Sonophotocatalytic Degradation of Malachite Green by Nanocrystalline Chitosan-Ascorbic Acid@NiFe2O4 Spinel Ferrite
Abstract
:1. Introduction
2. Methods and Material
2.1. Chemicals and Reagents
2.2. Synthesis of CTAS@NIFE Nanocomposite
2.3. Characterization of Nickel Ferrite Nanoparticles
2.4. Sonophotocatalysis Experiment
3. Results and Discussion
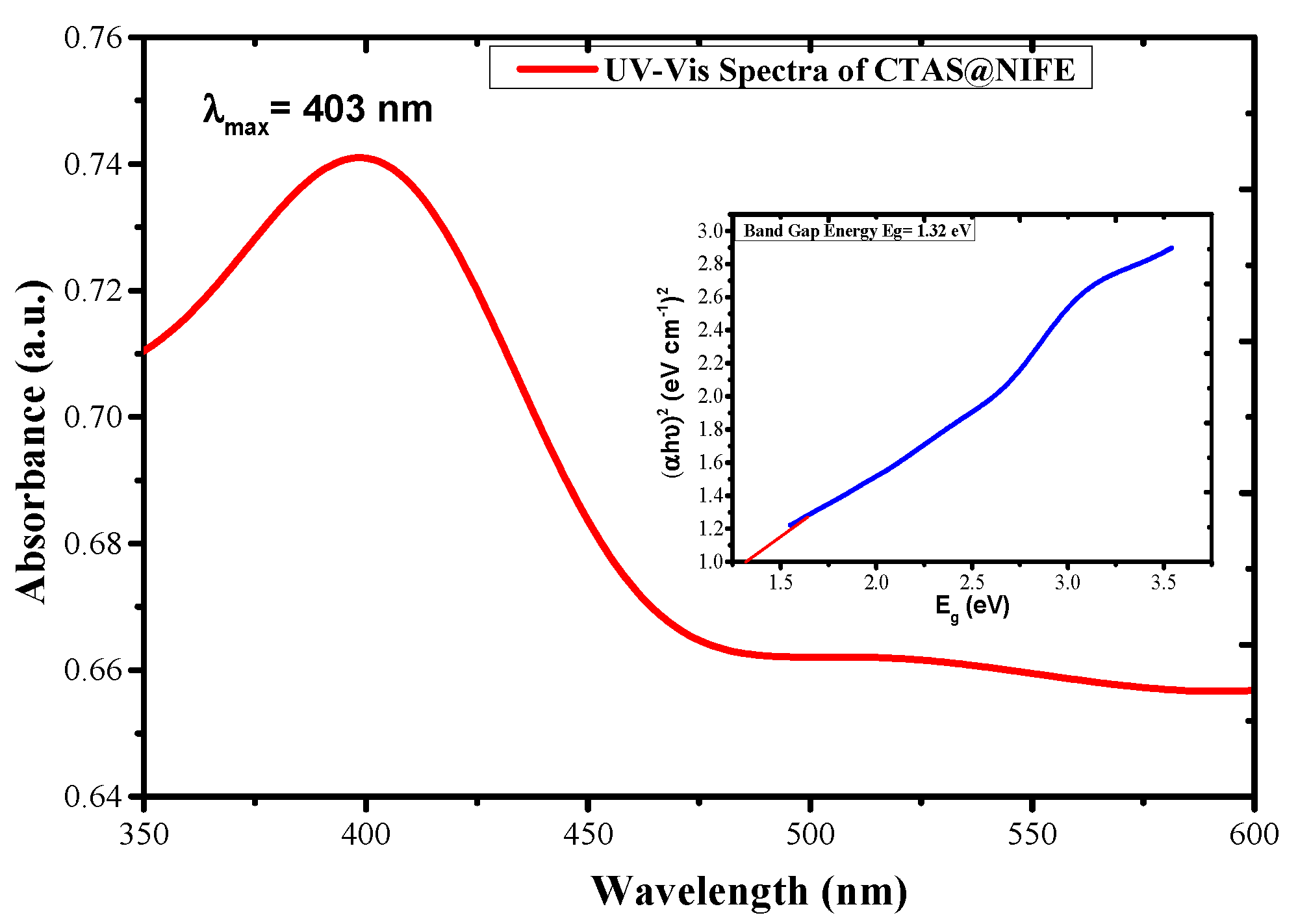
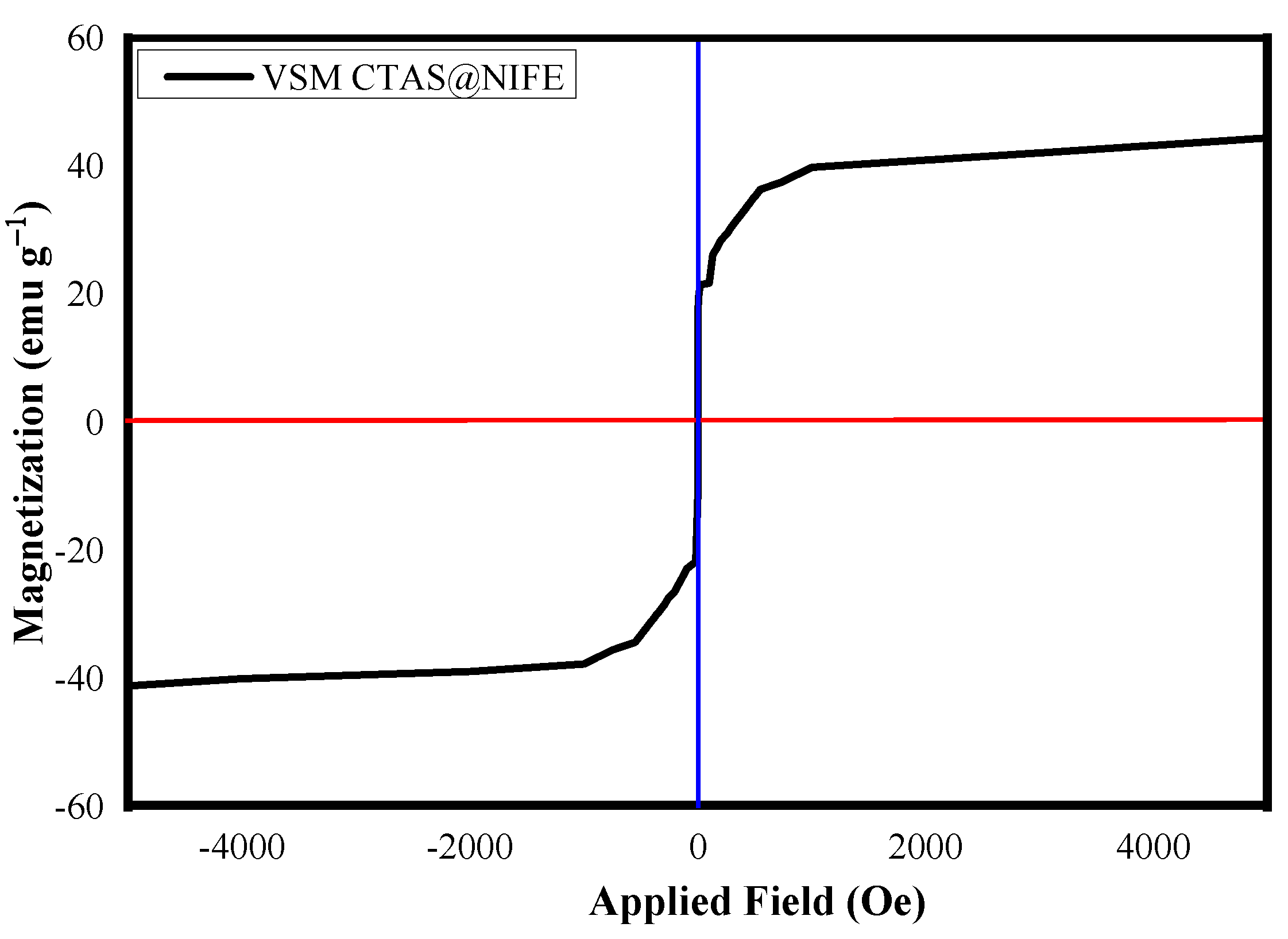
3.1. Analytical Techniques for Material Characterization
3.2. Sonophotocatalysis and Optimization of Reaction Parameters
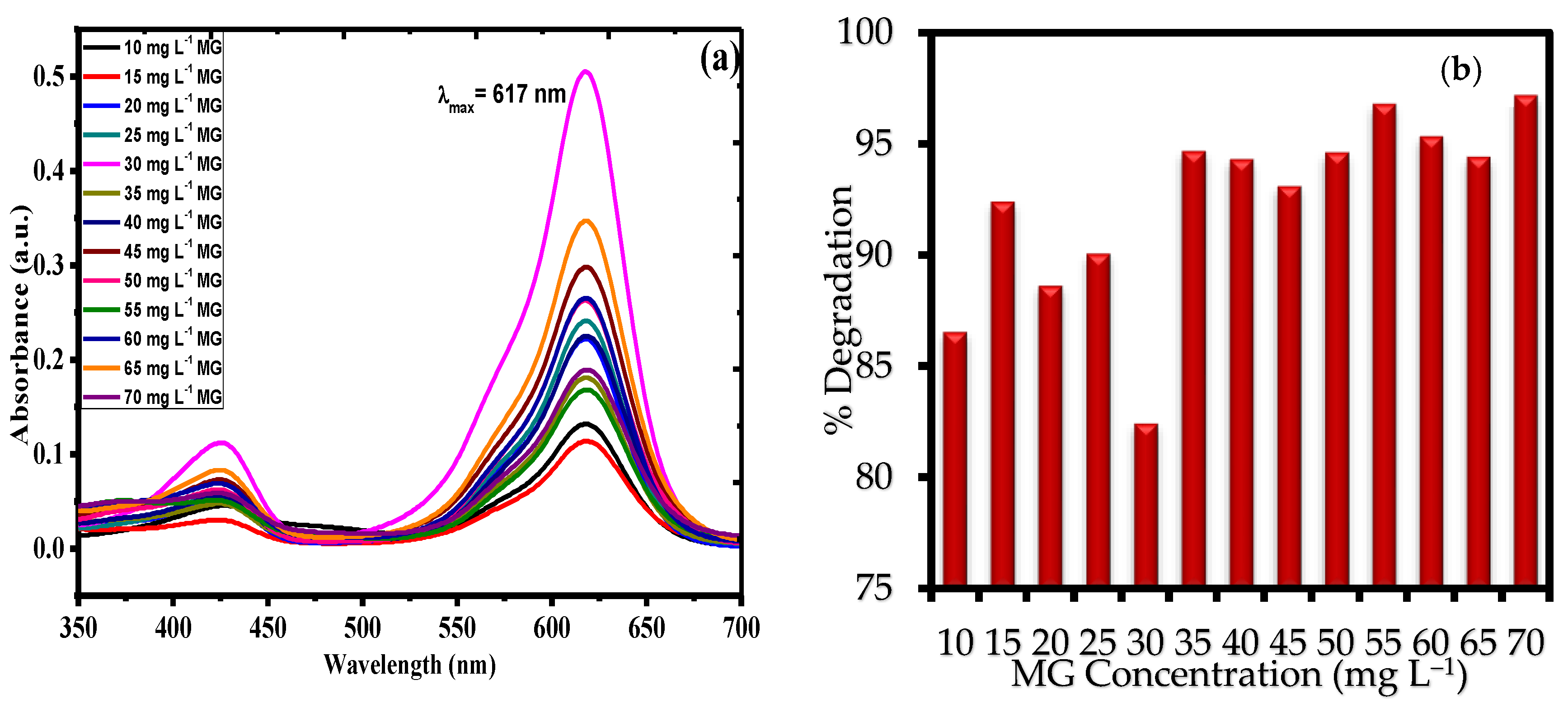
3.2.1. Sonophotocatalysis with Variable MG Concentration
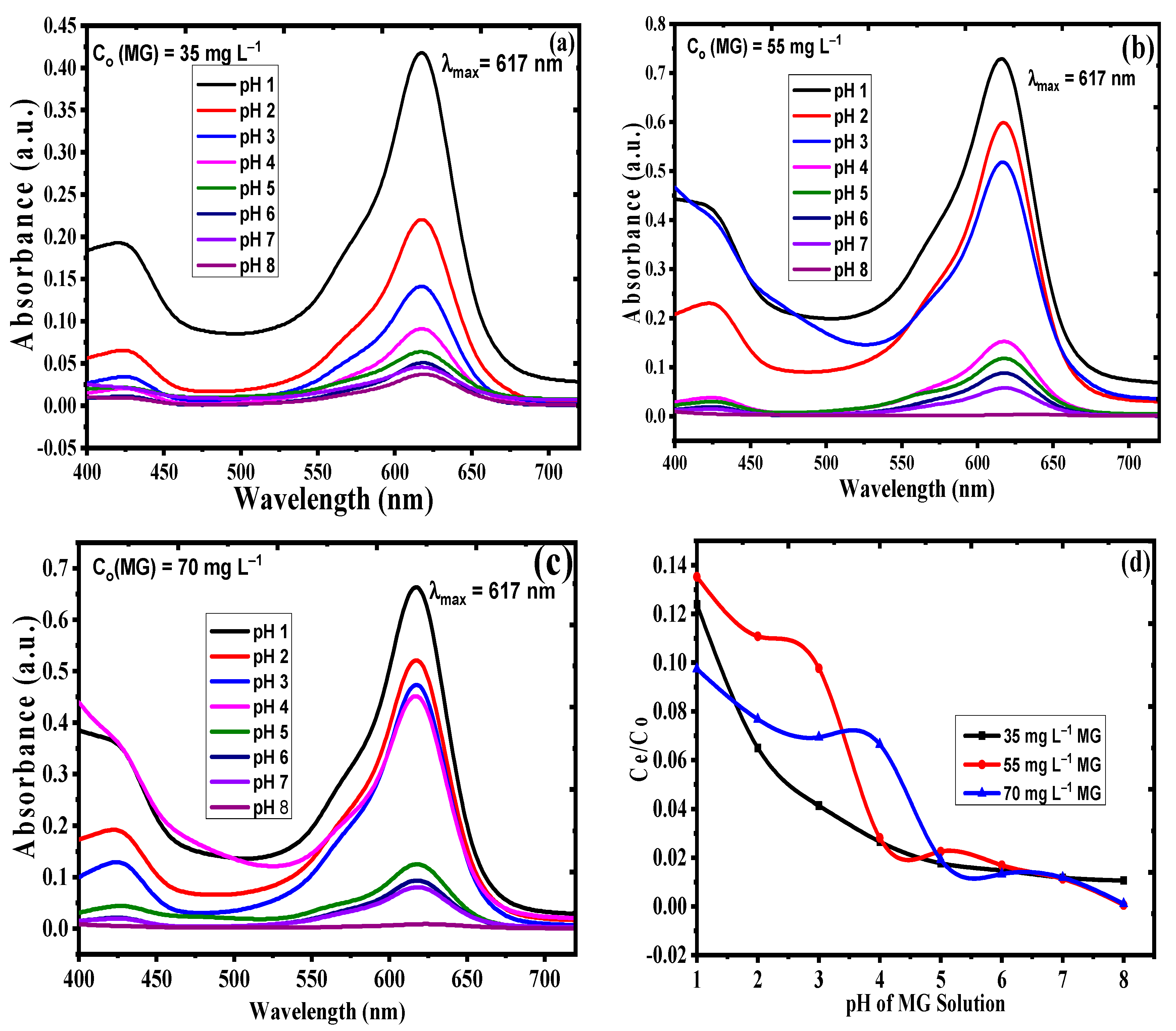
3.2.2. Sonophotocatalysis with Variable MG Solution pH
3.2.3. Sonophotocatalysis with Variable Irradiation Time
3.2.4. Sonophotocatalysis with Variable Ultrasonic Power Intensities
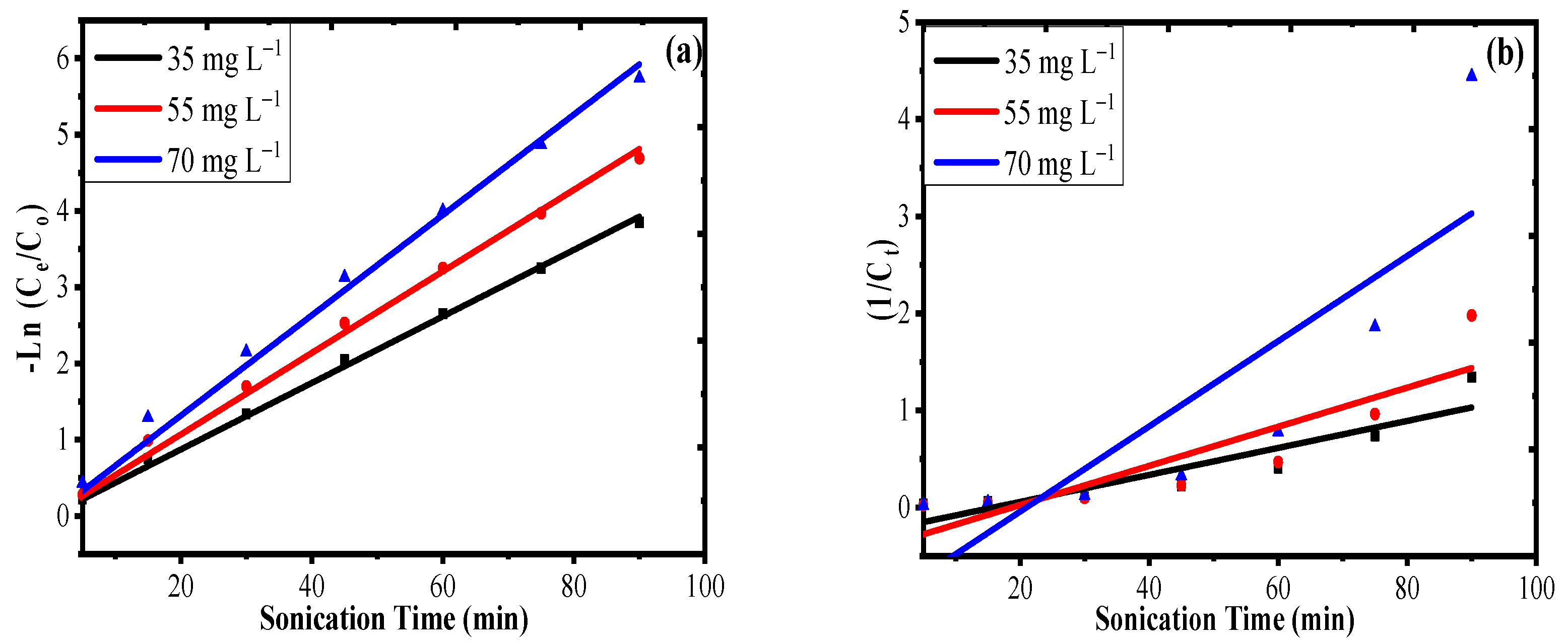
3.3. Sonophotocatalysis Kinetics
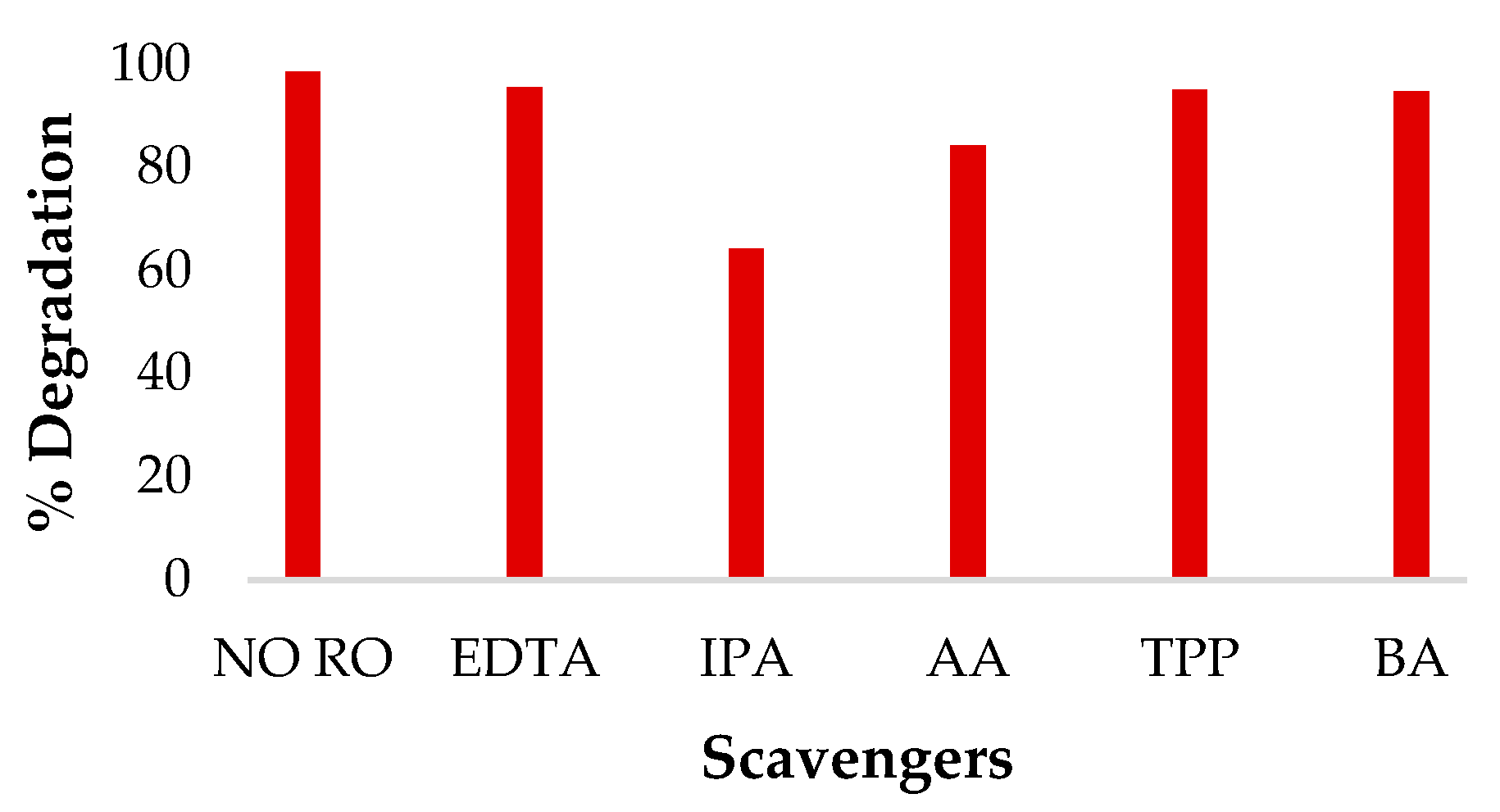
3.4. Effect of Scavengers and Mechanism of Sonophotocatalysis
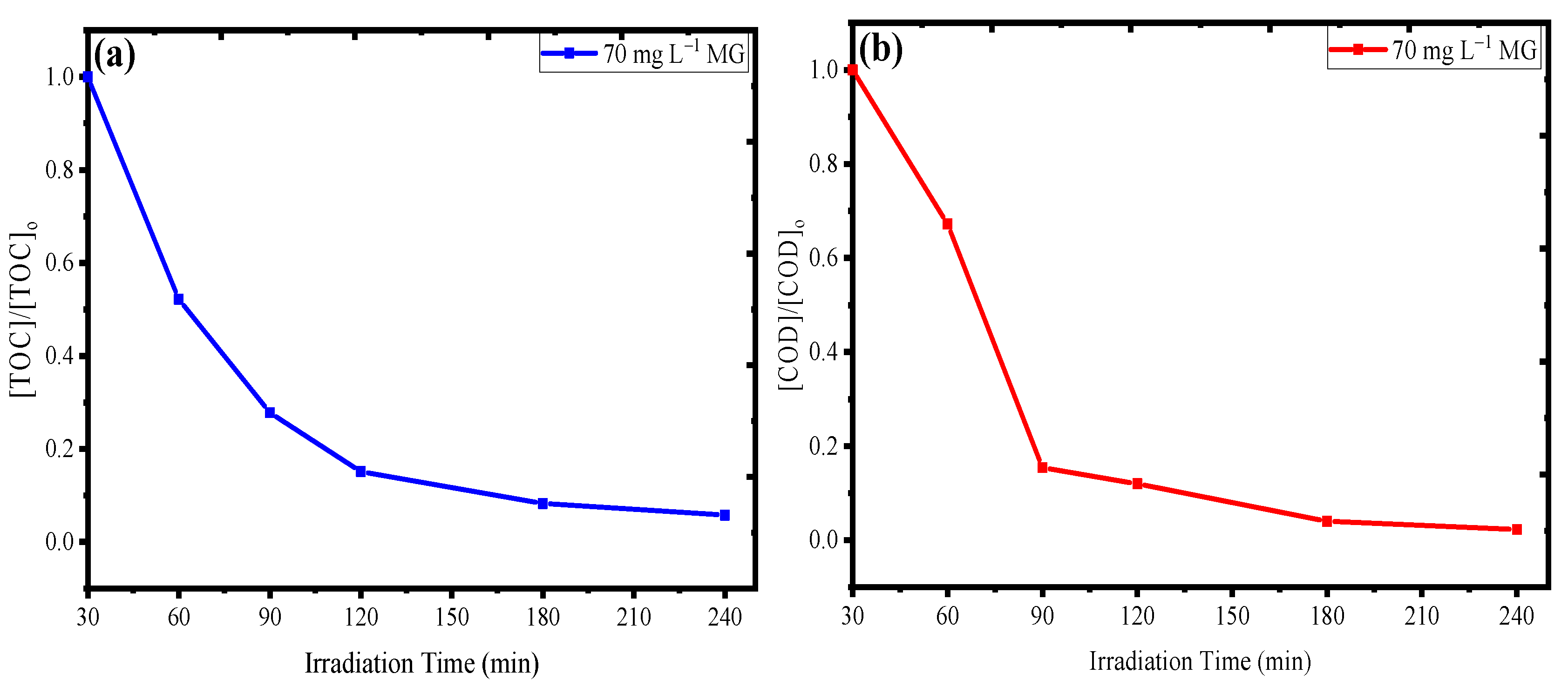
3.5. Mineralization of MG
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mohanta, J.; Dey, B.; Dey, S. Sucrose-triggered, self-sustained combustive synthesis of magnetic nickel oxide nanoparticles and efficient removal of malachite green from water. ACS Omega 2020, 5, 16510–16520. [Google Scholar] [CrossRef]
- Hasan, I.; Bhatia, D.; Walia, S.; Singh, P. Removal of malachite green by polyacrylamide-g-chitosan γ-Fe2O3 nanocomposite—An application of central composite design. Groundw. Sustain. Dev. 2020, 11, 100378. [Google Scholar] [CrossRef]
- Bakhshi Nejad, S.; Mohammadi, A. Epoxy-triazinetrione-functionalized magnetic nanoparticles as an efficient magnetic nanoadsorbent for the removal of malachite green and Pb(II) from aqueous solutions. J. Chem. Eng. Data 2020, 65, 2731–2742. [Google Scholar] [CrossRef]
- Ali, I.; Peng, C.; Ye, T.; Naz, I. Sorption of cationic malachite green dye on phytogenic magnetic nanoparticles functionalized by 3-marcaptopropanic acid. RSC Adv. 2018, 8, 8878–8897. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Mao, Y.; Wang, W.; Yang, S.; Song, Z.; Zhao, X. Coal fly ash/CoFe2O4 composites: A magnetic adsorbent for the removal of malachite green from aqueous solution. RSC Adv. 2016, 6, 93564–93574. [Google Scholar] [CrossRef]
