Microbial Community and Abundance of Selected Antimicrobial Resistance Genes in Poultry Litter from Conventional and Antibiotic-Free Farms
Abstract
:1. Introduction
2. Results
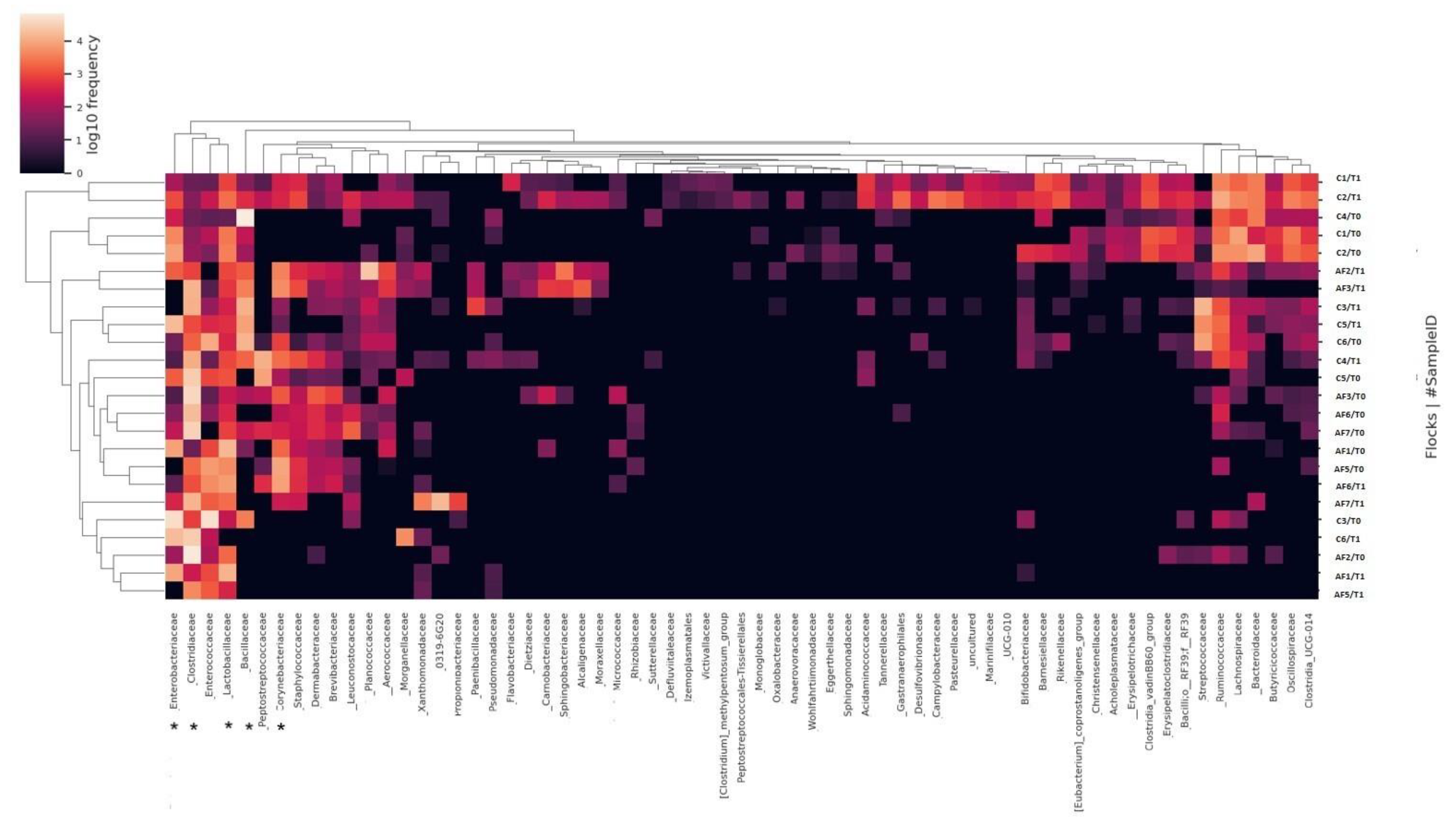
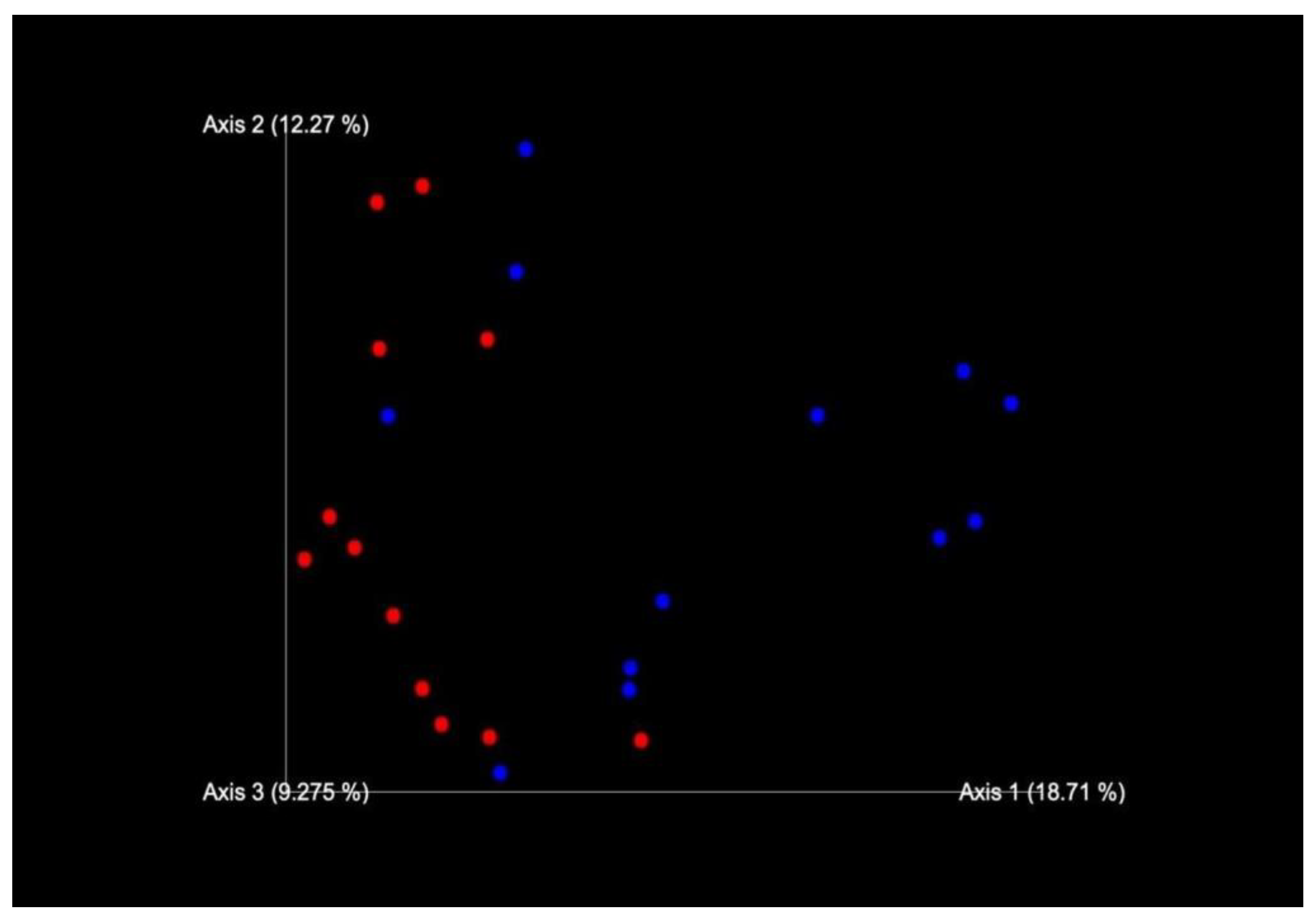
2.1. Sequencing of 16S rRNA Gene and Data Analysis
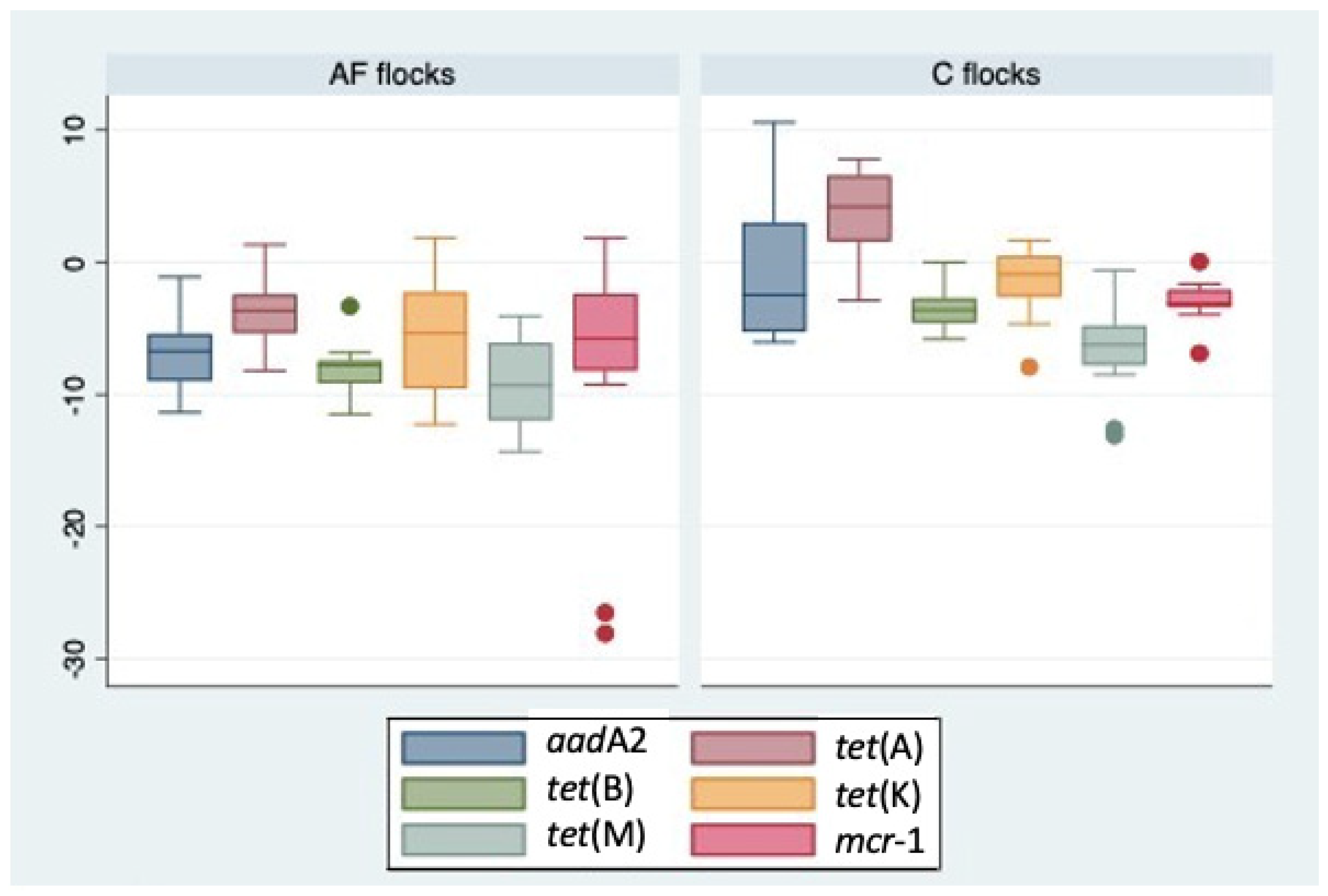
2.2. Quantitative PCR Analysis of ARGs
2.3. Statistical Analysis
3. Discussion
4. Materials and Methods
4.1. Study Area and Sampling Design
4.2. Extraction of DNA
4.3. Sequencing of 16S rRNA Gene and Data Analysis
4.4. Quantitative PCR Analysis of ARGs
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kirchhelle, C. Pharming animals: A global history of antibiotics in food production (1935–2017). Palgrave Commun. 2018, 4, 96. [Google Scholar] [CrossRef]
