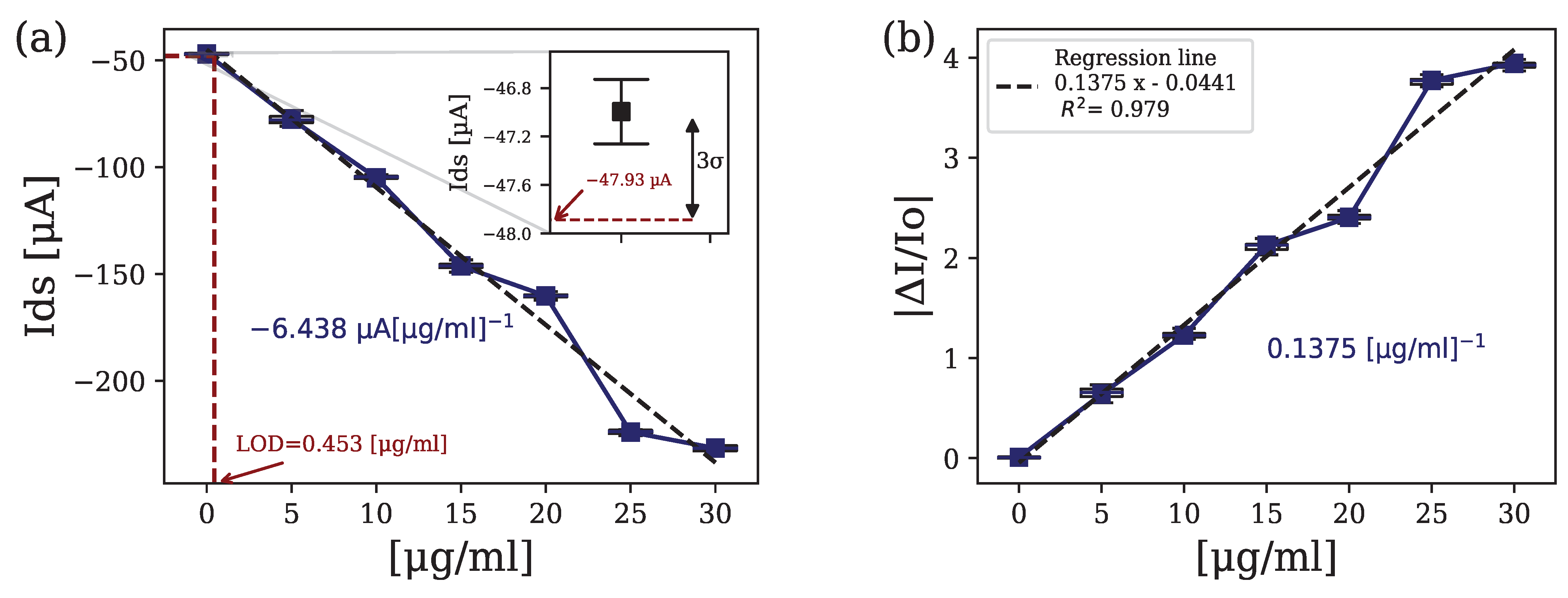
4.2. Immuno-Sensing
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LD | linear dichroism |
| OECT | organic electrochemical transistor |
| H-IgG | human immunoglobulin G |
| FDA | US Food and Drug Administration |
| RT-PCR | real-time reverse-transcription polymerase chain reaction |
| GO | graphene oxide |
| rGO | reduced graphene oxide |
| BSA | bovine serum albumin |
| FET | field effect transistor |
| PEDOT:PSS | poly(3,4-ethylenedioxythiophene)-poly(styrenesulfonate) |
| PBS | phosphate buffered saline |
| DI | deionized water |
| SCPI | Standard Commands for Programmable Instruments |
| RBD | receptor binding domain |
| LOD | detection limit |
References
- Chau, C.H.; Strope, J.D.; Figg, W.D. COVID-19 clinical diagnostics and testing technology. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2020, 40, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Moreira, V.M.; Mascarenhas, P.; Machado, V.; Botelho, J.; Mendes, J.J.; Taveira, N.; Almeida, M.G. Diagnosis of SARS-Cov-2 infection by RT-PCR using specimens other than naso-and oropharyngeal swabs: A systematic review and meta-analysis. Diagnostics 2021, 11, 363. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.P.; Lin, R.T.; Renia, L.; Ng, L.F. Serological approaches for COVID-19: Epidemiologic perspective on surveillance and control. Front. Immunol. 2020, 11, 879. [Google Scholar] [CrossRef] [PubMed]
- Lieberth, K.; Romele, P.; Torricelli, F.; Koutsouras, D.A.; Brückner, M.; Mailänder, V.; Gkoupidenis, P.; Blom, P.W. Current-Driven Organic Electrochemical Transistors for Monitoring Cell Layer Integrity with Enhanced Sensitivity. Adv. Healthc. Mater. 2021, 10, 2100845. [Google Scholar] [CrossRef] [PubMed]
- Decataldo, F.; Barbalinardo, M.; Tessarolo, M.; Vurro, V.; Calienni, M.; Gentili, D.; Valle, F.; Cavallini, M.; Fraboni, B. Organic electrochemical transistors: Smart devices for real-time monitoring of cellular vitality. Adv. Mater. Technol. 2019, 4, 1900207. [Google Scholar] [CrossRef]
- Rivnay, J.; Leleux, P.; Ferro, M.; Sessolo, M.; Williamson, A.; Koutsouras, D.A.; Khodagholy, D.; Ramuz, M.; Strakosas, X.; Owens, R.M.; et al. High-performance transistors for bioelectronics through tuning of channel thickness. Sci. Adv. 2015, 1, e1400251. [Google Scholar] [CrossRef] [PubMed]
- Cea, C.; Spyropoulos, G.D.; Jastrzebska-Perfect, P.; Ferrero, J.J.; Gelinas, J.N.; Khodagholy, D. Enhancement-mode ion-based transistor as a comprehensive interface and real-time processing unit for in vivo electrophysiology. Nat. Mater. 2020, 19, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Currano, L.J.; Sage, F.C.; Hagedon, M.; Hamilton, L.; Patrone, J.; Gerasopoulos, K. Wearable sensor system for detection of lactate in sweat. Sci. Rep. 2018, 8, 15890. [Google Scholar] [CrossRef] [PubMed]
