Assessment of Streptococcus Mutans Adhesion to the Surface of Biomimetically-Modified Orthodontic Archwires
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrate
2.2. Silica sol Synthesis
2.3. Surface Modification
2.4. Surface Characterization
2.5. Bacterial Adhesion
2.6. Statistical Analysis
3. Results
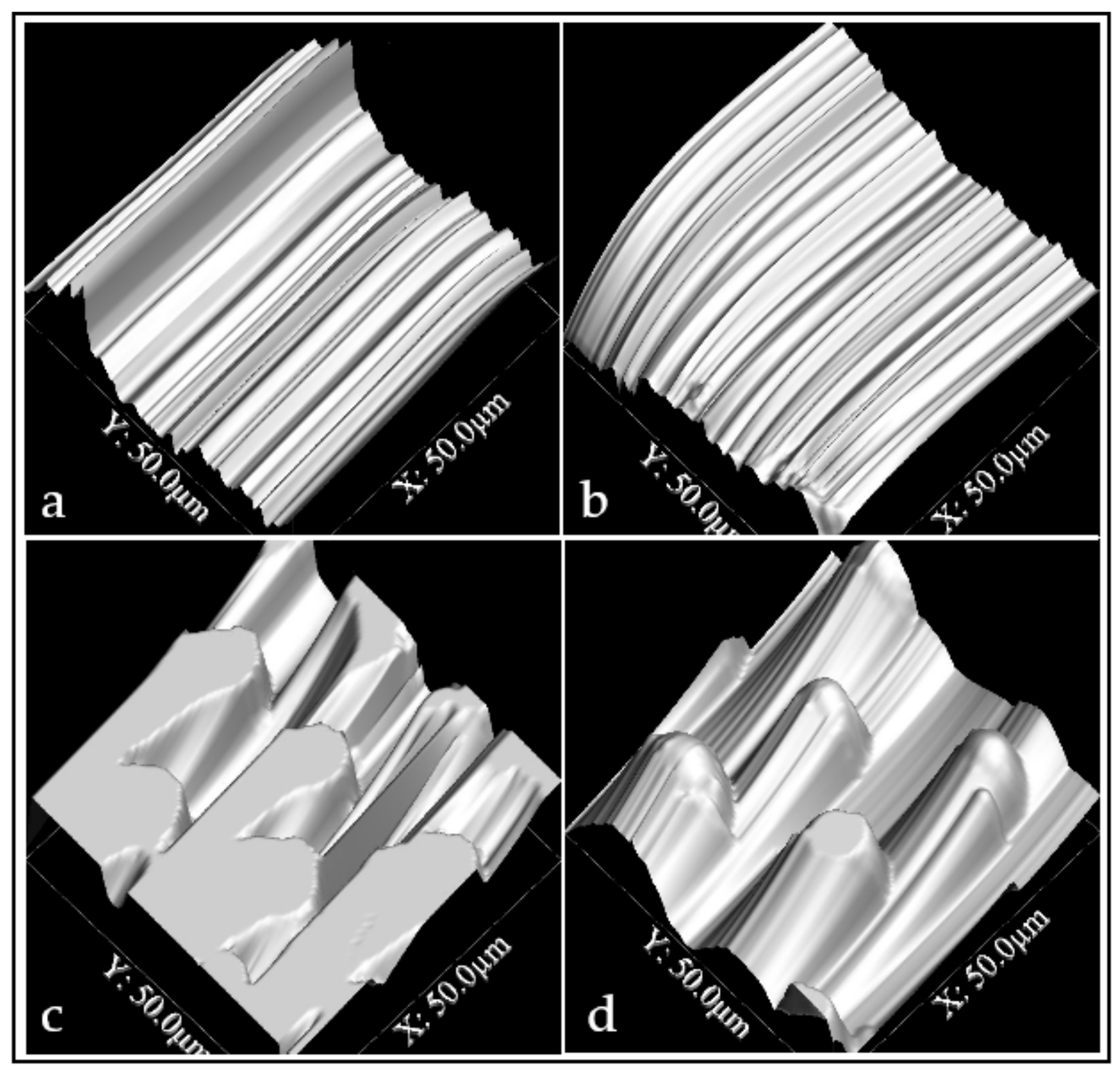
3.1. Surface Modification
3.2. Surface Characterization
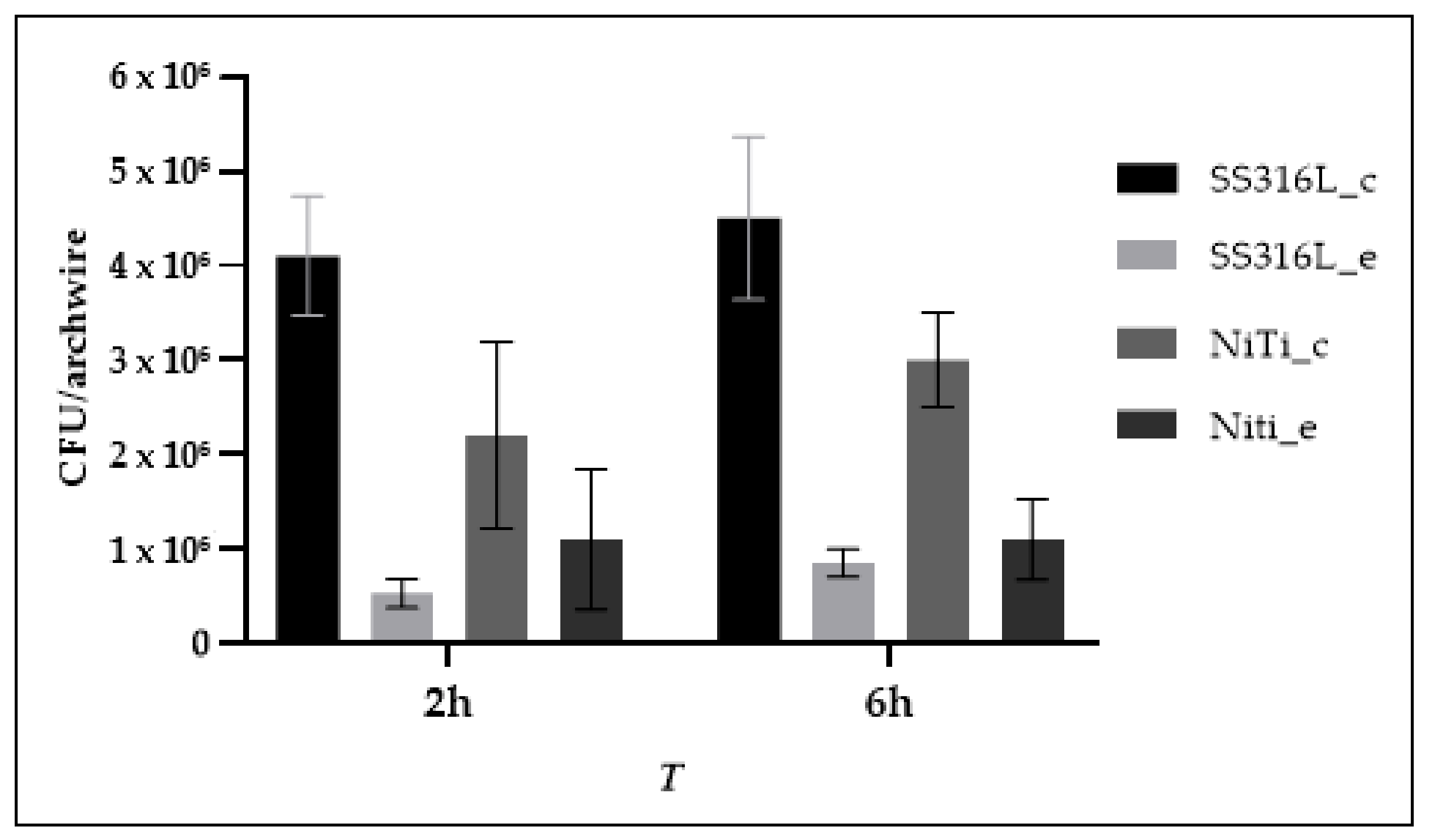
3.3. Bacterial Adhesion
4. Discussion
5. Conclusions and Considerations
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arango-Santander, S.; Ramírez-Vega, C. Titanio: Aspectos del material para uso en ortodoncia. Rev. Nac. Odontol. 2016, 12, 63–71. [Google Scholar] [CrossRef]
- Arango Santander, S.; Luna Ossa, C.M. Stainless Steel: Material Facts for the Orthodontic Practitioner. Rev. Nac. Odontol. 2015, 11, 71–82. [Google Scholar] [CrossRef] [Green Version]
- Bahije, L.; Benyahia, H.; El Hamzaoui, S.; Ebn Touhami, M.; Bengueddour, R.; Rerhrhaye, W.; Abdallaoui, F.; Zaoui, F. Behavior of NiTi in the presence of oral bacteria: Corrosion by Streptococcus mutans. Int. Orthod. 2011, 9, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Renner, L.D.; Weibel, D.B. Physicochemical regulation of biofilm formation. MRS Bull. 2011, 36, 347–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. A review of the biomaterials technologies for infection-resistant surfaces. Biomaterials 2013, 34, 8533–8554. [Google Scholar] [CrossRef] [PubMed]
- Patil, P.; Kharbanda, O.P.; Duggal, R.; Das, T.K.; Kalyanasundaram, D. Surface deterioration and elemental composition of retrieved orthodontic miniscrews. Am. J. Orthod. Dentofac. Orthop. 2015, 147, S88–S100. [Google Scholar] [CrossRef]
- Mystkowska, J.; Niemirowicz-Laskowska, K.; Łysik, D.; Tokajuk, G.; Dąbrowski, J.R.; Bucki, R. The role of oral cavity biofilm on metallic biomaterial surface destruction–corrosion and friction aspects. Int. J. Mol. Sci. 2018, 19, 743. [Google Scholar] [CrossRef] [Green Version]
- Øilo, M.; Bakken, V. Biofilm and dental biomaterials. Materials (Basel) 2015, 8, 2887–2900. [Google Scholar] [CrossRef]
- Al Qahtani, W.M.S.; Schille, C.; Spintzyk, S.; Al Qahtani, M.S.A.; Engel, E.; Geis-Gerstorfer, J.; Rupp, F.; Scheideler, L. Effect of surface modification of zirconia on cell adhesion, metabolic activity and proliferation of human osteoblasts. Biomed. Tech. 2017, 62, 75–87. [Google Scholar] [CrossRef]