- Landers, T.F.; Cohen, B.; Wittum, T.E.; Larson, E.L. A review of antibiotic use in food animals: Perspective, policy, and potential. Public Health Rep. 2012, 127, 4–22. [Google Scholar] [CrossRef] [PubMed]
- McEwen, S.A.; Collignon, P.J. Antimicrobial Resistance: A One Health Perspective. Microbiol. Spectr. 2018, 6, 521–547. [Google Scholar] [CrossRef]
- Duan, M.; Gu, J.; Wang, X.; Li, Y.; Zhang, R.; Hu, T.; Zhou, B. Factors that affect the occurrence and distribution of antibiotic resistance genes in soils from livestock and poultry farms. Ecotoxicol. Environ. Saf. 2019, 180, 114–122. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hazards (BIOHAZ); Koutsoumanis, K.; Allende, A.; Álvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Herman, L.; et al. Role played by the environment in the emergence and spread of antimicrobial resistance (AMR) through the food chain. EFSA J. 2021, 19, e06651. [Google Scholar] [CrossRef]
- Schmerold, I.; van Geijlswijk, I.; Gehring, R. European regulations on the use of antibiotics in veterinary medicine. Eur. J. Pharm. Sci. 2023, 189, 106473. [Google Scholar] [CrossRef]
- World Health Organization. Critically Important Antimicrobials for Human Medicine, 6th ed.; GO: Geneva, Switzerland, 2019; Available online: https://www.who.int/publications/i/item/9789241515528 (accessed on 4 September 2023).
