Phenotypic and Genotypic Analysis of Bacterial Pathogens Recovered from Patients Diagnosed with Fever of Unknown Origin in Egypt
Abstract
:1. Introduction
2. Results
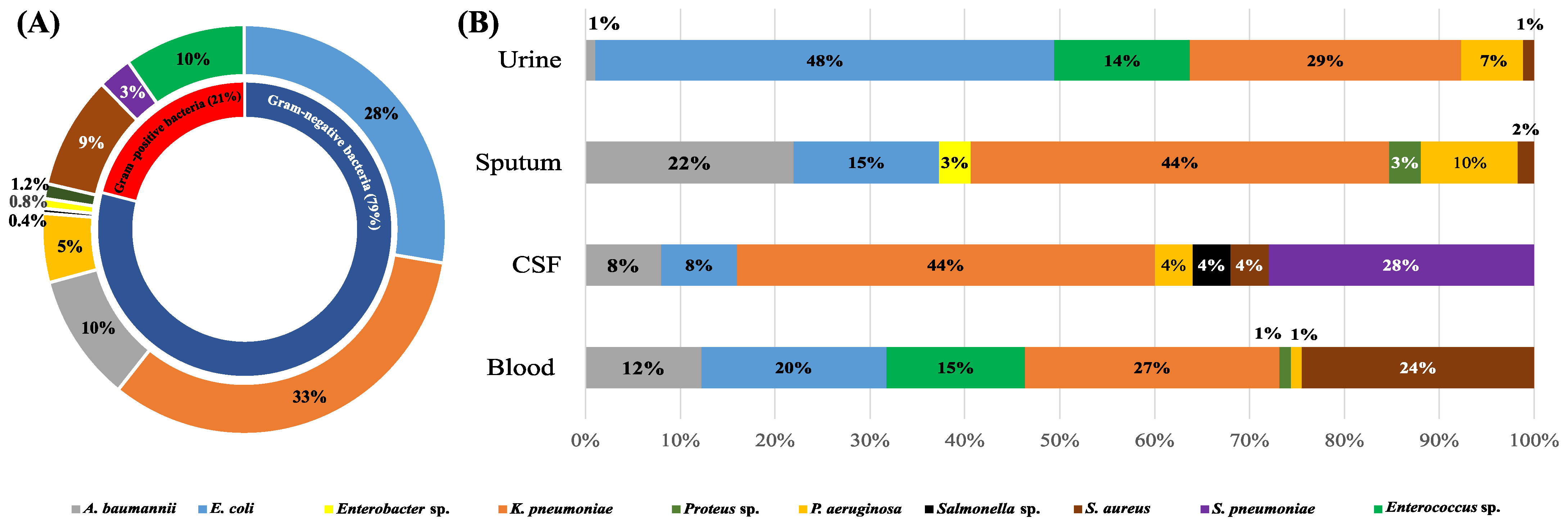
2.1. Fever of Unknown Origin (FUO) Caused by Bacterial Infections
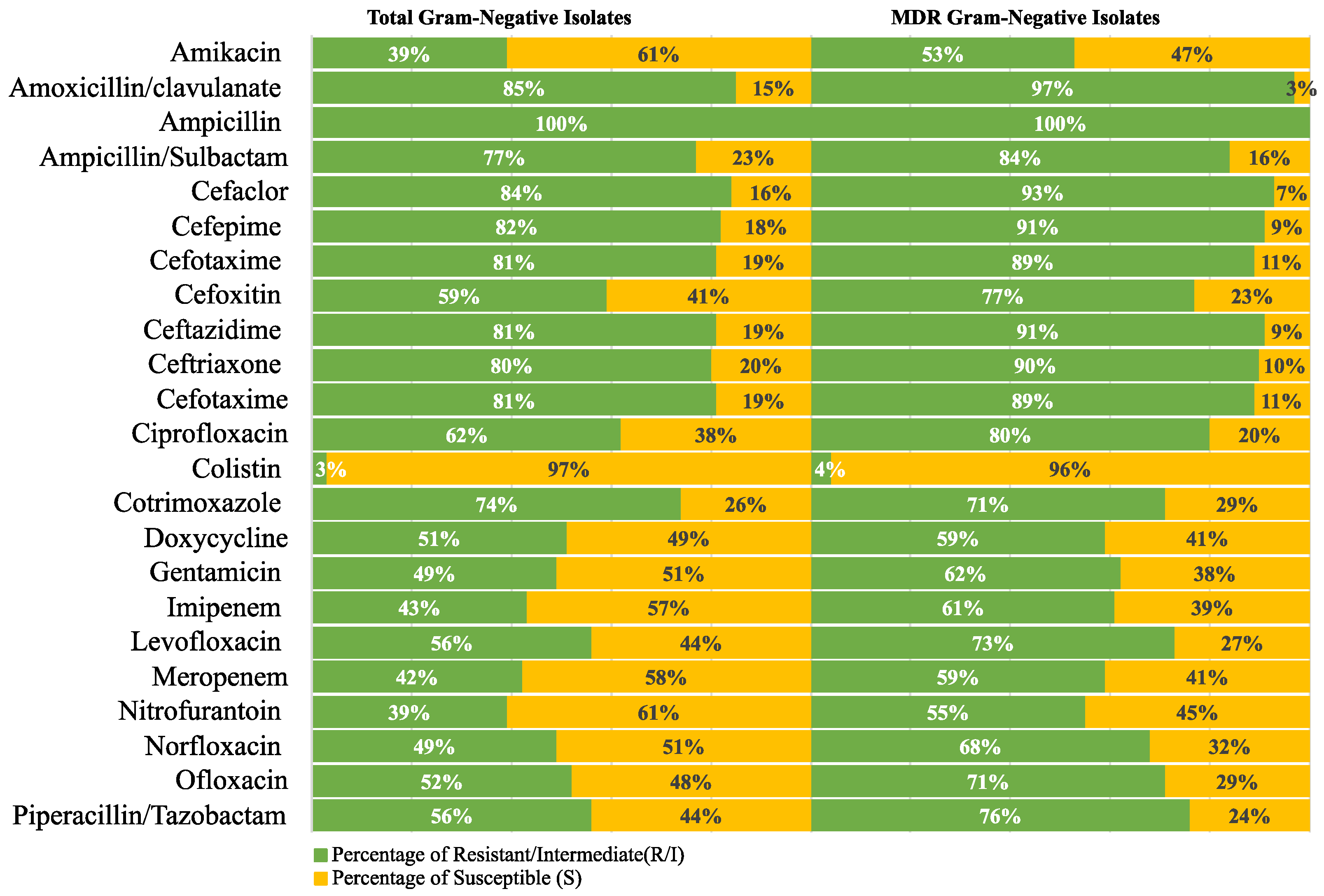
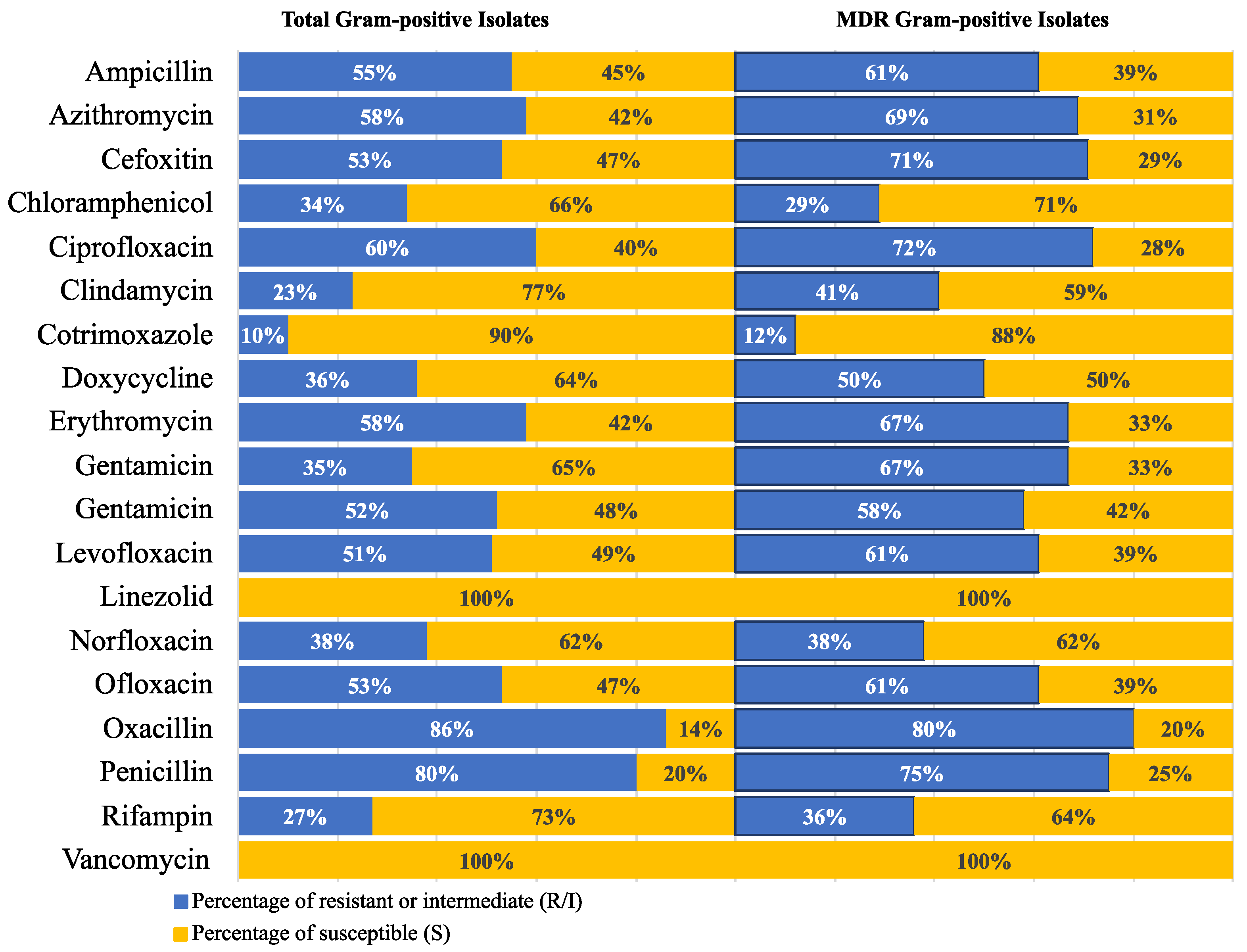
2.2. Antimicrobial Susceptibility Profiles
2.3. Minimum Inhibitory Concentrations of the Tested Antibiotics
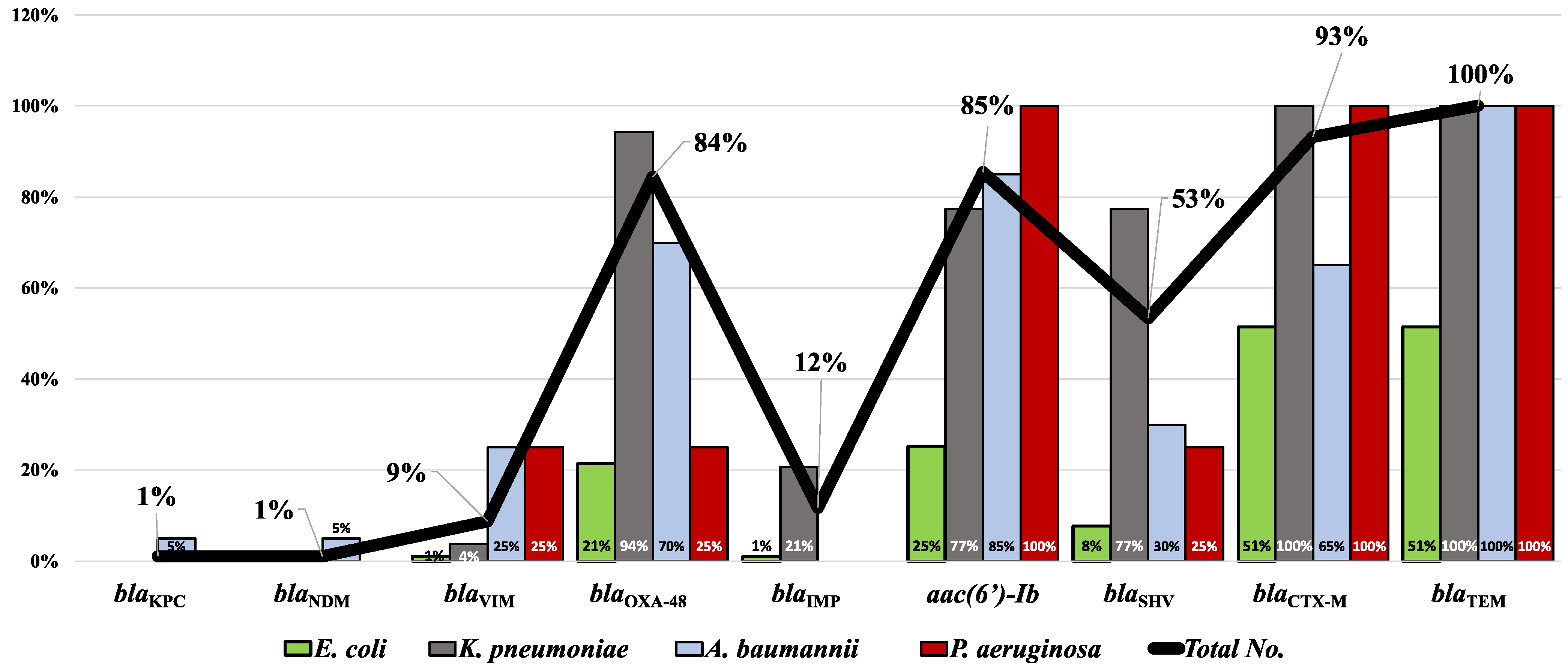
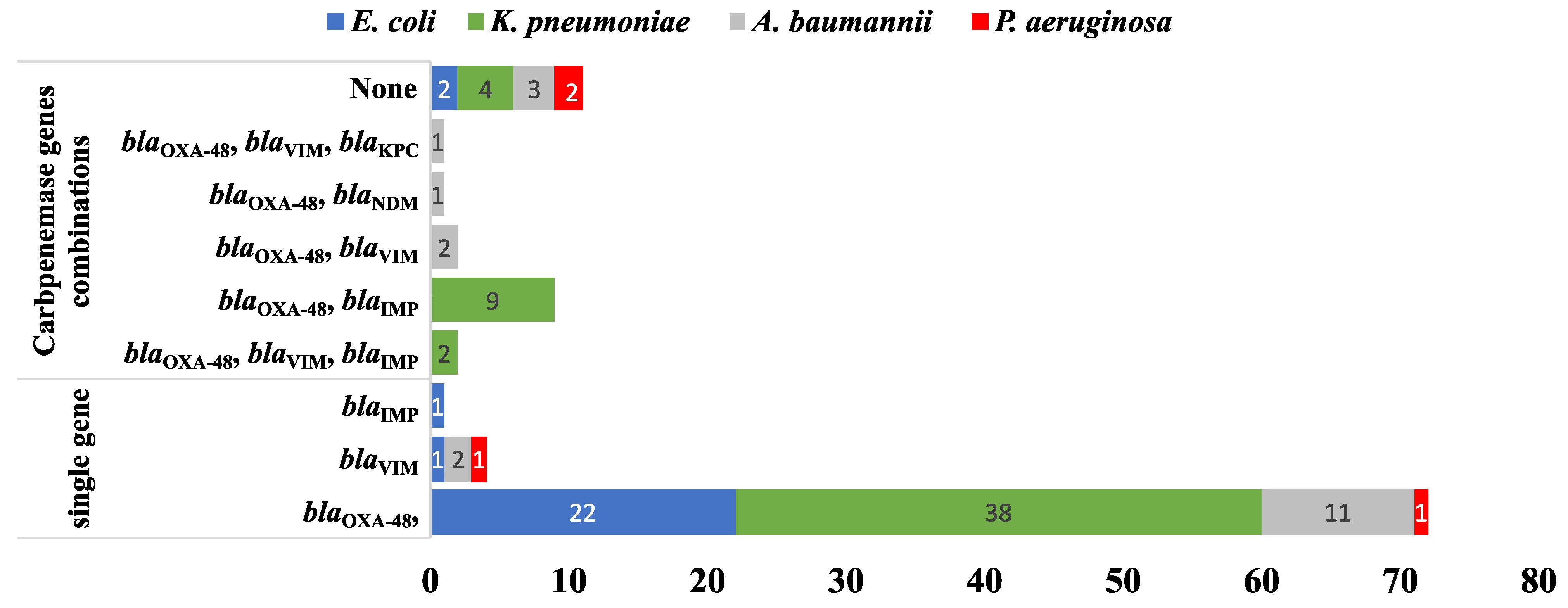
2.4. Molecular Detection of the Carbapenemase-, Extended-Spectrum β-Lactamase (ESBL)-, and aac(6′)-Ib-Coding Genes
2.5. Phenotypic and Genotypic Analysis Using Heatmap Analysis
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Microbiological Procedures
4.3. Antimicrobial Susceptibility Testing
4.4. Identification of MDR and ESBL Phenotypes
4.5. Identification of Carbapenemase-Coding and ESBL Genes
4.6. Phenotypic and Genotypic Analysis Using Heatmap Analysis:
4.7. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Unger, M.; Karanikas, G.; Kerschbaumer, A.; Winkler, S.; Aletaha, D. Fever of unknown origin (FUO) revised. Wien. Klin. Wochenschr. 2016, 128, 796–801. [Google Scholar] [CrossRef] [Green Version]
- Fusco, F.M.; Pisapia, R.; Nardiello, S.; Cicala, S.D.; Gaeta, G.B.; Brancaccio, G. Fever of unknown origin (FUO): Which are the factors influencing the final diagnosis? A 2005–2015 systematic review. BMC Infect. Dis. 2019, 19, 653. [Google Scholar] [CrossRef] [PubMed]
- Cunha, B.A. Fever of unknown origin: Focused diagnostic approach based on clinical clues from the history, physical examination, and laboratory tests. Infect. Dis. Clin. N. Am. 2007, 21, 1137–1187. [Google Scholar] [CrossRef]
- Parry, C.M.; Threlfall, E.J. Antimicrobial resistance in typhoidal and nontyphoidal salmonellae. Curr. Opin. Infect. Dis. 2008, 21, 531–538. [Google Scholar] [CrossRef]
- Partridge, S.R.; Kwong, S.M.; Firth, N.; Jensen, S.O. Mobile Genetic Elements Associated with Antimicrobial Resistance. Clin. Microbiol. Rev. 2018, 31, e00088-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, M.A.; Vianna, M.F.; Nishino, L.K.; Lazarini, P.R. Vestibular disorders in Bell’s palsy: A prospective study. Rev. Laryngol. Otol. Rhinol. 2015, 136, 29–31. [Google Scholar]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Mabrouk, S.S.; Abdellatif, G.R.; El-Ansary, M.R.; Aboshanab, K.M.; Ragab, Y.M. Carbapenemase Producers Among Extensive Drug-Resistant Gram-Negative Pathogens Recovered from Febrile Neutrophilic Patients in Egypt. Infect. Drug Resist. 2020, 13, 3113–3124. [Google Scholar] [CrossRef]
- Jean, S.S.; Harnod, D.; Hsueh, P.R. Global Threat of Carbapenem-Resistant Gram-Negative Bacteria. Front. Cell Infect. Microbiol. 2022, 12, 823684. [Google Scholar] [CrossRef]