- Carvalho, A.; Pelaez-Vargas, A.; Gallego-Perez, D.; Grenho, L.; Fernandes, M.H.; De Aza, A.H.; Ferraz, M.P.; Hansford, D.J.; Monteiro, F.J. Micropatterned silica thin films with nanohydroxyapatite micro-aggregates for guided tissue regeneration. Dent. Mater. 2012, 28, 1250–1260. [Google Scholar] [CrossRef]
- Laranjeira, M.S.; Carvalho, Â.; Pelaez-Vargas, A.; Hansford, D.; Ferraz, M.P.; Coimbra, S.; Costa, E.; Santos-Silva, A.; Fernandes, M.H.; Monteiro, F.J. Modulation of human dermal microvascular endothelial cell and human gingival fibroblast behavior by micropatterned silica coating surfaces for zirconia dental implant applications. Sci. Technol. Adv. Mater. 2014, 15, 025001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arango-Santander, S.; Pelaez-Vargas, A.; Freitas, S.C.; García, C. Surface Modification by Combination of Dip-Pen Nanolithography and Soft Lithography for Reduction of Bacterial Adhesion. J. Nanotechnol. 2018, 2018, 10. [Google Scholar] [CrossRef] [Green Version]
- Biswas, A.; Bayer, I.S.; Biris, A.S.; Wang, T.; Dervishi, E.; Faupel, F. Advances in top-down and bottom-up surface nanofabrication: Techniques, applications & future prospects. Adv. Colloid Interface Sci. 2012, 170, 2–27. [Google Scholar] [PubMed]
- Arango, S.; Peláez-Vargas, A.; García, C. Coating and surface treatments on orthodontic metallic materials. Coatings 2013, 3, 1–15. [Google Scholar] [CrossRef]
- Weibel, D.B.; DiLuzio, W.R.; Whitesides, G.M. Microfabrication meets microbiology. Nat. Rev. Microbiol. 2007, 5, 209–218. [Google Scholar] [CrossRef]
- Xia, Y.; Whitesides, G.M. Soft Lithography. Angew. Chemie Int. Ed. 1998, 37, 550–575. [Google Scholar] [CrossRef]
- Butler, R.T.; Ferrell, N.J.; Hansford, D.J. Spatial and geometrical control of silicification using a patterned poly-l-lysine template. Appl. Surf. Sci. 2006, 252, 7337–7342. [Google Scholar] [CrossRef]
- Pelaez-Vargas, A.; Gallego-Perez, D.; Fernandes, M.H.; Hansford, D.; Monteiro, F.J. Microstructured coatings to study the behavior of osteoblast-like cells on hard materials. Bone 2011, 48 (supp.2), s106. [Google Scholar] [CrossRef]
- Kitzmiller, J.; Beversdorf, D.; Hansford, D. Fabrication and testing of microelectrodes for small-field cortical surface recordings. Biomed. Microdevices 2006, 8, 81–85. [Google Scholar] [CrossRef]