- EMA. Website European Medicines Agency. Categorisation of Antibiotics Used in Animals Promotes Responsible Use to Protect Public and Animal Health. EMA/688114/2020, Media and Public Relations, Press release 28 January 2020. 2020. Available online: https://www.ema.europa.eu/en/news/categorisation-antibiotics-used-animals-promotes-responsible-use-protect-public-animal-health (accessed on 4 September 2023).
- Ministero della Salute. Piano Nazionale di Contrasto all’Antibiotico-Resistenza (PNCAR) 2022–2025. Available online: https://www.salute.gov.it/imgs/C_17_pubblicazioni_3294_allegato.pdf (accessed on 4 September 2023).
- Hafez, H.M.; Attia, Y.A. Challenges to the Poultry Industry: Current Perspectives and Strategic Future After the COVID-19 Outbreak. Front. Vet. Sci. 2020, 7, 516. [Google Scholar] [CrossRef]
- de Mesquita Souza Saraiva, M.; Lim, K.; do Monte, D.F.M.; Givisiez, P.E.N.; Alves, L.B.R.; de Freitas Neto, O.C.; Kariuki, S.; Júnior, A.B.; de Oliveira, C.J.B.; Gebreyes, W.A. Antimicrobial resistance in the globalized food chain: A One Health perspective applied to the poultry industry. Braz. J. Microbiol. 2022, 53, 465–486. [Google Scholar] [CrossRef]
- Kassem, I.I.; Kehinde, O.; Kumar, A.; Rajashekara, G. Antimicrobial-Resistant Campylobacter in Organically and Conventionally Raised Layer Chickens. Foodborne Pathog. Dis. 2017, 14, 29–34. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, H.S.; Yim, J.H.; Kim, Y.J.; Kim, D.H.; Chon, J.W.; Kim, H.; Om, A.S.; Seo, K.H. Comparison of the isolation rates and characteristics of Salmonella isolated from antibiotic-free and conventional chicken meat samples. Poult. Sci. 2017, 96, 2831–2838. [Google Scholar] [CrossRef]
- Musa, L.; Casagrande Proietti, P.; Branciari, R.; Menchetti, L.; Bellucci, S.; Ranucci, D.; Marenzoni, M.L.; Franciosini, M.P. Antimicrobial Susceptibility of Escherichia coli and ESBL-Producing Escherichia coli Diffusion in Conventional, Organic and Antibiotic-Free Meat Chickens at Slaughter. Animals 2020, 10, 1215. [Google Scholar] [CrossRef] [PubMed]
- De Cesare, A.; Oliveri, C.; Lucchi, A.; Savini, F.; Manfreda, G.; Sala, C. Pilot Study on Poultry Meat from Antibiotic Free and Conventional Farms: Can Metagenomics Detect Any Difference? Foods 2022, 11, 249. [Google Scholar] [CrossRef]
- Ferri, G.; Buonavoglia, A.; Farooq, M.; Festino, A.R.; Ruffini, F.; Paludi, D.; Di Francesco, C.E.; Vergara, A.; Smoglica, C. Antibiotic resistance in Italian poultry meat production chain: A one-health perspective comparing antibiotic free and conventional systems from the farming to the slaughterhouse. Front. Food Sci. Technol. 2023, 3, 1168896. [Google Scholar] [CrossRef]
- Gupta, C.L.; Blum, S.E.; Kattusamy, K.; Daniel, T.; Druyan, S.; Shapira, R.; Krifucks, O.; Zhu, Y.G.; Zhou, X.Y.; Su, J.Q.; et al. Longitudinal study on the effects of growth-promoting and therapeutic antibiotics on the dynamics of chicken cloacal and litter microbiomes and resistomes. Microbiome 2021, 9, 178. [Google Scholar] [CrossRef]
- He, L.Y.; Liu, Y.S.; Su, H.C.; Zhao, J.L.; Liu, S.S.; Chen, J.; Liu, W.R.; Ying, G.G. Dissemination of antibiotic resistance genes in representative broiler feedlots environments: Identification of indicator ARGs and correlations with environmental variables. Environ. Sci. Technol. 2014, 48, 13120–13129. [Google Scholar] [CrossRef]
- Clavijo, V.; Flórez, M.J.V. The gastrointestinal microbiome and its association with the control of pathogens in broiler chicken production: A review. Poult. Sci. 2018, 97, 1006–1021. [Google Scholar] [CrossRef]
- Laconi, A.; Mughini-Gras, L.; Tolosi, R.; Grilli, G.; Trocino, A.; Carraro, L.; Di Cesare, F.; Cagnardi, P.; Piccirillo, A. Microbial community composition and antimicrobial resistance in agricultural soils fertilized with livestock manure from conventional farming in Northern Italy. Sci. Total. Environ. 2021, 760, 143404. [Google Scholar] [CrossRef]
- Valeris-Chacin, R.; Weber, B.; Johnson, T.J.; Pieters, M.; Singer, R.S. Longitudinal Changes in Campylobacter and the Litter Microbiome throughout the Broiler Production Cycle. Appl. Environ. Microbiol. 2022, 88, e0066722. [Google Scholar] [CrossRef]
