Active Packaging—Poly(Vinyl Alcohol) Films Enriched with Tomato By-Products Extract
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization of Tomato Waste Extracts
2.2.1. Ultrasound-Assisted Extraction (UAE)
2.2.2. Quantitative and Qualitative Analysis of Carotenoids (Lycopene, Β-Carotene, and Lutein) by HPLC/DAD
2.2.3. Qualitative and Quantitative Analysis of Phenolic Compounds by HPLC-DAD-ESI-MS
2.3. Film Preparation
2.4. Shear Viscosity Measurement of Film Solutions
2.5. Total Phenolics
2.6. Antimicrobial Activity of the Film-Forming Solutions
2.7. Solid Film Characterization
2.7.1. Solid Film Measurements
2.7.2. Fourier-Transform Infrared analysis
2.8. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Tomato By-Products Extracts
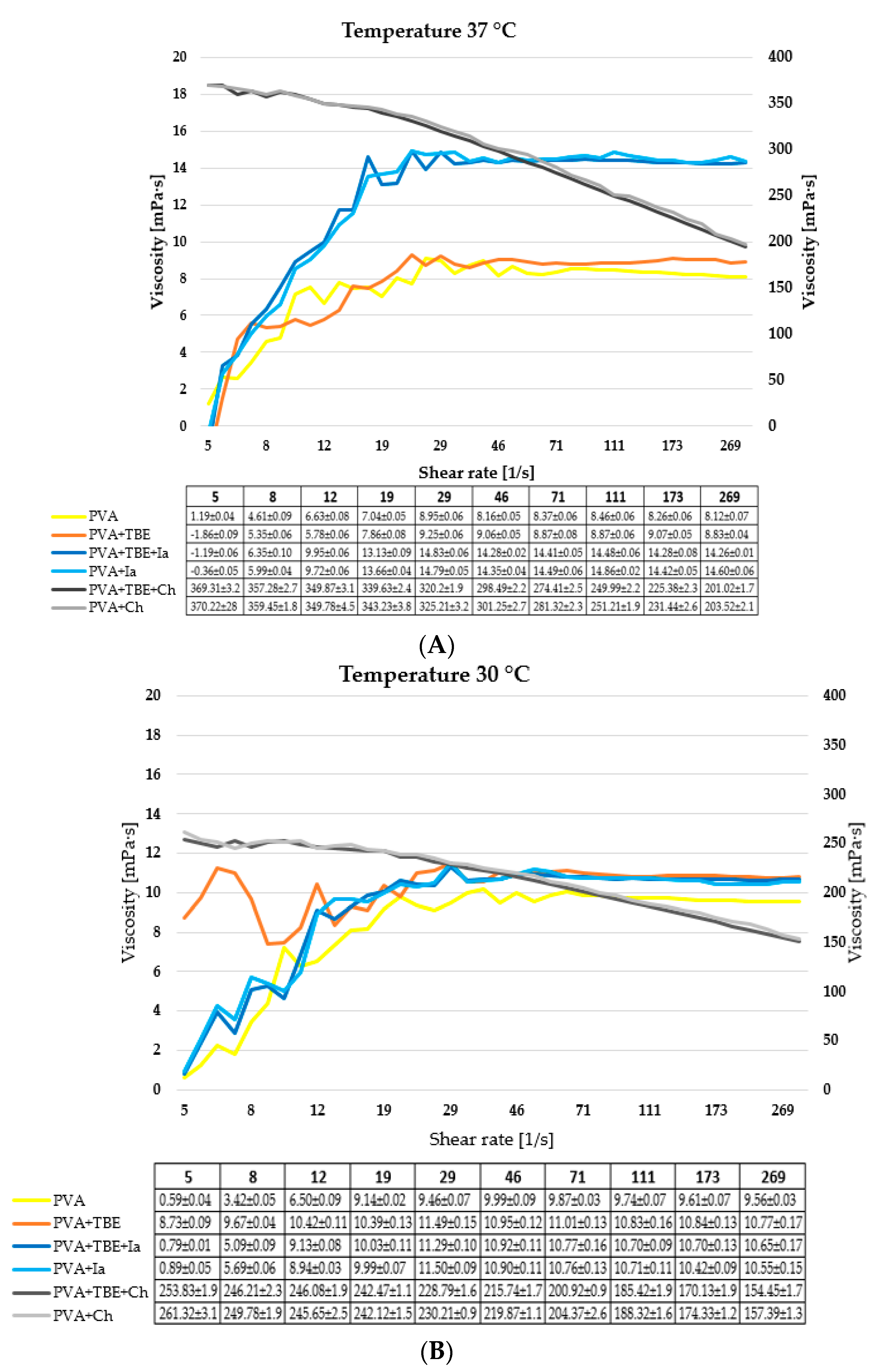
3.2. Shear Viscosity Measurement of Film-Forming Solutions
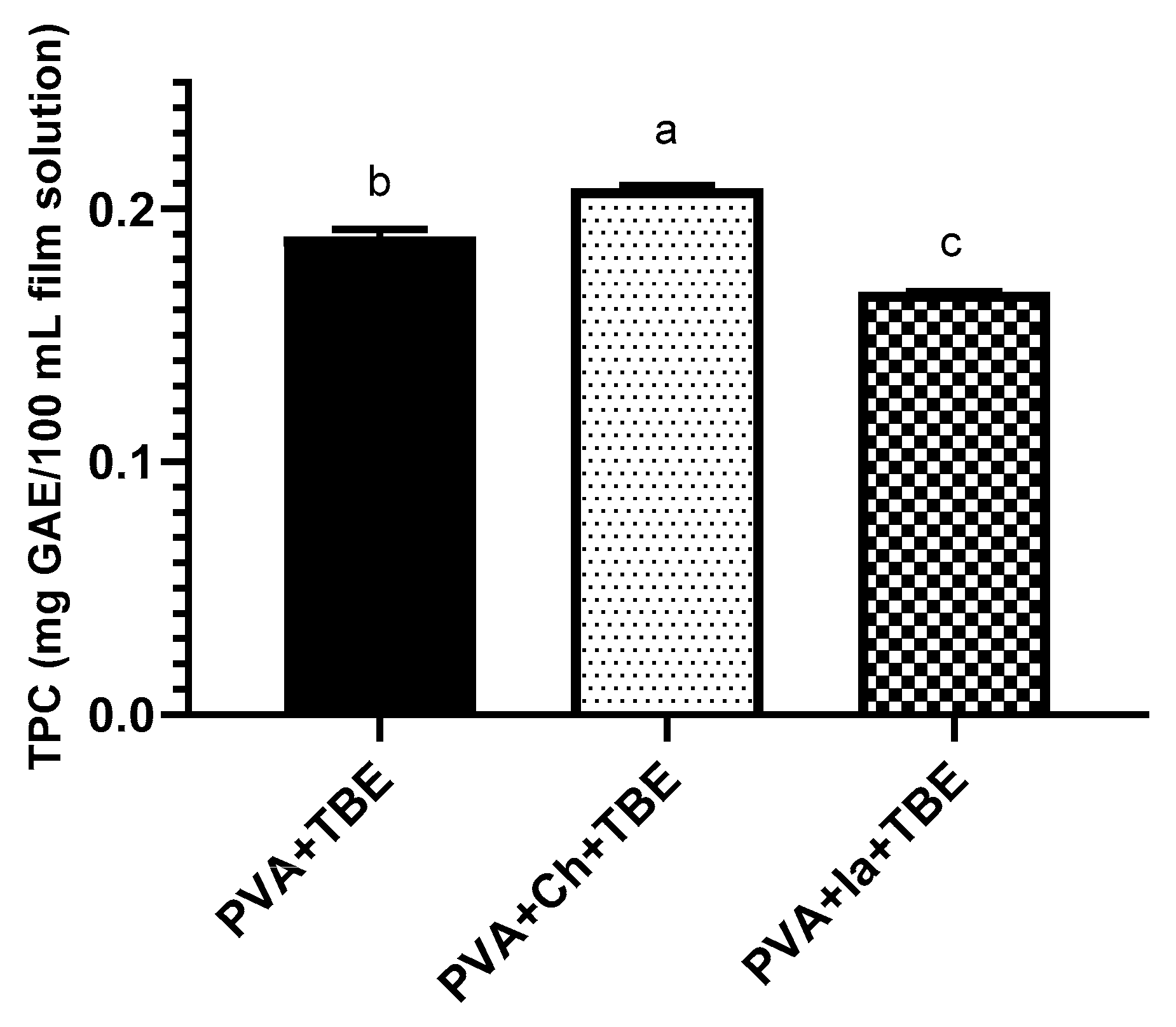
3.3. Total Phenolic Content (TPC)
3.4. Antimicrobial Activity
3.5. Physical Characterization of Solid Films
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Garavand, F.; Rouhi, M.; Razavi, S.H.; Cacciotti, I.; Mohammadi, R. Improving the integrity of natural biopolymer films used in food packaging by crosslinking approach: A review. Int. J. Biol. Macromol. 2017, 104, 687–707. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, T.D.; Hasselbalch, J.; Holmberg, K.; Stripple, J. Politics and the plastic crisis: A review throughout the plastic life cycle. Wires Energy Environ. 2019, 9, e360. [Google Scholar] [CrossRef] [Green Version]
- Raddadi, N.; Fava, F. Biodegradation of oil-based plastics in the environment: Existing knowledge and needs of research and innovation. Sci. Total Environ. 2019, 679, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Blettler, M.C.M.; Wantzen, K.M. Threats Underestimated in Freshwater Plastic Pollution: Mini-Review. Water Air Soil Pollut. 2019, 230, 174. [Google Scholar] [CrossRef]
- Wang, M.H.; He, Y.; Sen, B. Research and management of plastic pollution in coastal environments of China. Environ. Pollut. 2019, 248, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Available online: http://www3.weforum.org/docs/WEF_The_New_Plastics_Economy.pdf (accessed on 4 February 2020).