- Pelaez-Vargas, A.; Ferrel, N.; Fernandes, M.H.; Hansford, D.J.; Monteiro, F.J. Cellular Alignment Induction during Early In Vitro Culture Stages Using Micropatterned Glass Coatings Produced by Sol-Gel Process. Key Eng Mater 2009, 396–398, 303–306. [Google Scholar] [CrossRef]
- Ferrell, N.; Woodard, J.; Hansford, D. Fabrication of polymer microstructures for MEMS: Sacrificial layer micromolding and patterned substrate micromolding. Biomed. Microdevices 2007, 9, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Tran, K.T.M.; Nguyen, T.D. Lithography-based methods to manufacture biomaterials at small scales. J. Sci. Adv. Mater. Devices 2017, 2, 1–14. [Google Scholar] [CrossRef]
- Solga, A.; Cerman, Z.; Striffler, B.F.; Spaeth, M.; Barthlott, W. The dream of staying clean: Lotus and biomimetic surfaces. Bioinspir Biomim 2007, 2, S126–S134. [Google Scholar] [CrossRef] [PubMed]
- Koch, K.; Barthlott, W. Superhydrophobic and superhydrophilic plant surfaces: An inspiration for biomimetic materials. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2009, 367, 1487–1509. [Google Scholar] [CrossRef]
- Chung, K.K.; Schumacher, J.F.; Sampson, E.M.; Burne, R.A.; Antonelli, P.J.; Brennan, A.B. Impact of engineered surface microtopography on biofilm formation of Staphylococcus aureus. Biointerphases 2007, 2, 89–94. [Google Scholar] [CrossRef] [Green Version]
- Bixler, G.D.; Theiss, A.; Bhushan, B.; Lee, S.C. Anti-fouling properties of microstructured surfaces bio-inspired by rice leaves and butterfly wings. J. Colloid Interface Sci. 2014, 419, 114–133. [Google Scholar] [CrossRef]
- Bhadra, C.M.; Khanh Truong, V.; Pham, V.T.H.; Al Kobaisi, M.; Seniutinas, G.; Wang, J.Y.; Juodkazis, S.; Crawford, R.J.; Ivanova, E.P. Antibacterial titanium nano-patterned arrays inspired by dragonfly wings. Sci. Rep. 2015, 18, 16817. [Google Scholar] [CrossRef] [Green Version]
- Lim, T.K. Edible medicinal and non-medicinal plants. Modifed stems, roots, bulbs; Springer: London, UK, 2015; Volume 12. [Google Scholar]
- Neinhuis, C.; Barthlott, W. Characterization and distribution of water-repellent, self-cleaning plant surfaces. Ann. Bot. 1997, 79, 667–677. [Google Scholar] [CrossRef] [Green Version]
- Hüger, E.; Rothe, H.; Frant, M.; Grohmann, S.; Hildebrand, G.; Liefeith, K. Atomic force microscopy and thermodynamics on taro, a self-cleaning plant leaf. Appl. Phys. Lett. 2009, 95, 033702. [Google Scholar] [CrossRef]