- Greene, G.; Koolman, L.; Whyte, P.; Burgess, C.; Bolton, D. The Gut Microbiota of Broilers Reared with and without Antibiotic Treatment. Microorganisms 2023, 11, 876. [Google Scholar] [CrossRef]
- Farooq, M.; Smoglica, C.; Ruffini, F.; Soldati, L.; Marsilio, F.; Di Francesco, C.E. Antibiotic Resistance Genes Occurrence in Conventional and Antibiotic-Free Poultry Farming, Italy. Animals 2022, 12, 2310. [Google Scholar] [CrossRef]
- Bindari, Y.R.; Moore, R.J.; Van, T.T.H.; Hilliar, M.; Wu, S.B.; Walkden-Brown, S.W.; Gerber, P.F. Microbial communities of poultry house dust, excreta and litter are partially representative of microbiota of chicken caecum and ileum. PLoS ONE 2021, 16, e0255633. [Google Scholar] [CrossRef]
- Wei, S.; Morrison, M.; Yu, Z. Bacterial census of poultry intestinal microbiome. Poult. Sci. 2013, 92, 671–683. [Google Scholar] [CrossRef]
- Mohd Shaufi, M.A.; Sieo, C.C.; Chong, C.W.; Gan, H.M.; Ho, Y.W. Deciphering chicken gut microbial dynamics based on high-throughput 16S rRNA metagenomics analyses. Gut Pathog. 2015, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Greene, G.; Koolman, L.; Whyte, P.; Burgess, C.; Lynch, H.; Coffey, A.; Lucey, B.; O’Connor, L.; Bolton, D. Effect of Doxycycline Use in the Early Broiler Production Cycle on the Microbiome. Front. Microbiol. 2022, 13, 885862. [Google Scholar] [CrossRef] [PubMed]
- Videnska, P.; Faldynova, M.; Juricova, H.; Babak, V.; Sisak, F.; Havlickova, H.; Rychlik, I. Chicken faecal microbiota and disturbances induced by single or repeated therapy with tetracycline and streptomycin. BMC Vet. Res. 2013, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.S.; Rama Rao, S.V.; Hegde, N.; Williams, N.J.; Chatterjee, R.N.; Raju, M.V.L.N.; Reddy, G.N.; Kumar, V.; Phani Kumar, P.S.; Mallick, S.; et al. Effects of Dietary Antimicrobial Growth Promoters on Performance Parameters and Abundance and Diversity of Broiler Chicken Gut Microbiome and Selection of Antibiotic Resistance Genes. Front. Microbiol. 2022, 13, 905050. [Google Scholar] [CrossRef] [PubMed]
- Stanley, D.; Geier, M.S.; Denman, S.E.; Haring, V.R.; Crowley, T.M.; Hughes, R.J.; Moore, R.J. Identification of chicken intestinal microbiota correlated with the efficiency of energy extraction from feed. Vet. Microbiol. 2013, 164, 85–92. [Google Scholar] [CrossRef]
- Mignon-Grasteau, S.; Narcy, A.; Rideau, N.; Chantry-Darmon, C.; Boscher, M.Y.; Sellier, N.; Chabault, M.; Konsak-Ilievski, B.; Le Bihan-Duval, E.; Gabriel, I. Impact of Selection for Digestive Efficiency on Microbiota Composition in the Chicken. PLoS ONE 2015, 10, e0135488. [Google Scholar] [CrossRef]
- Wen, C.; Yan, W.; Mai, C.; Duan, Z.; Zheng, J.; Sun, C.; Yang, N. Joint contributions of the gut microbiota and host genetics to feed efficiency in chickens. Microbiome 2021, 9, 126. [Google Scholar] [CrossRef]
- Musa, L.; Proietti, P.C.; Marenzoni, M.L.; Stefanetti, V.; Kika, T.S.; Blasi, F.; Magistrali, C.F.; Toppi, V.; Ranucci, D.; Branciari, R.; et al. Susceptibility of Commensal E. coli Isolated from Conventional, Antibiotic-Free, and Organic Meat Chickens on Farms and at Slaughter toward Antimicrobials with Public Health Relevance. Antibiotics 2021, 10, 1321. [Google Scholar] [CrossRef]
- Le Roy, C.I.; Woodward, M.J.; Ellis, R.J.; La Ragione, R.M.; Claus, S.P. Antibiotic treatment triggers gut dysbiosis and modulates metabolism in a chicken model of gastro-intestinal infection. BMC Vet. Res. 2019, 15, 37. [Google Scholar] [CrossRef]
- Jankowski, J.; Tykałowski, B.; Stępniowska, A.; Konieczka, P.; Koncicki, A.; Matusevičius, P.; Ognik, K. Immune Parameters in Chickens Treated with Antibiotics and Probiotics during Early Life. Animals 2022, 12, 1133. [Google Scholar] [CrossRef]
- Feye, K.M.; Baxter, M.F.A.; Tellez-Isaias, G.; Kogut, M.H.; Ricke, S.C. Influential factors on the composition of the conventionally raised broiler gastrointestinal microbiomes. Poult. Sci. 2020, 99, 653–659. [Google Scholar] [CrossRef] [PubMed]
- AIFA. The Medicines Utilisation Monitoring Centre. National Report on Antibiotics Use in Italy. Year 2021; Italian Medicines Agency: Rome, Italy, 2023; ISBN 979-12-80335-28-9. Available online: https://www.aifa.gov.it/documents/20142/1740782/Rapporto-OsMed-2021.pdf (accessed on 4 September 2023).