- Adeyi, A.A.; Jamil, S.N.A.M.; Abdullah, L.C.; Choong, T.S.Y.; Lau, K.L.; Alias, N.H. Simultaneous adsorption of malachite green and methylene blue dyes in a fixed-bed column using poly(acrylonitrile-co-acrylic acid) modified with thiourea. Molecules 2020, 25, 2650. [Google Scholar] [CrossRef]
- Podasca, V.E.; Damaceanu, M.D. Photopolymerized films with ZnO and doped ZnO particles used as efficient photocatalysts in malachite green dye decomposition. Appl. Sci. 2020, 10, 1954. [Google Scholar] [CrossRef] [Green Version]
- Tayade, R.J.; Surolia, P.K.; Lazar, M.A.; Jasra, R.V. Enhanced photocatalytic activity by silver metal ion exchanged nay zeolite photocatalysts for the degradation of organic contaminants and dyes in aqueous medium. Ind. Eng. Chem. Res. 2008, 47, 7545–7551. [Google Scholar] [CrossRef]
- Sudha, M.; Saranya, A.; Selvakumar, G.; Sivakumar, N. Microbial degradation of azo dyes: A review. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 670–690. [Google Scholar]
- Hasan, I.; Walia, S.; Alharbi, K.H.; Khanjer, M.A.; Alsalme, A.; Khan, R.A. Multi-walled carbon nanotube coupled β-cyclodextrin/pani hybrid photocatalyst for advance oxidative degradation of crystal violet. J. Mol. Liq. 2020, 317, 114216. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, G.; Naushad, M.; Singh, P.; Kalia, S. Polyacrylamide/Ni0.02Zn0.98O nanocomposite with high solar light photocatalytic activity and efficient adsorption capacity for toxic dye removal. Ind. Eng. Chem. Res. 2014, 53, 15549–15560. [Google Scholar] [CrossRef]
- Wang, Q.; Cai, C.; Wang, M.; Guo, Q.; Wang, B.; Luo, W.; Wang, Y.; Zhang, C.; Zhou, L.; Zhang, D.; et al. Efficient photocatalytic degradation of malachite green in seawater by the hybrid of zinc-oxide nanorods grown on three-dimensional (3D) reduced graphene oxide (RGO)/Ni foam. Materials 2018, 11, 1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charanpahari, A.; Ghugal, S.G.; Umare, S.S.; Sasikala, R. Mineralization of malachite green dye over visible light responsive bismuth doped TiO2–ZrO2 ferromagnetic nanocomposites. New J. Chem. 2015, 39, 3629–3638. [Google Scholar] [CrossRef]