- Jagiello, Z.; Dylewski, L.; Tobolka, M.; Aguirre, J.I. Life in a polluted world: A global review of anthropogenic materials in bird nests. Environ. Pollut. 2019, 251, 717–722. [Google Scholar] [CrossRef]
- Cherubini, F.; Ulgiati, S. Crop residues as raw materials for biorefinery systems—A LCA case study. Appl. Energy 2010, 87, 47–57. [Google Scholar] [CrossRef]
- Available online: https://www.earthday.org/fact-sheet-single-use-plastics/ (accessed on 4 February 2020).
- Weiss, M.; Haufe, J.; Carus, M.; Brandão, M.; Bringezu, S.; Hermann, B.; Patel, M.K. A Review of the Environmental Impacts of Biobased Materials. J. Ind. Ecol. 2012, 16, S169–S181. [Google Scholar] [CrossRef]
- Hatti-Kaul, R.; Nilsson, L.J.; Zhang, B.; Rehnberg, N.; Lundmark, S. Designing Biobased Recyclable Polymers for Plastics. Trends Biotechnol. 2020, 38, 50–67. [Google Scholar] [CrossRef]
- Zhu, Y.; Romain, C.; Williams, C.K. Sustainable polymers from renewable resources. Nature 2016, 540, 354–362. [Google Scholar] [CrossRef]
- Iwata, T. Biodegradable and bio-based polymers: Future prospects of eco-friendly plastics. Angew. Chem. Int. Ed. Engl. 2015, 54, 3210–3215. [Google Scholar] [CrossRef] [PubMed]
- Mitrea, L.; Vodnar, D.C. Klebsiella pneumoniae—A Useful Pathogenic Strain for Biotechnological Purposes: Diols Biosynthesis under Controlled and Uncontrolled pH Levels. Pathogens 2019, 8, 293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Available online: http://www.fao.org/fileadmin/templates/agns/pdf/jecfa/cta/61/PVA.pdf (accessed on 4 February 2020).
- Wong, C.Y.; Wong, W.Y.; Loh, K.S.; Daud, W.R.W.; Lim, K.L.; Khalid, M.; Walvekar, R. Development of Poly(Vinyl Alcohol)-Based Polymers as Proton Exchange Membranes and Challenges in Fuel Cell Application: A Review. Polym. Rev. 2019, 171–202. [Google Scholar] [CrossRef]
- Kanatt, S.R.; Makwana, S.H. Development of active, water-resistant carboxymethyl cellulose-poly vinyl alcohol-Aloe vera packaging film. Carbohydr. Polym. 2020, 227, 115303. [Google Scholar] [CrossRef]
- Szabo, K.; Diaconeasa, Z.; Catoi, A.F.; Vodnar, D.C. Screening of Ten Tomato Varieties Processing Waste for Bioactive Components and Their Related Antioxidant and Antimicrobial Activities. Antioxidants 2019, 8, 292. [Google Scholar] [CrossRef] [Green Version]
- Călinoiu, L.F.; Mitrea, L.; Precup, G.; Bindea, M.; Rusu, B.; Szabo, K.; Dulf, F.V.; Ştefănescu, B.E.; Vodnar, D.C. Sustainable use of agro-industrial wastes for feeding 10 billion people by 2050. In Professionals in Food Chains: Ethics, Roles and Responsibilities; Wageningen Academic Publishers: Wageningen, The Netherlands, 2018. [Google Scholar]
- Bilal, M.; Iqbal, H.M.N. Naturally-derived biopolymers: Potential platforms for enzyme immobilization. Int. J. Biol. Macromol. 2019, 130, 462–482. [Google Scholar] [CrossRef]
- Calinoiu, L.F.; Catoi, A.F.; Vodnar, D.C. Solid-State Yeast Fermented Wheat and Oat Bran as A Route for Delivery of Antioxidants. Antioxidants 2019, 8, 372. [Google Scholar] [CrossRef] [Green Version]