- Bhushan, B.; Jung, Y.C. Wetting, adhesion and friction of superhydrophobic and hydrophilic leaves and fabricated micro/nanopatterned surfaces. J. Phys. Condens. Matter 2008, 20, 225010. [Google Scholar] [CrossRef] [Green Version]
- Vasudevan, R.; Kennedy, A.J.; Merritt, M.; Crocker, F.H.; Baney, R.H. Microscale patterned surfaces reduce bacterial fouling-microscopic and theoretical analysis. Colloids Surfaces B Biointerfaces 2014, 117, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Hochbaum, A.I.; Aizenberg, J. Bacteria pattern spontaneously on periodic nanostructure arrays. Nano Lett. 2010, 10, 3717–3721. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.C.; Siedlecki, C.A. Submicron-textured biomaterial surface reduces staphylococcal bacterial adhesion and biofilm formation. Acta Biomater. 2012, 8, 72–81. [Google Scholar] [CrossRef]
- Arango-Santander, S.; Pelaez-Vargas, A.; Freitas, S.C.; García, C. A novel approach to create an antibacterial surface using titanium dioxide and a combination of dip-pen nanolithography and soft lithography. Sci. Rep. 2018, 8, 15818. [Google Scholar] [CrossRef] [PubMed]
- Durán, A.; Conde, A.; Gómez Coedo, A.; Dorado, T.; García, C.; Ceré, S. Sol-gel coatings for protection and bioactivation of metals used in orthopaedic devices. J. Mater. Chem. 2004, 14, 2282–2290. [Google Scholar] [CrossRef] [Green Version]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Horcas, I.; Fernández, R.; Gómez-Rodríguez, J.M.; Colchero, J.; Gómez-Herrero, J.; Baro, A.M. WSXM: A software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instrum. 2007, 78, 013705. [Google Scholar] [CrossRef]
- Naghili, H.; Tajik, H.; Mardani, K.; Razavi Rouhani, S.M.; Ehsani, A.; Zare, P. Validation of drop plate technique for bacterial enumeration by parametric and nonparametric tests. Vet. Res. forum an Int. Q. J. 2013, 4, 179–183. [Google Scholar]
- Sfondrini, M.F.; Debiaggi, M.; Zara, F.; Brerra, R.; Comelli, M.; Bianchi, M.; Pollone, S.R.; Scribante, A. Influence of lingual bracket position on microbial and periodontal parameters in vivo. J Appl Oral Sci. 2012, 20, 357–361. [Google Scholar] [CrossRef] [Green Version]
- Türkkahraman, H.; Sayin, M.O.; Bozkurt, F.Y.; Yetkin, Z.; Kaya, S.; Onal, S. Archwire ligation techniques, microbial colonization, and periodontal status in orthodontically treated patients. Angle Orthod. 2005, 75, 231–236. [Google Scholar]