- Elshamy, A.A.; Aboshanab, K.M. A review on bacterial resistance to carbapenems: Epidemiology, detection and treatment options. Future Sci. OA 2020, 6, FSO438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zafer, M.M.; Hussein, A.F.A.; Al-Agamy, M.H.; Radwan, H.H.; Hamed, S.M. Genomic Characterization of Extensively Drug-Resistant NDM-Producing Acinetobacter baumannii Clinical Isolates With the Emergence of Novel bla (ADC-257). Front. Microbiol. 2021, 12, 736982. [Google Scholar] [CrossRef] [PubMed]
- Nordmann, P.; Poirel, L.; Carrer, A.; Toleman, M.A.; Walsh, T.R. How to detect NDM-1 producers. J. Clin. Microbiol. 2011, 49, 718–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGregor, A.C.; Moore, D.A. Infectious causes of fever of unknown origin. Clin. Med. 2015, 15, 285–287. [Google Scholar] [CrossRef] [Green Version]
- Albalakosy, A.; Hussein, M.; Khalil, M.; Hussein, H.; Abdallah, M.; Askar, S.R. Epidemiology of Fever of Unknown Origin (Fuo) at Imbaba Fever Hospital, Giza Governorate, Egypt. J. Egypt. Soc. Parasitol. 2022, 52, 169–176. [Google Scholar] [CrossRef]
- Abdelbaky, M.S.; Mansour, H.E.; Ibrahim, S.I.; Hassan, I.A. Prevalence of connective tissue diseases in egyptian patients presenting with Fever of unknown origin. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2011, 4, 33–41. [Google Scholar] [CrossRef] [Green Version]
- Ali-Eldin, F.A.; Abdelhakam, S.M.; Ali-Eldin, Z.A. Clinical spectrum of fever of unknown origin among adult Egyptian patients admitted to Ain Shams University Hospitals: A hospital based study. J. Egypt. Soc. Parasitol. 2011, 41, 379–386. [Google Scholar]
- Montasser, M.F.; Abdelkader, N.A.; Montasser, I.F.; El Khouly, A.M. Changing the face of fever of unknown origin in Egypt: A single hospital study. Braz. J. Infect. Dis. 2015, 19, 334–335. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, S.H.; Saleh, S.E.; Hamed, S.M.; Aboshanab, K.M. Febrile illness of bacterial etiology in a public fever hospital in Egypt: High burden of multidrug resistance and WHO priority Gram negative pathogens. Germs 2022, 12, 75–85. [Google Scholar] [CrossRef]
- El-Kholy, A.; El-Mahallawy, H.A.; Elsharnouby, N.; Abdel Aziz, M.; Helmy, A.M.; Kotb, R. Landscape of Multidrug-Resistant Gram-Negative Infections in Egypt: Survey and Literature Review. Infect. Drug Resist. 2021, 14, 1905–1920. [Google Scholar] [CrossRef]
- Fahim, N.A.E. Prevalence and antimicrobial susceptibility profile of multidrug-resistant bacteria among intensive care units patients at Ain Shams University Hospitals in Egypt-a retrospective study. J. Egypt. Public Health Assoc. 2021, 96, 7. [Google Scholar] [CrossRef]
- Taha, M.S.; Hagras, M.M.; Shalaby, M.M.; Zamzam, Y.A.; Elkolaly, R.M.; Abdelwahab, M.A.; Maxwell, S.Y. Genotypic Characterization of Carbapenem-Resistant Klebsiella pneumoniae Isolated from an Egyptian University Hospital. Pathogens 2023, 12, 121. [Google Scholar] [CrossRef]