- Mitrea, L.; Calinoiu, L.F.; Precup, G.; Bindea, M.; Rusu, B.; Trif, M.; Stefanescu, B.E.; Pop, I.D.; Vodnar, D.C. Isolated Microorganisms for Bioconversion of Biodiesel-Derived Glycerol Into 1,3-Propanediol. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca-Food Sci. Technol. 2017, 74, 43–49. [Google Scholar] [CrossRef] [Green Version]
- Saini, R.K.; Moon, S.H.; Keum, Y.S. An updated review on use of tomato pomace and crustacean processing waste to recover commercially vital carotenoids. Food Res. Int. 2018, 108, 516–529. [Google Scholar] [CrossRef]
- Trif, M.; Vodnar, D.C.; Mitrea, L.; Rusu, A.V.; Socol, C.T. Design and Development of Oleoresins Rich in Carotenoids Coated Microbeads. Coatings 2019, 9, 235. [Google Scholar] [CrossRef] [Green Version]
- Szabo, K.; Catoi, A.F.; Vodnar, D.C. Bioactive Compounds Extracted from Tomato Processing by-Products as a Source of Valuable Nutrients. Plant Foods Hum. Nutr. 2018, 73, 268–277. [Google Scholar] [CrossRef]
- Calinoiu, L.F.; Vodnar, D.C. Whole Grains and Phenolic Acids: A Review on Bioactivity, Functionality, Health Benefits and Bioavailability. Nutrients 2018, 10, 1615. [Google Scholar] [CrossRef] [Green Version]
- Strati, I.F.; Oreopoulou, V. Recovery of carotenoids from tomato processing by-products—A review. Food Res. Int. 2014, 65, 311–321. [Google Scholar] [CrossRef]
- Calinoiu, L.F.; Vodnar, D.C. Thermal Processing for the Release of Phenolic Compounds from Wheat and Oat Bran. Biomolecules 2019, 10, 21. [Google Scholar] [CrossRef] [Green Version]
- Kuenz, A.; Krull, S. Biotechnological production of itaconic acid-things you have to know. Appl. Microbiol. Biotechnol. 2018, 102, 3901–3914. [Google Scholar] [CrossRef]
- Weastra SRO. Available online: https://www.igb.fraunhofer.de/content/dam/igb/en/documents/publications/BioConSepT_Market-potential-for-selected-platform-chemicals_ppt1.pdf (accessed on 4 February 2020).
- Regestein, L.; Klement, T.; Grande, P.; Kreyenschulte, D.; Heyman, B.; Massmann, T.; Eggert, A.; Sengpiel, R.; Wang, Y.; Wierckx, N.; et al. From beech wood to itaconic acid: Case study on biorefinery process integration. Biotechnol. Biofuels 2018, 11, 279. [Google Scholar] [CrossRef]
- Teleky, B.E.; Vodnar, D.C. Biomass-Derived Production of Itaconic Acid as a Building Block in Specialty Polymers. Polymers 2019, 11, 1035. [Google Scholar] [CrossRef] [Green Version]
- Fuciños, C.F.P.; Amado, I.R.; Míguez, M.; Fajardo, P.; Pastrana, L.M.; Rúa, M.L. Smart Nanohydrogels for Controlled Release of Food Preservatives; Elseviser: Amsterdam, The Netherlands, 2016. [Google Scholar]
- González-Henríquez, C.M.; Sarabia-Vallejos, M.A.; Rodriguez-Hernandez, J. Polymers for additive manufacturing and 4D-printing: Materials, methodologies, and biomedical applications. Prog. Polym. Sci. 2019, 94, 57–116. [Google Scholar] [CrossRef]
- Top 5 Vendors in the Global Biopolymers Market From 2017–2021: Technavio. Available online: https://www.businesswire.com/news/home/20170112005066/en/Top-5-Vendors-Global-Biopolymers-Market-2017-2021 (accessed on 2 September 2019).