- Ajayan, J.; Mohankumar, P.; Mathew, R.; Thoutam, L.R.; Kaushik, B.K.; Nirmal, D. Organic Electrochemical Transistors (OECTs): Advancements and Exciting Prospects for Future Biosensing Applications. IEEE Trans. Electron Devices 2023, 70, 3401–3412. [Google Scholar] [CrossRef]
- Song, J.; Liu, H.; Zhao, Z.; Lin, P.; Yan, F. Flexible Organic Transistors for Biosensing: Devices and Applications. Adv. Mater. 2023, 2300034. [Google Scholar]
- Friedlein, J.T.; Mcleod, R.R.; Rivnay, J. Device physics of organic electrochemical transistors. Org. Electron. 2018, 63, 398–414. [Google Scholar] [CrossRef]
- Liv, L. Electrochemical immunosensor platform based on gold-clusters, cysteamine and glutaraldehyde modified electrode for diagnosing COVID-19. Microchem. J. 2021, 168, 106445. [Google Scholar] [CrossRef] [PubMed]
- Post, N.; Eddy, D.; Huntley, C.; van Schalkwyk, M.C.; Shrotri, M.; Leeman, D.; Rigby, S.; Williams, S.V.; Bermingham, W.H.; Kellam, P.; et al. Antibody response to SARS-CoV-2 infection in humans: A systematic review. PLoS ONE 2020, 15, e0244126. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Hsiung, J.; Zhao, S.; Kost, J.; Sreedhar, D.; Hanson, C.V.; Olson, K.; Keare, D.; Chang, S.T.; Bliden, K.P.; et al. Quantification of antibody avidities and accurate detection of SARS-CoV-2 antibodies in serum and saliva on plasmonic substrates. Nat. Biomed. Eng. 2020, 4, 1188–1196. [Google Scholar] [CrossRef] [PubMed]
- Amanat, F.; Stadlbauer, D.; Strohmeier, S.; Nguyen, T.H.; Chromikova, V.; McMahon, M.; Jiang, K.; Arunkumar, G.A.; Jurczyszak, D.; Polanco, J.; et al. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat. Med. 2020, 26, 1033–1036. [Google Scholar] [CrossRef] [PubMed]
- Gaebler, C.; Wang, Z.; Lorenzi, J.C.C.; Muecksch, F.; Finkin, S.; Tokuyama, M.; Cho, A.; Jankovic, M.; Schaefer-Babajew, D.; Oliveira, T.Y.; et al. Evolution of antibody immunity to SARS-CoV-2. Nature 2021, 591, 639–644. [Google Scholar] [CrossRef]
- Sun, B.; Feng, Y.; Mo, X.; Zheng, P.; Wang, Q.; Li, P.; Peng, P.; Liu, X.; Chen, Z.; Huang, H.; et al. Kinetics of SARS-CoV-2 specific IgM and IgG responses in COVID-19 patients. Emerg. Microbes Infect. 2020, 9, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Iyer, A.S.; Jones, F.K.; Nodoushani, A.; Kelly, M.; Becker, M.; Slater, D.; Mills, R.; Teng, E.; Kamruzzaman, M.; Garcia-Beltran, W.F.; et al. Persistence and decay of human antibody responses to the receptor binding domain of SARS-CoV-2 spike protein in COVID-19 patients. Sci. Immunol. 2020, 5, eabe0367. [Google Scholar] [CrossRef] [PubMed]
- Demonbreun, A.R.; Sancilio, A.; Velez, M.P.; Ryan, D.T.; Saber, R.; Vaught, L.A.; Reiser, N.L.; Hsieh, R.R.; D’aquila, R.T.; Mustanski, B.; et al. Comparison of IgG and neutralizing antibody responses after one or two doses of COVID-19 mRNA vaccine in previously infected and uninfected individuals. eClinicalMedicine 2021, 38, 101018. [Google Scholar] [CrossRef]