- Alfeky, A.E.; Tawfick, M.M.; Ashour, M.S.; El-Moghazy, A.A. High Prevalence of Multi-drug Resistant Methicillin-Resistant Staphylococcus aureus in Tertiary Egyptian Hospitals. J. Infect. Dev. Ctries. 2022, 16, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Elshimy, R.; Zedan, H.; Elmorsy, T.H.; Khattab, R.A. A Study on Multidrug-Resistant Escherichia coli Clinical Isolates from Different Hospitals in Greater Cairo. Microb. Drug Resist. 2021, 27, 1420–1432. [Google Scholar] [CrossRef] [PubMed]
- Catalano, A.; Iacopetta, D.; Ceramella, J.; Scumaci, D.; Giuzio, F.; Saturnino, C.; Aquaro, S.; Rosano, C.; Sinicropi, M.S. Multidrug Resistance (MDR): A Widespread Phenomenon in Pharmacological Therapies. Molecules 2022, 27, 616. [Google Scholar] [CrossRef]
- Salleh, M.Z.; Nik Zuraina, N.M.N.; Hajissa, K.; Ilias, M.I.; Banga Singh, K.K.; Deris, Z.Z. Prevalence of Multidrug-Resistant and Extended-Spectrum Beta-Lactamase-Producing Shigella Species in Asia: A Systematic Review and Meta-Analysis. Antibiotics 2022, 11, 11653. [Google Scholar] [CrossRef]
- Shbaklo, N.; Corcione, S.; Vicentini, C.; Giordano, S.; Fiorentino, D.; Bianco, G.; Cattel, F.; Cavallo, R.; Zotti, C.M.; De Rosa, F.G. An Observational Study of MDR Hospital-Acquired Infections and Antibiotic Use during COVID-19 Pandemic: A Call for Antimicrobial Stewardship Programs. Antibiotics 2022, 11, 695. [Google Scholar] [CrossRef] [PubMed]
- Bedenic, B.; Bratic, V.; Mihaljevic, S.; Lukic, A.; Vidovic, K.; Reiner, K.; Schoenthaler, S.; Barisic, I.; Zarfel, G.; Grisold, A. Multidrug-Resistant Bacteria in a COVID-19 Hospital in Zagreb. Pathogens 2023, 12, 117. [Google Scholar] [CrossRef]
- Rizk, N.A.; Moghnieh, R.; Haddad, N.; Rebeiz, M.C.; Zeenny, R.M.; Hindy, J.R.; Orlando, G.; Kanj, S.S. Challenges to Antimicrobial Stewardship in the Countries of the Arab League: Concerns of Worsening Resistance during the COVID-19 Pandemic and Proposed Solutions. Antibiotics 2021, 10, 1320. [Google Scholar] [CrossRef]
- Guarnera, L.; Trotta, G.E.; Boldrini, V.; Cardillo, L.; Cerroni, I.; Mezzanotte, V.; Pasqualone, G.; Savi, A.; Borsellino, B.; Buzzatti, E.; et al. Fever of Unknown Origin and Multidrug Resistant Organism Colonization in AML Patients. Mediterr. J. Hematol. Infect. Dis. 2023, 15, e2023013. [Google Scholar] [CrossRef]
- Grundmann, H.; Glasner, C.; Albiger, B.; Aanensen, D.M.; Tomlinson, C.T.; Andrasevic, A.T.; Canton, R.; Carmeli, Y.; Friedrich, A.W.; European Survey of Carbapenemase-Producing Enterobacteriaceae Working Group; et al. Occurrence of carbapenemase-producing Klebsiella pneumoniae and Escherichia coli in the European survey of carbapenemase-producing Enterobacteriaceae (EuSCAPE): A prospective, multinational study. Lancet Infect. Dis. 2017, 17, 153–163. [Google Scholar] [CrossRef] [Green Version]