- Hepyukselen, B.G.; Cesur, M.G. Comparison of the microbial flora from different orthodontic archwires using a cultivation method and PCR: A prospective study. Orthod Craniofac Res. 2019, 22, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Pi, P.; Cai, Z.Q.; Wen, X.; Wang, X.; Cheng, J.; Yang, Z. Facile preparation of super-hydrophobic and super-oleophilic silica film on stainless steel mesh via sol-gel process. Appl. Surf. Sci. 2010, 256, 4095–4102. [Google Scholar] [CrossRef]
- Hosseinalipour, S.M.; Ershad-langroudi, A.; Hayati, A.N.; Nabizade-Haghighi, A.M. Characterization of sol-gel coated 316L stainless steel for biomedical applications. Prog. Org. Coatings 2010, 67, 371–374. [Google Scholar] [CrossRef]
- Santos, O.; Nylander, T.; Rosmaninho, R.; Rizzo, G.; Yiantsios, S.; Andritsos, N.; Karabelas, A.; Müller-Steinhagen, H.; Melo, L.; Boulangé-Petermann, L.; et al. Modified stainless steel surfaces targeted to reduce fouling - Surface characterization. J. Food Eng. 2004, 64, 63–79. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Y.; Chen, Y.; Gu, H. Improving endothelialization on 316L stainless steel through wettability controllable coating by sol-gel technology. Appl. Surf. Sci. 2013, 268, 73–78. [Google Scholar] [CrossRef]
- Herminghaus, S. Roughness-induced non-wetting. Europhys. Lett. 2000, 52, 165. [Google Scholar] [CrossRef]
- Burton, Z.; Bhushan, B. Surface characterization and adhesion and friction properties of hydrophobic leaf surfaces. Ultramicroscopy 2006, 106, 709–719. [Google Scholar] [CrossRef]
- Grewal, H.S.; Cho, I.J.; Yoon, E.S. The role of bio-inspired hierarchical structures in wetting. Bioinspiration Biomim. 2015, 10, 026009. [Google Scholar] [CrossRef]
- Kim, I.H.; Park, H.S.; Kim, Y.K.; Kim, K.H.; Kwon, T.Y. Comparative short-term in vitro analysis of mutans streptococci adhesion on esthetic, nickel-titanium, and stainless-steel arch wires. Angle Orthod. 2014, 84, 680–686. [Google Scholar] [CrossRef] [Green Version]
- Satou, J.; Fukunaga, A.; Satou, N.; Shintani, H.; Okuda, K. Streptococcal Adherence on Various Restorative Materials. J. Dent. Res. 1988, 67, 588–591. [Google Scholar] [CrossRef]
- Busscher, H.J.; van Pelt, A.W.J.; de Boer, P.; de Jong, H.P.; Arends, J. The effect of surface roughening of polymers on measured contact angles of liquids. Colloids and Surfaces 1984, 9, 319–331. [Google Scholar] [CrossRef]