- Bernards, D.A.; Malliaras, G.G. Steady-state and transient behavior of organic electrochemical transistors. Adv. Funct. Mater. 2007, 17, 3538–3544. [Google Scholar] [CrossRef]
- Prigodin, V.N.; Hsu, F.C.; Kim, Y.M.; Park, J.H.; Waldmann, O.; Epstein, A.J. Electric field control of charge transport in doped polymers. Synth. Met. 2005, 153, 157–160. [Google Scholar] [CrossRef]
- Robinson, N.D.; Svensson, P.O.; Nilsson, D.; Berggren, M. On the Current Saturation Observed in Electrochemical Polymer Transistors. J. Electrochem. Soc. 2006, 153, H39. [Google Scholar] [CrossRef]
- Sophocleous, M.; Contat-Rodrigo, L.; Garcia-Breijo, E.; Georgiou, J. Organic electrochemical transistors as an emerging platform for bio-sensing applications: A review. IEEE Sens. J. 2021, 21, 3977–4006. [Google Scholar] [CrossRef]
- Yaghmazadeh, O.; Cicoira, F.; Bernards, D.A.; Yang, S.Y.; Bonnassieux, Y.; Malliaras, G.G. Optimization of Organic Electrochemical Transistors for Sensor Applications. J. Polym. Sci. Part B Polym. Phys. 2010, 49, 34–39. [Google Scholar] [CrossRef]
- Liao, J.; Si, H.; Zhang, X.; Lin, S. Functional sensing interfaces of PEDOT: PSS organic electrochemical transistors for chemical and biological sensors: A mini review. Sensors 2019, 19, 218. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Offenhäusser, A.; Ingebrandt, S.; Mayer, D. PEDOT:PSS-Based Bioelectronic Devices for Recording and Modulation of Electrophysiological and Biochemical Cell Signals. Adv. Healthc. Mater. 2021, 10, 2100061. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, A.; Song, J.; Wang, N.; Lam, P.; Li, Y.; Law, H.K.W.; Yan, F. Ultrafast, sensitive, and portable detection of COVID-19 IgG using flexible organic electrochemical transistors. Sci. Adv. 2021, 7, 8387–8402. [Google Scholar] [CrossRef] [PubMed]
- Barra, M.; Tomaiuolo, G.; Villella, V.R.; Esposito, S.; Liboà, A.; D’Angelo, P.; Marasso, S.L.; Cocuzza, M.; Bertana, V.; Camilli, E.; et al. Organic Electrochemical Transistor Immuno-Sensors for Spike Protein Early Detection. Biosensors 2023, 13, 739. [Google Scholar] [CrossRef] [PubMed]
- Bernards, D.A.; Macaya, D.J.; Nikolou, M.; Defranco, J.A.; Takamatsu, S.; Malliaras, G.G. Enzymatic sensing with organic electrochemical transistors. J. Mater. Chem. 2008, 18, 116–120. [Google Scholar] [CrossRef]
- Pappa, A.M.; Curto, V.F.; Braendlein, M.; Strakosas, X.; Donahue, M.J.; Fiocchi, M.; Malliaras, G.G.; Owens, R.M. Organic transistor arrays integrated with finger-powered microfluidics for multianalyte saliva testing. Adv. Healthc. Mater. 2016, 5, 2295–2302. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Dang, T.; Harrison, K.; Lill, A.; Dixon, A.; Lewis, E.; Vollbrecht, J.; Hachisu, T.; Biswas, S.; Visell, Y.; Nguyen, T.Q.; et al. Biomaterial-Based Solid-Electrolyte Organic Electrochemical Transistors for Electronic and Neuromorphic Applications. Adv. Electron. Mater. 2021, 7, 2100519. [Google Scholar] [CrossRef]