- Ghaith, D.M.; Zafer, M.M.; Said, H.M.; Elanwary, S.; Elsaban, S.; Al-Agamy, M.H.; Bohol, M.F.F.; Bendary, M.M.; Al-Qahtani, A.; Al-Ahdal, M.N. Genetic diversity of carbapenem-resistant Klebsiella Pneumoniae causing neonatal sepsis in intensive care unit, Cairo, Egypt. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Zafer, M.M.; El Bastawisie, M.M.; Wassef, M.; Hussein, A.F.; Ramadan, M.A. Epidemiological features of nosocomial Klebsiella pneumoniae: Virulence and resistance determinants. Future Microbiol. 2022, 17, 27–40. [Google Scholar] [CrossRef] [PubMed]
- El-Badawy, M.F.; El-Far, S.W.; Althobaiti, S.S.; Abou-Elazm, F.I.; Shohayeb, M.M. The First Egyptian Report Showing the Co-Existence of bla (NDM-25), bla (OXA-23), bla (OXA-181), and bla (GES-1) Among Carbapenem-Resistant K. pneumoniae Clinical Isolates Genotyped by BOX-PCR. Infect. Drug Resist. 2020, 13, 1237–1250. [Google Scholar] [CrossRef]
- Osama, D.; El-Mahallawy, H.; Mansour, M.T.; Hashem, A.; Attia, A.S. Molecular Characterization of Carbapenemase-Producing Klebsiella pneumoniae Isolated from Egyptian Pediatric Cancer Patients Including a Strain with a Rare Gene-Combination of beta-Lactamases. Infect. Drug Resist. 2021, 14, 335–348. [Google Scholar] [CrossRef] [PubMed]
- El Far, M.Y.; El-Mahallawy, H.A.; Attia, A.S. Tracing the dissemination of the international clones of multidrug-resistant Acinetobacter baumannii among cancer patients in Egypt using the PCR-based open reading frame typing (POT) method. J. Glob. Antimicrob. Resist. 2019, 19, 210–215. [Google Scholar] [CrossRef]
- Hamed, S.M.; Hussein, A.F.A.; Al-Agamy, M.H.; Radwan, H.H.; Zafer, M.M. Genetic Configuration of Genomic Resistance Islands in Acinetobacter baumannii Clinical Isolates from Egypt. Front. Microbiol. 2022, 13, 878912. [Google Scholar] [CrossRef]
- Wasfi, R.; Rasslan, F.; Hassan, S.S.; Ashour, H.M.; Abd El-Rahman, O.A. Co-Existence of Carbapenemase-Encoding Genes in Acinetobacter baumannii from Cancer Patients. Infect. Dis. Ther. 2021, 10, 291–305. [Google Scholar] [CrossRef]
- Higgins, P.G.; Dammhayn, C.; Hackel, M.; Seifert, H. Global spread of carbapenem-resistant Acinetobacter baumannii. J. Antimicrob. Chemother. 2010, 65, 233–238. [Google Scholar] [CrossRef] [Green Version]
- Pogue, J.M.; Mann, T.; Barber, K.E.; Kaye, K.S. Carbapenem-resistant Acinetobacter baumannii: Epidemiology, surveillance and management. Expert. Rev. Anti Infect. Ther. 2013, 11, 383–393. [Google Scholar] [CrossRef]
- Jalal, D.; Elzayat, M.G.; Diab, A.A.; El-Shqanqery, H.E.; Samir, O.; Bakry, U.; Hassan, R.; Elanany, M.; Shalaby, L.; Sayed, A.A. Deciphering Multidrug-Resistant Acinetobacter baumannii from a Pediatric Cancer Hospital in Egypt. mSphere 2021, 6, e0072521. [Google Scholar] [CrossRef]
- Borg, M.A.; de Kraker, M.; Scicluna, E.; van de Sande-Bruinsma, N.; Tiemersma, E.; Monen, J.; Grundmann, H.; ARMed Project Members and Collaborators. Prevalence of methicillin-resistant Staphylococcus aureus (MRSA) in invasive isolates from southern and eastern Mediterranean countries. J. Antimicrob. Chemother. 2007, 60, 1310–1315. [Google Scholar] [CrossRef] [Green Version]