- Minero, C.; Lucchiari, M.; Vione, D.; Maurino, V. Fe(III)-enhanced sonochemical degradation of methylene blue in aqueous solution. Environ. Sci. Technol. 2005, 39, 8936–8942. [Google Scholar] [CrossRef] [PubMed]
- Cabello-Alvarado, C.; Andrade-Guel, M.; Pérez-Alvarez, M.; Cadenas-Pliego, G.; Cortés-Hernández, D.A.; Bartolo-Pérez, P.; Ávila-Orta, C.A.; Cruz-Delgado, V.J.; Zepeda-Pedreguera, A. Graphene nanoplatelets modified with amino-groups by ultrasonic radiation of variable frequency for potential adsorption of uremic toxins. Nanomaterials 2019, 9, 1261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bejarano-Pérez, N.J.; Suárez-Herrera, M.F. Sonochemical and sonophotocatalytic degradation of malachite green: The effect of carbon tetrachloride on reaction rates. Ultrason. Sonochem. 2008, 15, 612–617. [Google Scholar] [CrossRef]
- Bazrafshan, A.A.; Hajati, S.; Ghaedi, M. Synthesis of regenerable Zn(OH)2 nanoparticle-loaded activated carbon for the ultrasound-assisted removal of malachite green: Optimization, isotherm and kinetics. RSC Adv. 2015, 5, 79119–79128. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, H.E.; Kim, J.H.; Lee, J.S. Ferrites: Emerging light absorbers for solar water splitting. J. Mater. Chem. A 2020, 8, 9447–9482. [Google Scholar] [CrossRef]
- Diodati, S.; Walton, R.I.; Mascotto, S.; Gross, S. Low-temperature wet chemistry synthetic approaches towards ferrites. Inorg. Chem. Front. 2020, 7, 3282–3314. [Google Scholar] [CrossRef]
- Riaz, U.; Ashraf, S.M.; Raza, R.; Kohli, K.; Kashyap, J. Sonochemical facile synthesis of self-assembled poly(o-phenylenediamine)/cobalt ferrite nanohybrid with enhanced photocatalytic activity. Ind. Eng. Chem. Res. 2016, 55, 6300–6309. [Google Scholar] [CrossRef]
- Jia, J.; Du, X.; Zhang, Q.; Liu, E.; Fan, J. Z-scheme MgFe2O4/Bi2MoO6 heterojunction photocatalyst with enhanced visible light photocatalytic activity for malachite green removal. Appl. Surf. Sci. 2019, 492, 527–539. [Google Scholar] [CrossRef]