- Cowen, L.M.; Atoyo, J.; Carnie, M.J.; Baran, D.; Schroeder, B.C. Review-Organic Materials for Thermoelectric Energy Generation. ECS J. Solid State Sci. Technol. 2017, 6, N3080. [Google Scholar] [CrossRef]
- Gao, W. The chemistry of graphene oxide. In Graphene Oxide: Reduction Recipes, Spectroscopy, and Applications; Springer: Cham, Switzerland, 2015; pp. 61–95. [Google Scholar]
- Bai, R.G.; Muthoosamy, K.; Manickam, S.; Hilal-Alnaqbi, A. Graphene-based 3D scaffolds in tissue engineering: Fabrication, applications, and future scope in liver tissue engineering. Int. J. Nanomed. 2019, 14, 5753–5783. [Google Scholar]
- Mao, S.; Lu, G.; Yu, K.; Bo, Z.; Chen, J. Specific protein detection using thermally reduced graphene oxide sheet decorated with gold nanoparticle-antibody conjugates. Adv. Mater. 2010, 22, 3521–3526. [Google Scholar] [CrossRef] [PubMed]
- Aspermair, P.; Mishyn, V.; Bintinger, J.; Happy, H.; Bagga, K.; Subramanian, P.; Knoll, W.; Boukherroub, R.; Szunerits, S. Reduced graphene oxide–based field effect transistors for the detection of E7 protein of human papillomavirus in saliva. Anal. Bioanal. Chem. 2021, 413, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Muda, M.R.; Ramli, M.M.; Isa, S.S.; Jamlos, M.F.; Murad, S.A.; Norhanisah, Z.; Isa, M.M.; Kasjoo, S.R.; Ahmad, N.; Nor, N.I.; et al. Fundamental study of reduction graphene oxide by sodium borohydride for gas sensor application. AIP Conf. Proc. 2017, 1808, 020034. [Google Scholar]
- Tarabella, G.; Santato, C.; Yang, S.Y.; Iannotta, S.; Malliaras, G.G.; Cicoira, F. Effect of the gate electrode on the response of organic electrochemical transistors. Appl. Phys. Lett. 2010, 97, 123304. [Google Scholar] [CrossRef]
- Macchia, E.; Romele, P.; Manoli, K.; Ghittorelli, M.; Magliulo, M.; Kovács-Vajna, Z.M.; Torricelli, F.; Torsi, L. Ultra-sensitive protein detection with organic electrochemical transistors printed on plastic substrates. Flex. Print. Electron. 2018, 3, 034002. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Algarín Pérez, A.; Acedo, P. An Organic Electrochemical Transistor-Based Sensor for IgG Levels Detection of Relevance in SARS-CoV-2 Infections. Biosensors 2024, 14, 207. https://doi.org/10.3390/bios14040207
Algarín Pérez A, Acedo P. An Organic Electrochemical Transistor-Based Sensor for IgG Levels Detection of Relevance in SARS-CoV-2 Infections. Biosensors. 2024; 14(4):207. https://doi.org/10.3390/bios14040207
Chicago/Turabian StyleAlgarín Pérez, Antonio, and Pablo Acedo. 2024. "An Organic Electrochemical Transistor-Based Sensor for IgG Levels Detection of Relevance in SARS-CoV-2 Infections" Biosensors 14, no. 4: 207. https://doi.org/10.3390/bios14040207