- Rodvold, K.A.; McConeghy, K.W. Methicillin-resistant Staphylococcus aureus therapy: Past, present, and future. Clin. Infect. Dis. 2014, 58 (Suppl. S1), S20–S27. [Google Scholar] [CrossRef]
- Cong, Y.; Yang, S.; Rao, X. Vancomycin resistant Staphylococcus aureus infections: A review of case updating and clinical features. J. Adv. Res. 2020, 21, 169–176. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2021. [Google Scholar]
- Wasfy, M.O.; Pimentel, G.; Abdel-Maksoud, M.; Russell, K.L.; Barrozo, C.P.; Klena, J.D.; Earhart, K.; Hajjeh, R. Antimicrobial susceptibility and serotype distribution of Streptococcus pneumoniae causing meningitis in Egypt, 1998–2003. J. Antimicrob. Chemother. 2005, 55, 958–964. [Google Scholar] [CrossRef] [Green Version]
- El-Kholy, A.; Badawy, M.; Gad, M.; Soliman, M. Serotypes and Antimicrobial Susceptibility of Nasopharyngeal Isolates of Streptococcus pneumoniae from Children Less Than 5 Years Old in Egypt. Infect. Drug Resist. 2020, 13, 3669–3677. [Google Scholar] [CrossRef]
- Willems, R.J.; van Schaik, W. Transition of Enterococcus faecium from commensal organism to nosocomial pathogen. Future Microbiol. 2009, 4, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- Herman, D.J.; Gerding, D.N. Screening and treatment of infections caused by resistant enterococci. Antimicrob. Agents Chemother. 1991, 35, 215–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamed, S.M.; Hussein, A.F.A.; Al-Agamy, M.H.; Radwan, H.H.; Zafer, M.M. Tn7382, a novel composite transposon harboring bla(NDM-1) and aphA6 in Acinetobacter baumannii. J. Glob. Antimicrob. Resist. 2022, 30, 414–417. [Google Scholar] [CrossRef]
- Pan, Y.; Zeng, J.; Li, L.; Yang, J.; Tang, Z.; Xiong, W.; Li, Y.; Chen, S.; Zeng, Z. Coexistence of Antibiotic Resistance Genes and Virulence Factors Deciphered by Large-Scale Complete Genome Analysis. mSystems 2020, 5, e00821-19. [Google Scholar] [CrossRef]
- Abdelaziz, N.A. Phenotype-genotype correlations among carbapenem-resistant Enterobacterales recovered from four Egyptian hospitals with the report of SPM carbapenemase. Antimicrob. Resist. Infect. Control 2022, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Hassan, R.M.; Salem, S.T.; Hassan, S.I.M.; Hegab, A.S.; Elkholy, Y.S. Molecular characterization of carbapenem-resistant Acinetobacter baumannii clinical isolates from Egyptian patients. PLoS ONE 2021, 16, e0251508. [Google Scholar] [CrossRef] [PubMed]
- El Bannah, A.M.S.; Nawar, N.N.; Hassan, R.M.M.; Salem, S.T.B. Molecular Epidemiology of Carbapenem-Resistant Acinetobacter baumannii in a Tertiary Care Hospital in Egypt: Clonal Spread of blaOXA-23. Microb. Drug Resist. 2018, 24, 269–277. [Google Scholar] [CrossRef]