- Nadumane, A.; Shetty, K.; Anantharaju, K.S.; Nagaswarupa, H.P.; Rangappa, D.; Vidya, Y.S.; Nagabhushana, H.; Prashantha, S.C. Sunlight photocatalytic performance of Mg-doped nickel ferrite synthesized by a green sol-gel route. J. Sci. Adv. Mater. Devices 2019, 4, 89–100. [Google Scholar] [CrossRef]
- Kaneva, N.V.; Dushkin, C.D. Tuning of the UV Photocatalytic activity of ZnO using zinc ferrite(III): Powders and thin films prepared of powders. Colloids Surfaces A Physicochem. Eng. Asp. 2011, 382, 211–218. [Google Scholar] [CrossRef]
- Shabbir, A.; Ajmal, S.; Shahid, M.; Shakir, I.; Agboola, P.O.; Warsi, M.F. Zirconium substituted spinel nano-ferrite Mg0.2Co0.8Fe2O4 particles and their hybrids with reduced graphene oxide for photocatalytic and other potential applications. Ceram. Int. 2019, 45, 16121–16129. [Google Scholar] [CrossRef]
- Kefeni, K.K.; Mamba, B.B. Photocatalytic application of spinel ferrite nanoparticles and nanocomposites in wastewater treatment: Review. Sustain. Mater. Technol. 2020, 23, e00140. [Google Scholar] [CrossRef]
- Reddy, D.H.K.; Yun, Y.S. Spinel ferrite magnetic adsorbents: Alternative future materials for water purification? Coord. Chem. Rev. 2016, 315, 90–111. [Google Scholar] [CrossRef]
- Liu, S.Q.; Xiao, B.; Feng, L.R.; Zhou, S.S.; Chen, Z.G.; Liu, C.B.; Chen, F.; Wu, Z.Y.; Xu, N.; Oh, W.C.; et al. Graphene oxide enhances the fenton-like photocatalytic activity of nickel ferrite for degradation of dyes under visible light irradiation. Carbon N. Y. 2013, 64, 197–206. [Google Scholar] [CrossRef]
- Hasmath Farzana, M.; Meenakshi, S. Synergistic effect of chitosan and titanium dioxide on the removal of toxic dyes by the photodegradation technique. Ind. Eng. Chem. Res. 2014, 53, 55–63. [Google Scholar] [CrossRef]
- Zhang, S.; Li, J.; Li, J.; Du, N.; Li, D.; Li, F.; Man, J. Application status and technical analysis of chitosan-based medical dressings: A review. RSC Adv. 2020, 10, 34308–34322. [Google Scholar] [CrossRef]
- Foszpańczyk, M.; Bilińska, L.; Gmurek, M.; Ledakowicz, S. Heterogeneous oxidation of phenolic compounds with photosensitizing catalysts incorporated into chitosan. Catalysts 2019, 9, 891. [Google Scholar] [CrossRef] [Green Version]