- Calinoiu, L.F.; Vodnar, D.; Precup, G. A Review: The Probiotic Bacteria Viability under Different Conditions. Bulletin of University of Agricultural Sciences and Veterinary Medicine Cluj-Napoca. Food Sci. Technol. 2016, 73. [Google Scholar] [CrossRef] [Green Version]
- Martau, G.A.; Mihai, M.; Vodnar, D.C. The Use of Chitosan, Alginate, and Pectin in the Biomedical and Food Sector-Biocompatibility, Bioadhesiveness, and Biodegradability. Polymers 2019, 11, 1837. [Google Scholar] [CrossRef] [Green Version]
- Sinha, V.R.; Singla, A.K.; Wadhawan, S.; Kaushik, R.; Kumria, R.; Bansal, K.; Dhawan, S. Chitosan microspheres as a potential carrier for drugs. Int. J. Pharm. 2004, 274, 1–33. [Google Scholar] [CrossRef]
- Cavallaro, G.; Lazzara, G.; Milioto, S. Sustainable nanocomposites based on halloysite nanotubes and pectin/polyethylene glycol blend. Polym. Degrad. Stab. 2013, 98, 2529–2536. [Google Scholar] [CrossRef] [Green Version]
- Sanuja, S.; Agalya, A.; Umapathy, M.J. Studies on Magnesium Oxide Reinforced Chitosan Bionanocomposite Incorporated with Clove Oil for Active Food Packaging Application. Int. J. Polym. Mater. Polym. Biomater. 2014, 63, 733–740. [Google Scholar] [CrossRef]
- Guerreiro, A.C.; Gago, C.M.L.; Miguel, M.G.C.; Faleiro, M.L.; Antunes, M.D.C. The influence of edible coatings enriched with citral and eugenol on the raspberry storage ability, nutritional and sensory quality. Food Packag. Shelf Life 2016, 9, 20–28. [Google Scholar] [CrossRef]
- Kanetis, L.; Exarchou, V.; Charalambous, Z.; Goulas, V. Edible coating composed of chitosan and Salvia fruticosa Mill. extract for the control of grey mould of table grapes. JSCI Food Agric. 2017, 97, 452–460. [Google Scholar] [CrossRef]
- Călinoiu, L.-F.; Ştefănescu, B.; Pop, I.; Muntean, L.; Vodnar, D. Chitosan Coating Applications in Probiotic Microencapsulation. Coatings 2019, 9, 194. [Google Scholar] [CrossRef] [Green Version]
- Krisanti, E.A.; Naziha, G.M.; Amany, N.S.; Mulia, K.; Handayani, N.A. Effect of biopolymers composition on release profile of iron(II) fumarate from chitosan-alginate microparticles. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2019. [Google Scholar]
- Estevinho, B.N.; Rocha, F.; Santos, L.; Alves, A. Microencapsulation with chitosan by spray drying for industry applications—A review. Trends Food Sci. Technol. 2013, 31, 138–155. [Google Scholar] [CrossRef]
- Gonçalves, A.; Estevinho, B.N.; Rocha, F. Microencapsulation of vitamin A: A review. Trends Food Sci. Technol. 2016, 51, 76–87. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.B.; Ahn, J.; Lee, J.; Kwak, H.S. The microencapsulated ascorbic acid release in vitro and its effect on iron bioavailability. Arch. Pharm. Res. 2003, 26, 874–879. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, R.; Chen, L.; McClements, D.J. Encapsulation of lactase (beta-galactosidase) into kappa-carrageenan-based hydrogel beads: Impact of environmental conditions on enzyme activity. Food Chem. 2016, 200, 69–75. [Google Scholar] [CrossRef] [Green Version]
- Gupta, C.; Chawla, P.; Arora, S.; Tomar, S.K.; Singh, A.K. Iron microencapsulation with blend of gum arabic, maltodextrin and modified starch using modified solvent evaporation method—Milk fortification. Food Hydrocoll. 2015, 43, 622–628. [Google Scholar] [CrossRef]