- Chen, Z.; Qiu, S.; Wang, Y.; Wang, Y.; Liu, S.; Wang, Z.; Du, X.; Wang, L.; Guo, J.; Wang, Z.; et al. Coexistence of blaNDM-1 with the prevalent blaOXA23 and blaIMP in pan-drug resistant Acinetobacter baumannii isolates in China. Clin. Infect. Dis. 2011, 52, 692–693. [Google Scholar] [CrossRef] [Green Version]
- Urmi, U.L.; Nahar, S.; Rana, M.; Sultana, F.; Jahan, N.; Hossain, B.; Alam, M.S.; Mosaddek, A.S.M.; McKimm, J.; Rahman, N.A.A.; et al. Genotypic to Phenotypic Resistance Discrepancies Identified Involving beta-Lactamase Genes, blaKPC, blaIMP, blaNDM-1, and blaVIM in Uropathogenic Klebsiella pneumoniae. Infect. Drug Resist. 2020, 13, 2863–2875. [Google Scholar] [CrossRef]
- Cornaglia, G.; Giamarellou, H.; Rossolini, G.M. Metallo-beta-lactamases: A last frontier for beta-lactams? Lancet Infect. Dis. 2011, 11, 381–393. [Google Scholar] [CrossRef]
- Naas, T.; Dortet, L.; Iorga, B.I. Structural and Functional Aspects of Class A Carbapenemases. Curr. Drug Targets 2016, 17, 1006–1028. [Google Scholar] [CrossRef] [PubMed]
- Al-Agamy, M.H.; Jeannot, K.; El-Mahdy, T.S.; Shibl, A.M.; Kattan, W.; Plesiat, P.; Courvalin, P. First Detection of GES-5 Carbapenemase-Producing Acinetobacter baumannii Isolate. Microb. Drug Resist. 2017, 23, 556–562. [Google Scholar] [CrossRef]
- Gatya Al-Mayahie, S.M.; Al-Guranie, D.R.T.; Hussein, A.A.; Bachai, Z.A. Prevalence of common carbapenemase genes and multidrug resistance among uropathogenic Escherichia coli phylogroup B2 isolates from outpatients in Wasit Province/Iraq. PLoS ONE 2022, 17, e0262984. [Google Scholar] [CrossRef]
- Ortega, A.; Saez, D.; Bautista, V.; Fernandez-Romero, S.; Lara, N.; Aracil, B.; Perez-Vazquez, M.; Campos, J.; Oteo, J.; Spanish Collaborating Group for the Antibiotic Resistance Surveillance Programme. Carbapenemase-producing Escherichia coli is becoming more prevalent in Spain mainly because of the polyclonal dissemination of OXA-48. J. Antimicrob. Chemother. 2016, 71, 2131–2138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gauthier, L.; Dortet, L.; Cotellon, G.; Creton, E.; Cuzon, G.; Ponties, V.; Bonnin, R.A.; Naas, T. Diversity of Carbapenemase-Producing Escherichia coli Isolates in France in 2012–2013. Antimicrob. Agents Chemother. 2018, 62, e00266-18. [Google Scholar] [CrossRef] [Green Version]
- Kayama, S.; Koba, Y.; Shigemoto, N.; Kuwahara, R.; Kakuhama, T.; Kimura, K.; Hisatsune, J.; Onodera, M.; Yokozaki, M.; Ohge, H.; et al. Imipenem-susceptible, meropenem-resistant Klebsiella pneumoniae producing OXA-181 in Japan. Antimicrob. Agents Chemother. 2015, 59, 1379–1380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, C.H.; Tuckman, M.; Keeney, D.; Ruzin, A.; Bradford, P.A. Characterization and sequence analysis of extended-spectrum-beta-lactamase-encoding genes from Escherichia coli, Klebsiella pneumoniae, and Proteus mirabilis isolates collected during tigecycline phase 3 clinical trials. Antimicrob. Agents Chemother. 2009, 53, 465–475. [Google Scholar] [CrossRef] [Green Version]