- Othman, N.; Masarudin, M.; Kuen, C.; Dasuan, N.; Abdullah, L.; Md. Jamil, S. Synthesis and optimization of chitosan nanoparticles loaded with l-ascorbic acid and thymoquinone. Nanomaterials 2018, 8, 920. [Google Scholar] [CrossRef] [Green Version]
- Todorov, A.R.; Aikonen, S.; Muuronen, M.; Helaja, J. Visible-light-photocatalyzed reductions of N-heterocyclic nitroaryls to anilines utilizing ascorbic acid reductant. Org. Lett. 2019, 21, 3764–3768. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, C.A.; Giovannetti, R.; Zannotti, M.; Rommozzi, E.; Minicucci, M.; Gunnella, R.; Di Cicco, A. Band gap implications on nano-TiO2 surface modification with ascorbic acid for visible light-active polypropylene coated photocatalyst. Nanomaterials 2018, 8, 599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gioria, E.; Marchesini, F.A.; Soldati, A.; Giorello, A.; Hueso, J.L.; Gutierrez, L. Green synthesis of a Cu/SiO2 catalyst for efficient H2-SCR of NO. Appl. Sci. 2019, 9, 4075. [Google Scholar] [CrossRef] [Green Version]
- Leybo, D.; Tagirov, M.; Permyakova, E.; Konopatsky, A.; Firestein, K.; Tuyakova, F.; Arkhipov, D.; Kuznetsov, D. Ascorbic acid-assisted polyol synthesis of iron and Fe/Go, Fe/h-BN Composites for Pb2+ removal from wastewaters. Nanomaterials 2020, 10, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scherrer, P. Estimation of the size and internal structure of colloidal particles by means of rontgen rays. Nachr. Ges. Wiss. Göttingen 1918, 26, 98–100. [Google Scholar]
- Rodrigues, A.R.O.; Gomes, I.T.; Almeida, B.G.; Araújo, J.P.; Castanheira, E.M.S.; Coutinho, P.J.G. Magnetic liposomes based on nickel ferrite nanoparticles for biomedical applications. Phys. Chem. Chem. Phys. 2015, 17, 18011–18021. [Google Scholar] [CrossRef]
- Ortiz-Quiñonez, J.L.; Pal, U.; Villanueva, M.S. Structural, magnetic, and catalytic evaluation of spinel Co, Ni, and Co–Ni ferrite nanoparticles fabricated by low-temperature solution combustion process. ACS Omega 2018, 3, 14986–15001. [Google Scholar] [CrossRef]