- Valenzuela, C.; Hernández, V.; Morales, M.S.; Neira-Carrillo, A.; Pizarro, F. Preparation and characterization of heme iron-alginate beads. LWT Food Sci. Technol. 2014, 59, 1283–1289. [Google Scholar] [CrossRef]
- Nasui, L.; Vodnar, D.; Socaciu, C. Bioactive Labels for Fresh Fruits and Vegetables. Bulletin of University of Agricultural Sciences and Veterinary Medicine Cluj-Napoca. Food Sci. Technol. 2013, 70, 74–82. [Google Scholar] [CrossRef] [Green Version]
- Dong, H.; Cheng, L.; Tan, J.; Zheng, K.; Jiang, Y. Effects of chitosan coating on quality and shelf life of peeled litchi fruit. J. Food Eng. 2004, 64, 355–358. [Google Scholar] [CrossRef]
- Guo, Z.; Xing, R.; Liu, S.; Zhong, Z.; Ji, X.; Wang, L.; Li, P. The influence of molecular weight of quaternized chitosan on antifungal activity. Carbohydr. Polym. 2008, 71, 694–697. [Google Scholar] [CrossRef]
- Athayde, A.J.A.A.; de Oliveira, P.D.L.; Guerra, I.C.D.; da Conceição, M.L.; de Lima, M.A.B.; Arcanjo, N.M.O.; Madruga, M.S.; Berger, L.R.R.; de Souza, E.L. A coating composed of chitosan and Cymbopogon citratus(Dc. Ex Nees) essential oil to control Rhizopus soft rot and quality in tomato fruit stored at room temperature. J. Hortic. Sci. Biotechnol. 2016, 91, 582–591. [Google Scholar] [CrossRef]
- Dulf, F.V.; Vodnar, D.C.; Dulf, E.H.; Tosa, M.I. Total phenolic contents, antioxidant activities, and lipid fractions from berry pomaces obtained by solid-state fermentation of two Sambucus species with Aspergillus niger. J. Agric. Food Chem. 2015, 63, 3489–3500. [Google Scholar] [CrossRef]
- Vodnar, D.C.; Calinoiu, L.F.; Dulf, F.V.; Stefanescu, B.E.; Crisan, G.; Socaciu, C. Identification of the bioactive compounds and antioxidant, antimutagenic and antimicrobial activities of thermally processed agro-industrial waste. Food Chem. 2017, 231, 131–140. [Google Scholar] [CrossRef]
- O’Donnell, F.; Smyth, T.J.; Ramachandran, V.N.; Smyth, W.F. A study of the antimicrobial activity of selected synthetic and naturally occurring quinolines. Int. J. Antimicrob. Agents 2010, 35, 30–38. [Google Scholar] [CrossRef] [Green Version]
- Szabo, K.; Dulf, F.V.; Diaconeasa, Z.; Vodnar, D.C. Antimicrobial and antioxidant properties of tomato processing byproducts and their correlation with the biochemical composition. LWT 2019, 116, 108558. [Google Scholar] [CrossRef]
- Strati, I.F.; Gogou, E.; Oreopoulou, V. Enzyme and high pressure assisted extraction of carotenoids from tomato waste. Food Bioprod. Process. 2015, 94, 668–674. [Google Scholar] [CrossRef]
- Ding, J.; Chen, S.-C.; Wang, X.-L.; Wang, Y.-Z. Preparation and Rheological Behaviors of Thermoplastic Poly(vinyl alcohol) Modified by Lactic Acid. Ind. Eng. Chem. Res. 2011, 50, 9123–9130. [Google Scholar] [CrossRef]
- Muresan, V.; Danthine, S.; Racolta, E.; Muste, S.; Blecker, C. The Influence of Particle Size Distribution on Sunflower Tahini Rheology and Structure. J. Food Process Eng. 2014, 37, 411–426. [Google Scholar] [CrossRef]
- Merlusca, I.P.; Ibanescu, C.; Tuchilus, C.; Danu, M.; Atanase, L.I.; Popa, I.M. Characterization of Neomycin-Loaded Xanthan-Chitosan Hydrogels for Topical Applications. Cellul. Chem. Technol. 2019, 53, 709–719. [Google Scholar] [CrossRef]
- Arcan, I.; Yemenicioğlu, A. Incorporating phenolic compounds opens a new perspective to use zein films as flexible bioactive packaging materials. Food Res. Int. 2011, 44, 550–556. [Google Scholar] [CrossRef] [Green Version]