- EMA. European Medicines Agency, European Surveillance of Veterinary Antimicrobial Consumption. 2022. ‘Sales of Veterinary Antimicrobial Agents in 31 European Countries in 2021’ (EMA/795956/2022). 2022. Available online: https://www.ema.europa.eu/en/documents/report/sales-veterinary-antimicrobial-agents-31-european-countries-2021-trends-2010-2021-twelfth-esvac_en.pdf (accessed on 4 September 2023).
- Amador, P.; Fernandes, R.; Prudêncio, C.; Duarte, I. Prevalence of antibiotic resistance genes in multidrug-resistant Enterobacteriaceae on Portuguese livestock manure. Antibiotics 2019, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Di Francesco, C.E.; Smoglica, C.; Profeta, F.; Farooq, M.; Di Giannatale, E.; Toscani, T.; Marsilio, F. Research Note: Detection of antibiotic-resistance genes in commercial poultry and turkey flocks from Italy. Poult. Sci. 2021, 100, 101084. [Google Scholar] [CrossRef]
- Gupta, C.L.; Avidov, R.; Kattusamy, K.; Saadi, I.; Varma, V.S.; Blum, S.E.; Zhu, Y.G.; Zhou, X.Y.; Su, J.Q.; Laor, Y.; et al. Spatial and temporal dynamics of microbiomes and resistomes in broiler litter stockpiles. Comput. Struct. Biotechnol. J. 2021, 19, 6201–6211. [Google Scholar] [CrossRef] [PubMed]
- Bailey, M.A.; Taylor, R.M.; Brar, J.S.; Corkran, S.C.; Velásquez, C.; Novoa Rama, E.; Oliver, H.F.; Singh, M. Prevalence and antimicrobial resistance of Campylobacter from antibiotic-free broilers during organic and conventional processing. Poult. Sci. 2019, 98, 1447–1454. [Google Scholar] [CrossRef] [PubMed]
- Salerno, B.; Furlan, M.; Sabatino, R.; Di Cesare, A.; Leati, M.; Volanti, M.; Barco, L.; Orsini, M.; Losasso, C.; Cibin, V. Antibiotic resistance genes load in an antibiotic free organic broiler farm. Poult. Sci. 2022, 101, 101675. [Google Scholar] [CrossRef]
- Partridge, S.R.; Kwong, S.M.; Firth, N.; Jensen, S.O. Mobile Genetic Elements Associated with Antimicrobial Resistance. Clin. Microbiol. Rev. 2018, 31, e00088-17. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Qi, X.; Wang, R.; Jin, L.; Zhao, M.; Zhang, Y.; Wang, Q.; Chen, H.; Wang, H. Molecular epidemiology of colistin resistant Enterobacteriaceae in inpatient and avian isolates from China: High prevalence of mcrnegative Klebsiella pneumoniae. Int. J. Antimicrob. Agents 2017, 50, 536–541. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Wang, Y.; Walsh, T.R.; Yi, L.X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Perrin-Guyomard, A.; Bruneau, M.; Houée, P.; Deleurme, K.; Legrandois, P.; Poirier, C.; Soumet, C.; Sanders, P. Prevalence of mcr-1 in commensal Escherichia coli from French livestock, 2007 to 2014. Eurosurveillance 2016, 21, 30135. [Google Scholar] [CrossRef] [PubMed]
- Timmermans, M.; Wattiau, P.; Denis, O.; Boland, C. Colistin resistance genes mcr-1 to mcr-5, including a case of triple occurrence (mcr-1, -3 and -5), in Escherichia coli isolates from faeces of healthy pigs, cattle and poultry in Belgium, 2012–2016. Int. J. Antimicrob. Agents 2021, 57, 106350. [Google Scholar] [CrossRef]