- Xia, Y.; He, Z.; Su, J.; Tang, B.; Hu, K.; Lu, Y.; Sun, S.; Li, X. Fabrication of magnetically separable NiFe2O4/BiOI nanocomposites with enhanced photocatalytic performance under visible-light irradiation. RSC Adv. 2018, 8, 4284–4294. [Google Scholar] [CrossRef] [Green Version]
- Ramezani, S.; Ghazitabar, A.; Sadrnezhaad, S.K. Synthesis and characterization of chitosan coating of NiFe2O4 nanoparticles for biomedical applications. J. Iran. Chem. Soc. 2016, 13, 2069–2076. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Hao, X.P.; Mo, L.P.; Liu, S.S.; Zhang, W.B.; Zhang, Z.H. A magnetic metal-organic framework as a highly active heterogeneous catalyst for one-pot synthesis of 2-Substituted alkyl and aryl(indolyl)kojic acid derivatives. New J. Chem. 2017, 41, 7108–7115. [Google Scholar] [CrossRef]
- Bindra Narang, S.; Pubby, K. Nickel spinel ferrites: A review. J. Magn. Magn. Mater. 2020, 167163. [Google Scholar]
- Kombaiah, K.; Vijaya, J.J.; Kennedy, L.J.; Kaviyarasu, K. Catalytic studies of NiFe2O4 nanoparticles prepared by conventional and microwave combustion method. Mater. Chem. Phys. 2019, 221, 11–28. [Google Scholar] [CrossRef]
- Sivakumar, P.; Ramesh, R.; Ramanand, A.; Ponnusamy, S.; Muthamizhchelvan, C. Synthesis and characterization of NiFe2O4 nanoparticles and nanorods. J. Alloys Compd. 2013, 563, 6–11. [Google Scholar] [CrossRef]
- Iacovita, C.; Stiufiuc, G.F.; Dudric, R.; Vedeanu, N.; Tetean, R.; Stiufiuc, R.I.; Lucaciu, C.M. Saturation of specific absorption rate for soft and hard spinel ferrite nanoparticles synthesized by polyol process. Magnetochemistry 2020, 6, 23. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, J.; Gao, D.; Zhu, Z.; Yang, Z.; Zhang, Z. Giant magnetoelectric coupling observed at high frequency in NiFe2O4–BaTiO3 particulate composite. RSC Adv. 2020, 10, 27242–27248. [Google Scholar] [CrossRef]
- Sellmyer, D.; Skomski, R. Advanced Magnetic Nanostructures; Springer: New York, NY, USA, 2006. [Google Scholar]
- Skomski, R.; Zhou, J. Nanomagnetic Models. In Advanced Magnetic Nanostructures; Springer: New York, NY, USA, 2006; pp. 41–90. [Google Scholar]