- Shahidi, F.; Arachchi, J.K.V.; Jeon, Y.-J. Food applications of chitin and chitosans. Trends Food Sci. Technol. 1999, 10, 37–51. [Google Scholar] [CrossRef]
- Kanatt, S.R.; Rao, M.S.; Chawla, S.P.; Sharma, A. Active chitosan-polyvinyl alcohol films with natural extracts. Food Hydrocoll. 2012, 29, 290–297. [Google Scholar] [CrossRef]
- Birajdar, M.S.; Cho, H.; Seo, Y.; Choi, J.; Park, H. Surface conjugation of poly(dimethyl siloxane) with itaconic acid-based materials for antibacterial effects. Appl. Surf. Sci. 2018, 437, 245–256. [Google Scholar] [CrossRef]
- Sakthivel, M.; Franklin, D.S.; Sudarsan, S.; Chitra, G.; Sridharan, T.B.; Guhanathan, S. Investigation on pH/salt-responsive multifunctional itaconic acid based polymeric biocompatible, antimicrobial and biodegradable hydrogels. React. Funct. Polym. 2018, 122, 9–21. [Google Scholar] [CrossRef]
- Ouattara, B.; Simard, R.E.; Piette, G.; Begin, A.; Holley, R.A. Diffusion of Acetic and Propionic Acids from Chitosan-based Antimicrobial Packaging Films. J. Food Sci. 2000, 65, 768–773. [Google Scholar] [CrossRef]
- Rao, M.S.; Kanatt, S.R.; Chawla, S.P.; Sharma, A. Chitosan and guar gum composite films: Preparation, physical, mechanical and antimicrobial properties. Carbohydr. Polym. 2010, 82, 1243–1247. [Google Scholar] [CrossRef]
- Mathew, S.; Abraham, T.E. Characterisation of ferulic acid incorporated starch–chitosan blend films. Food Hydrocoll. 2008, 22, 826–835. [Google Scholar] [CrossRef]
- Pranoto, Y.; Rakshit, S.K.; Salokhe, V.M. Enhancing antimicrobial activity of chitosan films by incorporating garlic oil, potassium sorbate and nisin. LWT Food Sci. Technol. 2005, 38, 859–865. [Google Scholar] [CrossRef]
- Sand, A.; Kniivilä, J.; Toivakka, M.; Hjelt, T. Structure formation mechanisms in consolidating pigment coatings—Simulation and visualisation. Chem. Eng. Process. Process Intensif. 2011, 50, 574–582. [Google Scholar] [CrossRef]
- Luzi, F.; Pannucci, E.; Santi, L.; Kenny, J.M.; Torre, L.; Bernini, R.; Puglia, D. Gallic Acid and Quercetin as Intelligent and Active Ingredients in Poly(vinyl alcohol) Films for Food Packaging. Polymers 2019, 11, 1999. [Google Scholar] [CrossRef] [Green Version]
- Mitrea, L.; Ranga, F.; Fetea, F.; Dulf, F.V.; Rusu, A.; Trif, M.; Vodnar, D.C. Biodiesel-Derived Glycerol Obtained from Renewable Biomass—A Suitable Substrate for the Growth of Candida zeylanoides Yeast Strain ATCC 20367. Microorganisms 2019, 7, 265. [Google Scholar] [CrossRef] [Green Version]
- Harun-or-Rashid, M.D.; Saifur Rahaman, M.D.; Enamul Kabir, S.; Khan, M.A. Effect of hydrochloric acid on the properties of biodegradable packaging materials of carboxymethylcellulose/poly(vinyl alcohol) blends. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Li, R.; Wang, Y.; Xu, J.; Ahmed, S.; Liu, Y. Preparation and Characterization of Ultrasound Treated Polyvinyl Alcohol/Chitosan/DMC Antimicrobial Films. Coatings 2019, 9, 582. [Google Scholar] [CrossRef] [Green Version]
- MilosavljevicÌ, N.B.; KljajevicÌ, L.M.; PopovicÌ, I.G.; FilipovicÌ, J.M.; Kalagasidis KrusÌŒicÌ, M.T. Chitosan, itaconic acid and poly(vinyl alcohol) hybrid polymer networks of high degree of swelling and good mechanical strength. Polym. Int. 2009, 59, 686–694. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, S.; Lan, W.; Qin, W. Fabrication and Testing of PVA/Chitosan Bilayer Films for Strawberry Packaging. Coatings 2017, 7, 109. [Google Scholar] [CrossRef]