- Irrgang, A.; Roschanski, N.; Tenhagen, B.A.; Grobbel, M.; Skladnikiewicz-Ziemer, T.; Thomas, K.; Roesler, U.; Käsbohrer, A. Prevalence of mcr-1 in E. coli from Livestock and Food in Germany, 2010–2015. PLoS ONE 2019, 11, e0159863. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed Ahmed, M.A.E.; Zhong, L.L.; Shen, C.; Yang, Y.; Doi, Y.; Tian, G.B. Colistin and its role in the Era of antibiotic resistance: An extended review (2000–2019). Emerg. Microbes Infect. 2020, 9, 868–885. [Google Scholar] [CrossRef]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Author Correction: Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 1091. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Yilmaz, P.; Parfrey, L.W.; Yarza, P.; Gerken, J.; Pruesse, E.; Quast, C.; Schweer, T.; Peplies, J.; Ludwig, W.; Glöckner, F.O. The SILVA and “All-species Living Tree Project (LTP)” taxonomic frameworks. Nucleic Acids Res. 2014, 42, D643–D648. [Google Scholar] [CrossRef]
- Zakrzewski, M.; Proietti, C.; Ellis, J.J.; Hasan, S.; Brion, M.J.; Berger, B.; Krause, L. Calypso: A user-friendly web-server for mining and visualizing microbiome-environment interactions. Bioinformatics 2017, 33, 782–783. [Google Scholar] [CrossRef]
- Ewbank, A.C.; Esperón, F.; Sacristán, C.; Sacristán, I.; Krul, R.; Cavalcante de Macedo, E.; Calatayud, O.; Bueno, I.; de Francisco Strefezzi, R.; Catão-Dias, J.L. Seabirds as anthropization indicators in two different tropical biotopes: A One Health approach to the issue of antimicrobial resistance genes pollution in oceanic islands. Sci. Total. Environ. 2021, 754, 142141. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Hu, X.; Xu, T.; Zhang, H.; Sheng, D.; Yin, D. Prevalence of antibiotic resistance genes and their relationship with antibiotics in the Huangpu River and the drinking water sources, Shanghai, China. Sci. Total Environ. 2013, 458–460, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Claudin, A.; Deem, S.L.; Rodríguez, C.; Cano, S.; Moity, N.; Cabrera, F.; Esperón, F. Antimicrobial resistance in Galapagos tortoises as an indicator of the growing human footprint. Environ. Pollut. 2021, 284, 117453. [Google Scholar] [CrossRef] [PubMed]
- StataCorp LLC. Stata Statistical Software: Release 17; StataCorp LLC.: College Station, TX, USA, 2021. [Google Scholar]

| Flock/Sample | aadA2 | tet(A) | tet(B) | tet(K) | tet(M) | mcr-1 |
|---|---|---|---|---|---|---|
| AF1/T0 | 4.76 × 10−4 | 4.35 × 10−3 | 1.49 × 10−4 | 1.58 × 10−4 | 2.30 × 10−6 | 9.6 × 10−5 |
| AF1/T1 | 3.71 × 10−3 | 7.84 × 10−2 | 1.05 × 10−4 | 4.76 × 10−6 | 9.18 × 10−5 | 3.0 × 10−1 |
| AF2/T0 | 1.32 × 10−3 | 1.84 × 10−2 | 4.92 × 10−4 | 1.16 × 10−4 | 1.88 × 10−6 | 6.5 × 10−1 |
| AF2/T1 | 3.29 × 10−1 | 3.80 × 10 | 3.58 × 10−2 | 6.97 × 10−2 | 3.83 x10−4 | 6.45 × 10 |
| AF3/T0 | 1.10 × 10−3 | 2.76 × 10−4 | 1.04 × 10−5 | 3.68 × 10−5 | 6.01 × 10−7 | 8.2 x10−4 |