- Shin, S.Y.; Kwon, K.C.; Park, J.W.; Song, J.H.; Ko, Y.H.; Sung, J.Y.; Shin, H.W.; Koo, S.H. Characteristics of aac(6′)-Ib-cr gene in extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae isolated from Chungnam area. Korean J. Lab. Med. 2009, 29, 541–550. [Google Scholar] [CrossRef] [Green Version]
- Hamed, S.M.; Elkhatib, W.F.; El-Mahallawy, H.A.; Helmy, M.M.; Ashour, M.S.; Aboshanab, K.M.A. Multiple mechanisms contributing to ciprofloxacin resistance among Gram negative bacteria causing infections to cancer patients. Sci. Rep. 2018, 8, 12268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamed, S.M.; Aboshanab, K.M.A.; El-Mahallawy, H.A.; Helmy, M.M.; Ashour, M.S.; Elkhatib, W.F. Plasmid-Mediated Quinolone Resistance in Gram-Negative Pathogens Isolated from Cancer Patients in Egypt. Microb. Drug Resist. 2018, 24, 1316–1325. [Google Scholar] [CrossRef] [PubMed]
- Matuschek, E.; Ahman, J.; Webster, C.; Kahlmeter, G. Antimicrobial susceptibility testing of colistin—Evaluation of seven commercial MIC products against standard broth microdilution for Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Acinetobacter spp. Clin. Microbiol. Infect. 2018, 24, 865–870. [Google Scholar] [CrossRef] [Green Version]
- Chew, K.L.; La, M.V.; Lin, R.T.P.; Teo, J.W.P. Colistin and Polymyxin B Susceptibility Testing for Carbapenem-Resistant and mcr-Positive Enterobacteriaceae: Comparison of Sensititre, MicroScan, Vitek 2, and Etest with Broth Microdilution. J. Clin. Microbiol. 2017, 55, 2609–2616. [Google Scholar] [CrossRef] [Green Version]
- Sharafi, T.; Ardebili, A. Plastic binding feature of polymyxins: The effect on MIC susceptibility measurements. Infect. Drug Resist. 2019, 12, 2649–2653. [Google Scholar] [CrossRef] [Green Version]
- Sood, S. Chloramphenicol—A Potent Armament Against Multi-Drug Resistant (MDR) Gram Negative Bacilli? J. Clin. Diagn. Res. 2016, 10, DC01–DC03. [Google Scholar] [CrossRef]
- Bergen, P.J.; Landersdorfer, C.B.; Lee, H.J.; Li, J.; Nation, R.L. ‘Old’ antibiotics for emerging multidrug-resistant bacteria. Curr. Opin. Infect. Dis. 2012, 25, 626–633. [Google Scholar] [CrossRef] [Green Version]
- Theuretzbacher, U.; Van Bambeke, F.; Canton, R.; Giske, C.G.; Mouton, J.W.; Nation, R.L.; Paul, M.; Turnidge, J.D.; Kahlmeter, G. Reviving old antibiotics. J. Antimicrob. Chemother. 2015, 70, 2177–2181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adeniji, O.O.; Nontongana, N.; Okoh, J.C.; Okoh, A.I. The Potential of Antibiotics and Nanomaterial Combinations as Therapeutic Strategies in the Management of Multidrug-Resistant Infections: A Review. Int. J. Mol. Sci. 2022, 23, 15038. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.; Cerceo, E. Trends, Epidemiology, and Management of Multi-Drug Resistant Gram-Negative Bacterial Infections in the Hospitalized Setting. Antibiotics 2020, 9, 196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [Green Version]