| Class of Compounds | Peak No. | Rt (min) | λmax (nm) | [M + H]+ (m/z) | Compound |
|---|---|---|---|---|---|
| Carotenoids | 1 | 6.41 | 448, 474 | Lutein | |
| 2 | 13.42 | 446, 473 | Lycopene | ||
| 3 | 14.51 | 455, 480 | β-Carotene | ||
| Phenolics | 1 | 11.12 | 292, 245 | 343 | Caffeic acid-glucoside isomer |
| 2 | 11.93 | 326, 248 | 355 | 5-Caffeoylquinic acid | |
| 3 | 14.09 | 355, 259 | 627 | Quercetin-diglucoside | |
| 4 | 14.87 | 355, 259 | 478 | Quercetin-glucuronide | |
| 5 | 15.34 | 354, 256 | 611 | Quercetin-3-rutinoside | |
| 6 | 16.98 | 328, 250 | 517 | Di-Caffeoylquinic acid | |
| 7 | 20.33 | 328, 250 | 679 | Tri-Caffeoylquinic acid | |
| 8 | 23.10 | 366, 250 | 273 | Naringenin chalcone |
| Peak | Carotenoids | mg/100 DW |
|---|---|---|
| 1 | Lutein | 1.549 ± 0.04 |
| 2 | Lycopene | 0.127 ± 0.01 |
| 3 | β-Carotene | 1.597 ± 0.01 |
| Sum of identified carotenoids | 3.273 ± 0.05 |
| Peak | Phenolic Compounds | mg/100 DW |
|---|---|---|
| 1 | Caffeic acid-glucoside isomer | 2.284 ± 0.02 |
| 2 | 5-Caffeoylquinic acid | 2.623 ± 0.02 |
| 3 | Quercetin-diglucoside | 7.150 ± 0.05 |
| 4 | Quercetin-glucuronide | 24.427 ± 0.04 |
| 5 | Quercetin-3-rutinoside | 6.061 ± 0.03 |
| 6 | Di-Caffeoylquinic acid | 2.092 ± 0.02 |
| 7 | Tri-Caffeoylquinic acid | 1.782 ± 0.01 |
| 8 | Naringenin chalcone | 34.178 ± 0.02 |
| Sum of identified phenolics | 80.596 ± 0.20 |
| G (+) Bacteria | G (−) Bacteria | ||||
|---|---|---|---|---|---|
| Samples | S. aureus | E. coli | P. aeruginosa | S. enterica Enteritidis | S. enterica Typhimurium |
| PVA | n.b. | n.b. | n.b. | n.b. | n.b. |
| PVA + TBE | n.b. | n.b. | n.b. | n.b. | n.b. |
| PVA + Ch + TBE | <0.078 | 5.00 | <0.078 | 0.312 | 5.00 |
| PVA + Ch | 0.156. | 10 | 0.156 | 0.624 | 10 |
| PVA + Ia + TBE | n.b. | n.b. | 2.5 | n.b. | n.b. |
| PVA + Ia | n.b. | n.b. | 5.00 | n.b. | n.b. |
| Sample | Diameter (cm) | Weight (g) | Thickness (mm) | Density (g/cm3) |
|---|---|---|---|---|
| PVA | 8.33 ± 0.15 | 0.44 ± 0.00 | 0.03 ± 0.01 | 2.40 ± 0.79 |
| PVA + TBE | 7.95 ± 0.07 | 0.46 ± 0.00 | 0.04 ± 0.01 | 3.70 ± 1.35 |
| PVA + Ia | 8.27 ± 0.06 | 0.61 ± 0.04 | 0.04 ± 0.01 | 4.58 ± 1.33 |
| PVA + Ia + TBE | 7.87 ± 0.06 | 0.61 ± 0.02 | 0.04 ± 0.01 | 4.63 ± 1.44 |
| PVA + Ch | 8.00 ± 0.00 | 0.57 ± 0.01 | 0.06 ± 0.01 | 6.79 ± 1.08 |
| PVA + Ch + TBE | 8.07 ± 0.12 | 0.64 ± 0.03 | 0.06 ± 0.01 | 7.55 ± 1.40 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szabo, K.; Teleky, B.-E.; Mitrea, L.; Călinoiu, L.-F.; Martău, G.-A.; Simon, E.; Varvara, R.-A.; Vodnar, D.C. Active Packaging—Poly(Vinyl Alcohol) Films Enriched with Tomato By-Products Extract. Coatings 2020, 10, 141. https://doi.org/10.3390/coatings10020141
Szabo K, Teleky B-E, Mitrea L, Călinoiu L-F, Martău G-A, Simon E, Varvara R-A, Vodnar DC. Active Packaging—Poly(Vinyl Alcohol) Films Enriched with Tomato By-Products Extract. Coatings. 2020; 10(2):141. https://doi.org/10.3390/coatings10020141
Chicago/Turabian StyleSzabo, Katalin, Bernadette-Emoke Teleky, Laura Mitrea, Lavinia-Florina Călinoiu, Gheorghe-Adrian Martău, Elemer Simon, Rodica-Anita Varvara, and Dan Cristian Vodnar. 2020. "Active Packaging—Poly(Vinyl Alcohol) Films Enriched with Tomato By-Products Extract" Coatings 10, no. 2: 141. https://doi.org/10.3390/coatings10020141