| AF3/T1 | 3.33 × 10−2 | 2.97 × 10−1 | 1.28 × 10−2 | 7.24 × 10−3 | 3.27 × 10−4 | 5.31 × 10−1 |
| AF4/T0 | 2.39 × 10−3 | 3.74 × 10−4 | 1.58 × 10−5 | 1.72 × 10−3 | 2.12 × 10−6 | 9.2 × 10−3 |
| AF4/T1 | 1.16 × 10−5 | 1.67 × 10−2 | 6.23 × 10−5 | 4.33 × 10−5 | 1.86 × 10−5 | 2.15 × 10−3 |
| AF5/T0 | 1.11 × 10−4 | 2.41 × 10−3 | 3.35 × 10−4 | 5.64 × 10−2 | 1.27 × 10−3 | 4.45 × 10−3 |
| AF5/T1 | 1.25 × 10−4 | 5.39 × 10−2 | 5.91 × 10−4 | 2.33 × 10−3 | 4.58 × 10−3 | 8.03 × 10−4 |
| AF6/T0 | 2.57 × 10−3 | 9.60 × 10−2 | 4.19 × 10−4 | 1.77 × 10−1 | 8.79 × 10−5 | 1.54 × 10−2 |
| AF6/T1 | 7.51 × 10−2 | 5.22 × 10−3 | 4.13 × 10−4 | 6.25 × 10 | 1.71 × 10−2 | 2.82 × 10−2 |
| AF7/T0 | 5.37 × 10−3 | 3.61 × 10−2 | Neg | 1.54 × 10−1 | 3.95 × 10−3 | Neg |
| AF7/T1 | 1.26 × 10−4 | 1.57 × 10−1 | 1.07 × 10−3 | 9.25 × 10−3 | 7.88 × 10−5 | Neg |
| C1/T0 | 1.12 × 10−1 | 1.87 × 101 | 7.20 × 10−2 | 9.51 × 10−1 | 5.56 × 10−1 | 3.18 × 10−2 |
| C1/T1 | 1.98 × 103 | 3.65 × 101 | 3.09 × 10−2 | 9.49 × 10−3 | 1.65 × 10−3 | 1.01 × 10−3 |
| C2/T0 | 2.80 × 10−2 | 1.29 × 101 | 2.5 × 10−2 | 5.81 × 10−2 | 8.67 × 10−4 | 8.41 × 10−2 |
| C2/T1 | 6.14 × 10−3 | 7.97 × 102 | 2.96 × 10−3 | 8.04 × 10−1 | 1.69 × 10−3 | 3.39 × 10−2 |
| C3/T0 | 1.92 × 10−1 | 1.87 × 10 | 1.18 × 10−2 | 9.04 × 10−2 | 1.25 × 10−2 | 1.91 × 10−2 |
| C3/T1 | 2.02 × 10−1 | 1.88 × 103 | 1.02 × 10 | 5.27 × 10 | 4.85 × 10−2 | 1.05 × 10 |
| C4/T0 | 2.36 × 10−3 | 1.38 × 102 | 9.17 × 10−3 | 3.30 × 10−1 | 2.24 × 10−3 | 3.42 × 10−2 |
| C4/T1 | 3.36 × 10−3 | 2.39 × 103 | 1.85 × 10−1 | 3.62 × 10−4 | 5.50 × 10−3 | 1.53 × 10−1 |
| C5/T0 | 2.97 × 104 | 5.59 × 10−2 | 2.88 × 10−2 | 4.97 × 10−1 | 2.01 × 10−4 | 3.91 × 10−2 |
| C5/T1 | 3.97 × 104 | 1.06 × 10 | 3.72 × 10−3 | 4.96 × 10 | 3.84 × 10−3 | 9.97 × 10−2 |
| C6/T0 | 4.37 × 10−3 | 1.22 × 102 | 5.90 × 10−2 | 2.84 × 10 | 2.02 × 10−6 | 5.32 × 10−2 |
| C6/T1 | 6.51 × 10−2 | 6.24 × 102 | 1.57 × 10−2 | 2.58 × 10−1 | 3.39 × 10−6 | 1.91 × 10−1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smoglica, C.; Farooq, M.; Ruffini, F.; Marsilio, F.; Di Francesco, C.E. Microbial Community and Abundance of Selected Antimicrobial Resistance Genes in Poultry Litter from Conventional and Antibiotic-Free Farms. Antibiotics 2023, 12, 1461. https://doi.org/10.3390/antibiotics12091461
Smoglica C, Farooq M, Ruffini F, Marsilio F, Di Francesco CE. Microbial Community and Abundance of Selected Antimicrobial Resistance Genes in Poultry Litter from Conventional and Antibiotic-Free Farms. Antibiotics. 2023; 12(9):1461. https://doi.org/10.3390/antibiotics12091461
Chicago/Turabian StyleSmoglica, Camilla, Muhammad Farooq, Fausto Ruffini, Fulvio Marsilio, and Cristina Esmeralda Di Francesco. 2023. "Microbial Community and Abundance of Selected Antimicrobial Resistance Genes in Poultry Litter from Conventional and Antibiotic-Free Farms" Antibiotics 12, no. 9: 1461. https://doi.org/10.3390/antibiotics12091461