- Hasan, I.; Khan, R.A.; Alharbi, W.; Alharbi, K.H.; Abu Khanjer, M.; Alslame, A. Synthesis, characterization and photo-catalytic activity of guar-gum: G-aliginate@silver bionanocomposite material. RSC Adv. 2020, 10, 7898–7911. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, A.; Wei, Y.; Syed, F.; Imran, M.; Khan, Z.U.H.; Tahir, K.; Khan, A.U.; Raza, M.; Khan, Q.; Yuan, Q. Size dependent catalytic activities of green synthesized gold nanoparticles and electro-catalytic oxidation of catechol on gold nanoparticles modified electrode. RSC Adv. 2015, 5, 99364–99377. [Google Scholar] [CrossRef]
- Maria Magdalane, C.; Kaviyarasu, K.; Judith Vijaya, J.; Jayakumar, C.; Maaza, M.; Jeyaraj, B. Photocatalytic degradation effect of malachite green and catalytic hydrogenation by UV–Illuminated CeO2/CdO multilayered nanoplatelet arrays: Investigation of antifungal and antimicrobial activities. J. Photochem. Photobiol. B Biol. 2017, 169, 110–123. [Google Scholar] [CrossRef]
- Kaviyarasu, K.; Ayeshamariam, A.; Manikandan, E.; Kennedy, J.; Ladchumananandasivam, R.; Umbelino Gomes, U.; Jayachandran, M.; Maaza, M. Solution Processing of CuSe quantum dots: Photocatalytic activity under RhB for UV and visible-light solar irradiation. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2016, 210, 1–9. [Google Scholar] [CrossRef]
- Yong, L.; Zhanqi, G.; Yuefei, J.; Xiaobin, H.; Cheng, S.; Shaogui, Y.; Lianhong, W.; Qingeng, W.; Die, F. Photodegradation of malachite green under simulated and natural irradiation: Kinetics, products, and pathways. J. Hazard. Mater. 2015, 285, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Kundu, A.; Mondal, A. Kinetics, isotherm, and thermodynamic studies of methylene blue selective adsorption and photocatalysis of malachite green from aqueous solution using layered Na-intercalated Cu-doped Titania. Appl. Clay Sci. 2019, 183, 105323. [Google Scholar] [CrossRef]
- Deivasigamani, P.; Maheswari, M.A. Soft synthesis of potassium Co-Doped Al-ZnO nanocomposites: A comprehensive study on their visible-light driven photocatalytic activity on dye degradation. Artic. J. Mater. Sci. 2016, 51, 8187–8208. [Google Scholar]