- May, R.M.; Hoffman, M.G.; Sogo, M.J.; Parker, A.E.; O’Toole, G.A.; Brennan, A.B.; Reddy, S.T. Micro-patterned surfaces reduce bacterial colonization and biofilm formation in vitro: Potential for enhancing endotracheal tube designs. Clin. Transl. Med. 2014, 3, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Group | CA (°) | Ra (nm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Min | Median | Max | Mean | SD | Min | Median | Max | |
| SS316L_c | 76.8 | 6.9 | 64.0 | 79.5 | 83.0 | 102.8 | 28.7 | 64.0 | 100.5 | 162.0 |
| SS316L_e | 110.8 | 7.1 | 96.0 | 113.5 | 118.0 | 140.4 | 65.2 | 54.0 | 134.0 | 251.0 |
| NiTi_c | 94.9 | 2.2 | 90.5 | 95.5 | 97.5 | 34.1 | 4.9 | 29.2 | 31.7 | 42.8 |
| NiTi_e | 129.4 | 4.8 | 120.2 | 130.8 | 135.4 | 142.3 | 31.9 | 119.9 | 125.1 | 204.5 |
| Group | 2h | 6h | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Min | Median | Max | Mean | SD | Min | Median | Max | |
| SS316L_c | 4.1 × 106 | 6.3 × 105 | 3.4 × 106 | 4.2 × 106 | 5.0 × 106 | 4.5 × 106 | 8.6 × 105 | 3.2 × 106 | 4.6 × 106 | 5.6 × 106 |
| SS316L_e | 5.3 × 105 | 1.5 × 105 | 3.5 × 105 | 5.0 × 105 | 7.5 × 105 | 8.5 × 105 | 1.5 × 105 | 6.5 × 105 | 8.0 × 105 | 1.0 × 106 |
| NiTi_c | 2.2 × 106 | 9.9 × 105 | 1.4 × 106 | 1.9 × 106 | 3.8 × 106 | 3.0 × 106 | 5.0 × 105 | 2.3 × 106 | 3.0 × 106 | 3.6 × 106 |
| NiTi_e | 1.1 × 106 | 7.4 × 105 | 3.0 × 105 | 9.0 × 105 | 2.3 × 106 | 1.1 × 106 | 4.3 × 105 | 3.5 × 105 | 1.3 × 106 | 1.5 × 106 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arango-Santander, S.; Gonzalez, C.; Aguilar, A.; Cano, A.; Castro, S.; Sanchez-Garzon, J.; Franco, J. Assessment of Streptococcus Mutans Adhesion to the Surface of Biomimetically-Modified Orthodontic Archwires. Coatings 2020, 10, 201. https://doi.org/10.3390/coatings10030201
Arango-Santander S, Gonzalez C, Aguilar A, Cano A, Castro S, Sanchez-Garzon J, Franco J. Assessment of Streptococcus Mutans Adhesion to the Surface of Biomimetically-Modified Orthodontic Archwires. Coatings. 2020; 10(3):201. https://doi.org/10.3390/coatings10030201
Chicago/Turabian StyleArango-Santander, Santiago, Carolina Gonzalez, Anizac Aguilar, Alejandro Cano, Sergio Castro, Juliana Sanchez-Garzon, and John Franco. 2020. "Assessment of Streptococcus Mutans Adhesion to the Surface of Biomimetically-Modified Orthodontic Archwires" Coatings 10, no. 3: 201. https://doi.org/10.3390/coatings10030201