- Doyle, D.; Peirano, G.; Lascols, C.; Lloyd, T.; Church, D.L.; Pitout, J.D. Laboratory detection of Enterobacteriaceae that produce carbapenemases. J. Clin. Microbiol. 2012, 50, 3877–3880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poirel, L.; Naas, T.; Nicolas, D.; Collet, L.; Bellais, S.; Cavallo, J.D.; Nordmann, P. Characterization of VIM-2, a carbapenem-hydrolyzing metallo-beta-lactamase and its plasmid- and integron-borne gene from a Pseudomonas aeruginosa clinical isolate in France. Antimicrob. Agents Chemother. 2000, 44, 891–897. [Google Scholar] [CrossRef] [Green Version]
- Woodford, N.; Tierno, P.M., Jr.; Young, K.; Tysall, L.; Palepou, M.F.; Ward, E.; Painter, R.E.; Suber, D.F.; Shungu, D.; Silver, L.L.; et al. Outbreak of Klebsiella pneumoniae producing a new carbapenem-hydrolyzing class A beta-lactamase, KPC-3, in a New York Medical Center. Antimicrob. Agents Chemother. 2004, 48, 4793–4799. [Google Scholar] [CrossRef] [Green Version]
- Hamed, S.M.; Aboshanab, K.M.A.; Elkhatib, W.F.; Ashour, M.S. Aminoglycoside Resistance Patterns of Certain Gram Negative Uropathogens Recovered from Hospitalized Egyptian Patients. Microbiol. Res. J. Int. 2013, 3, 678–691. [Google Scholar] [CrossRef]
- Rasheed, J.K.; Jay, C.; Metchock, B.; Berkowitz, F.; Weigel, L.; Crellin, J.; Steward, C.; Hill, B.; Medeiros, A.A.; Tenover, F.C. Evolution of extended-spectrum beta-lactam resistance (SHV-8) in a strain of Escherichia coli during multiple episodes of bacteremia. Antimicrob. Agents Chemother. 1997, 41, 647–653. [Google Scholar] [CrossRef]
- Bonnet, R.; Dutour, C.; Sampaio, J.L.; Chanal, C.; Sirot, D.; Labia, R.; De Champs, C.; Sirot, J. Novel cefotaximase (CTX-M-16) with increased catalytic efficiency due to substitution Asp-240-->Gly. Antimicrob. Agents Chemother. 2001, 45, 2269–2275. [Google Scholar] [CrossRef] [Green Version]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mostafa, S.H.; Saleh, S.E.; Khaleel, E.F.; Badi, R.M.; Aboshanab, K.M.; Hamed, S.M. Phenotypic and Genotypic Analysis of Bacterial Pathogens Recovered from Patients Diagnosed with Fever of Unknown Origin in Egypt. Antibiotics 2023, 12, 1294. https://doi.org/10.3390/antibiotics12081294
Mostafa SH, Saleh SE, Khaleel EF, Badi RM, Aboshanab KM, Hamed SM. Phenotypic and Genotypic Analysis of Bacterial Pathogens Recovered from Patients Diagnosed with Fever of Unknown Origin in Egypt. Antibiotics. 2023; 12(8):1294. https://doi.org/10.3390/antibiotics12081294
Chicago/Turabian StyleMostafa, Shimaa H., Sarra E. Saleh, Eman F. Khaleel, Rehab Mustafa Badi, Khaled M. Aboshanab, and Samira M. Hamed. 2023. "Phenotypic and Genotypic Analysis of Bacterial Pathogens Recovered from Patients Diagnosed with Fever of Unknown Origin in Egypt" Antibiotics 12, no. 8: 1294. https://doi.org/10.3390/antibiotics12081294