- Shandilya, P.; Mittal, D.; Soni, M.; Raizada, P.; Lim, J.H.; Jeong, D.Y.; Dewedi, R.P.; Saini, A.K.; Singh, P. Islanding of EuVO4 on high-dispersed fluorine doped few layered graphene sheets for efficient photocatalytic mineralization of phenolic compounds and bacterial disinfection. J. Taiwan Inst. Chem. Eng. 2018, 93, 528–542. [Google Scholar] [CrossRef]
- Thejaswini, T.V.L.; Prabhakaran, D.; Maheswari, M.A. Ultrasound assisted synthesis of nano-rod embedded petal designed α-bi2o3-zno nanoparticles and their ultra-responsive visible light induced photocatalytic properties. J. Photochem. Photobiol. A Chem. 2017, 335, 217–229. [Google Scholar] [CrossRef]






| Initial Temperature (°C) | Final Temperature (°C) | Enthalpy of Fusion (J/g) | Heat Capacity at Ti (J/g·°C) | Enthalpy of Crystallization (J/g) |
|---|---|---|---|---|
| 276 | 292 | 186.68 | 9.91 | 650.43 |
| S.N. | Concentration (mg·L−1) | Pseudo First Order | Pseudo Second Order | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Rate Constant (K1) (min−1) | Half-Life t1/2 (min) | R2 | RMSE | Rate Constant (K2) (L·mg−1·min−1) | Half-Life t1/2 (min) | R2 | RMSE | ||
| 1 | 35 | 0.04 | 17.32 | 0.99 | 0.06 | 0.013 | 2.05 | 0.80 | 0.21 |
| 2 | 55 | 0.05 | 13.86 | 0.99 | 0.11 | 0.020 | 0.90 | 0.74 | 0.37 |
| 3 | 70 | 0.06 | 11.55 | 0.94 | 0.18 | 0.044 | 0.33 | 0.67 | 0.93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasan, I.; Bassi, A.; Alharbi, K.H.; BinSharfan, I.I.; Khan, R.A.; Alslame, A. Sonophotocatalytic Degradation of Malachite Green by Nanocrystalline Chitosan-Ascorbic Acid@NiFe2O4 Spinel Ferrite. Coatings 2020, 10, 1200. https://doi.org/10.3390/coatings10121200
Hasan I, Bassi A, Alharbi KH, BinSharfan II, Khan RA, Alslame A. Sonophotocatalytic Degradation of Malachite Green by Nanocrystalline Chitosan-Ascorbic Acid@NiFe2O4 Spinel Ferrite. Coatings. 2020; 10(12):1200. https://doi.org/10.3390/coatings10121200
Chicago/Turabian StyleHasan, Imran, Akshara Bassi, Khadijah H. Alharbi, Ibtisam I. BinSharfan, Rais Ahmad Khan, and Ali Alslame. 2020. "Sonophotocatalytic Degradation of Malachite Green by Nanocrystalline Chitosan-Ascorbic Acid@NiFe2O4 Spinel Ferrite" Coatings 10, no. 12: 1200. https://doi.org/10.3390/coatings10121200







