Increasing the Efficiency of Dye-Sensitized Solar Cells by Adding Nickel Oxide Nanoparticles to Titanium Dioxide Working Electrodes
Abstract
:1. Introduction
2. Experiments
2.1. Preparing the Materials
2.2. Preparing the DCCS Devices
2.3. Characteristics of the Working Electrode and the DSSC Devices
3. Results and Discussion
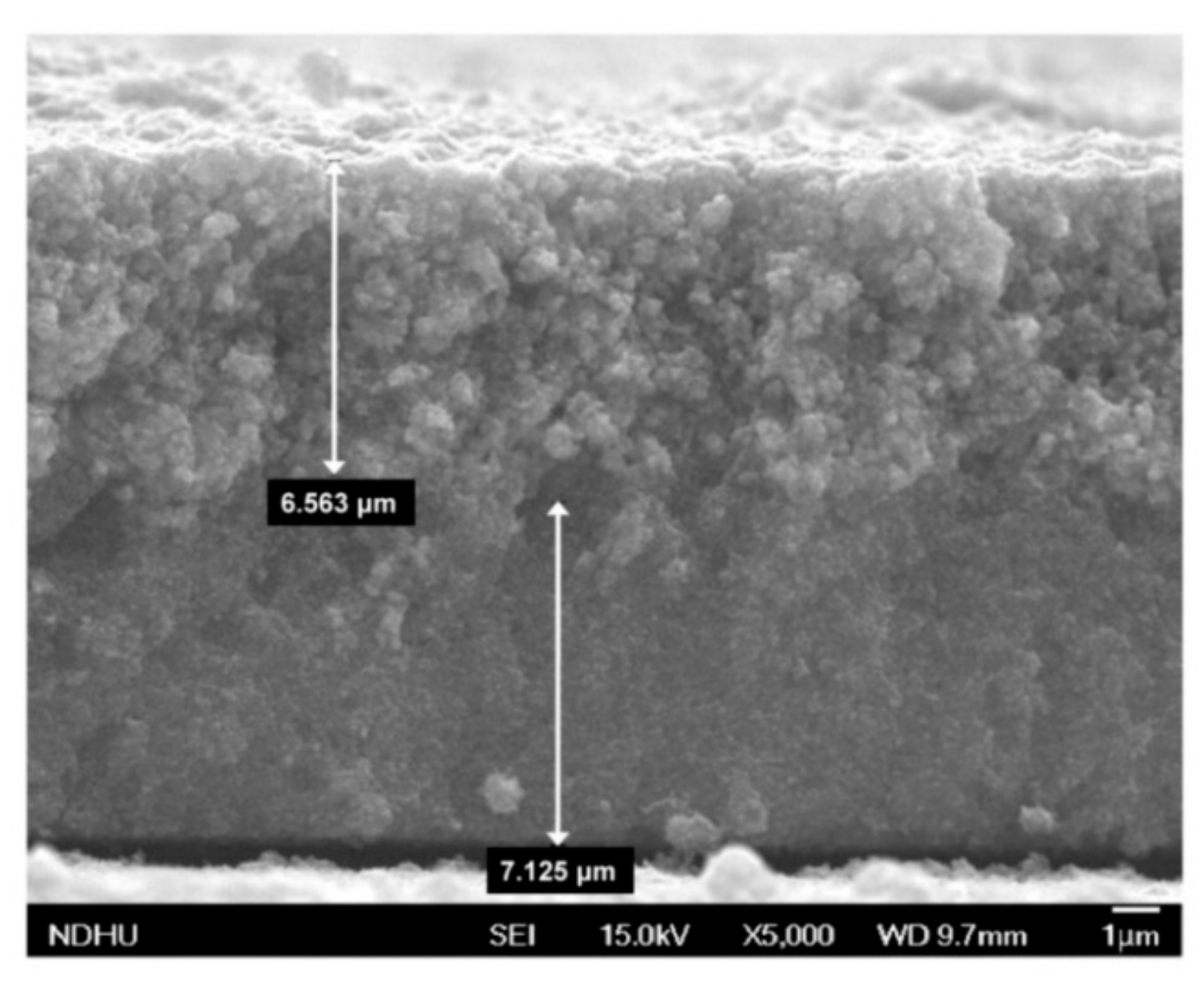
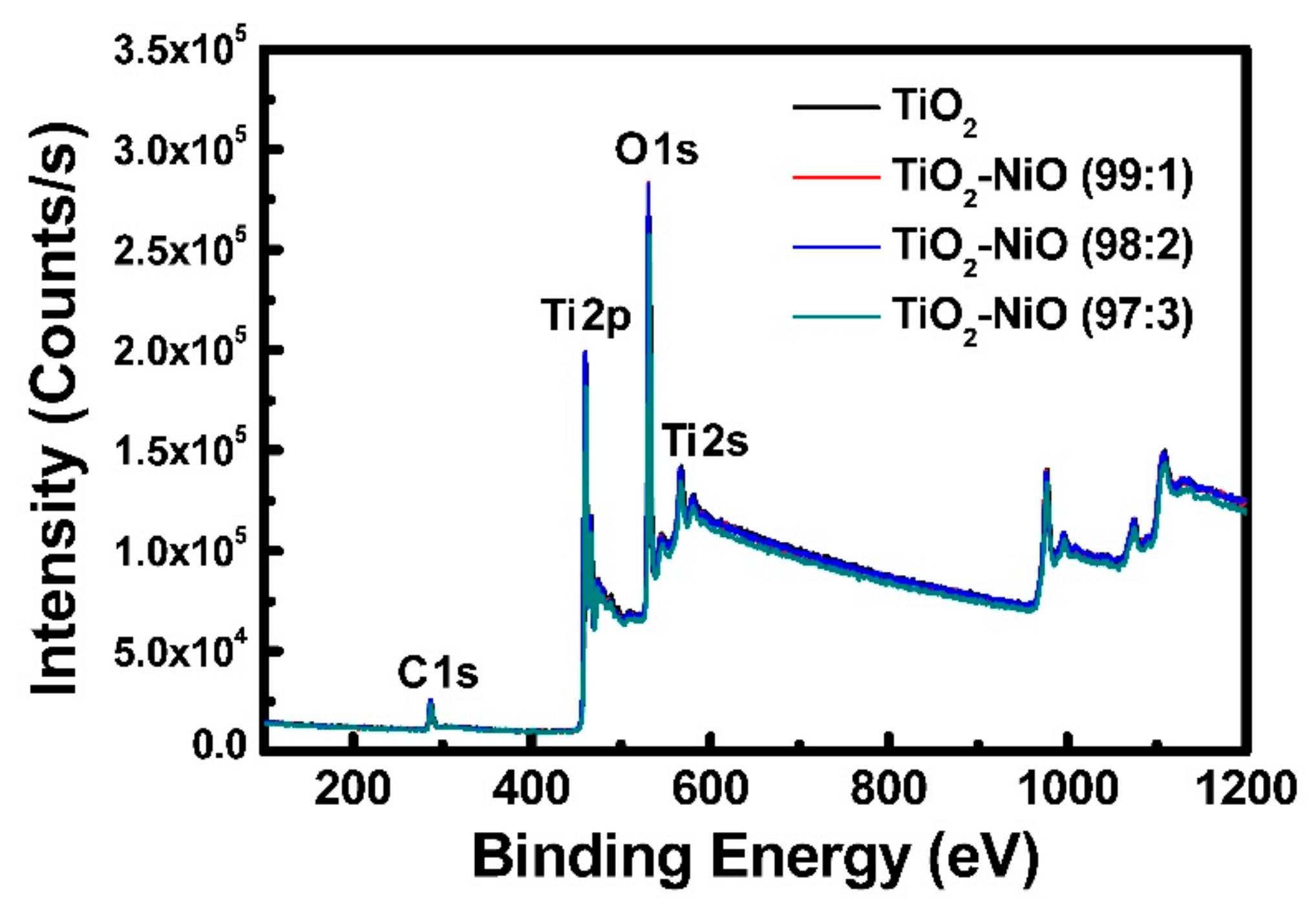
3.1. Characteristics of the Working Electrode
3.2. Characteristics of the DSSC Devices
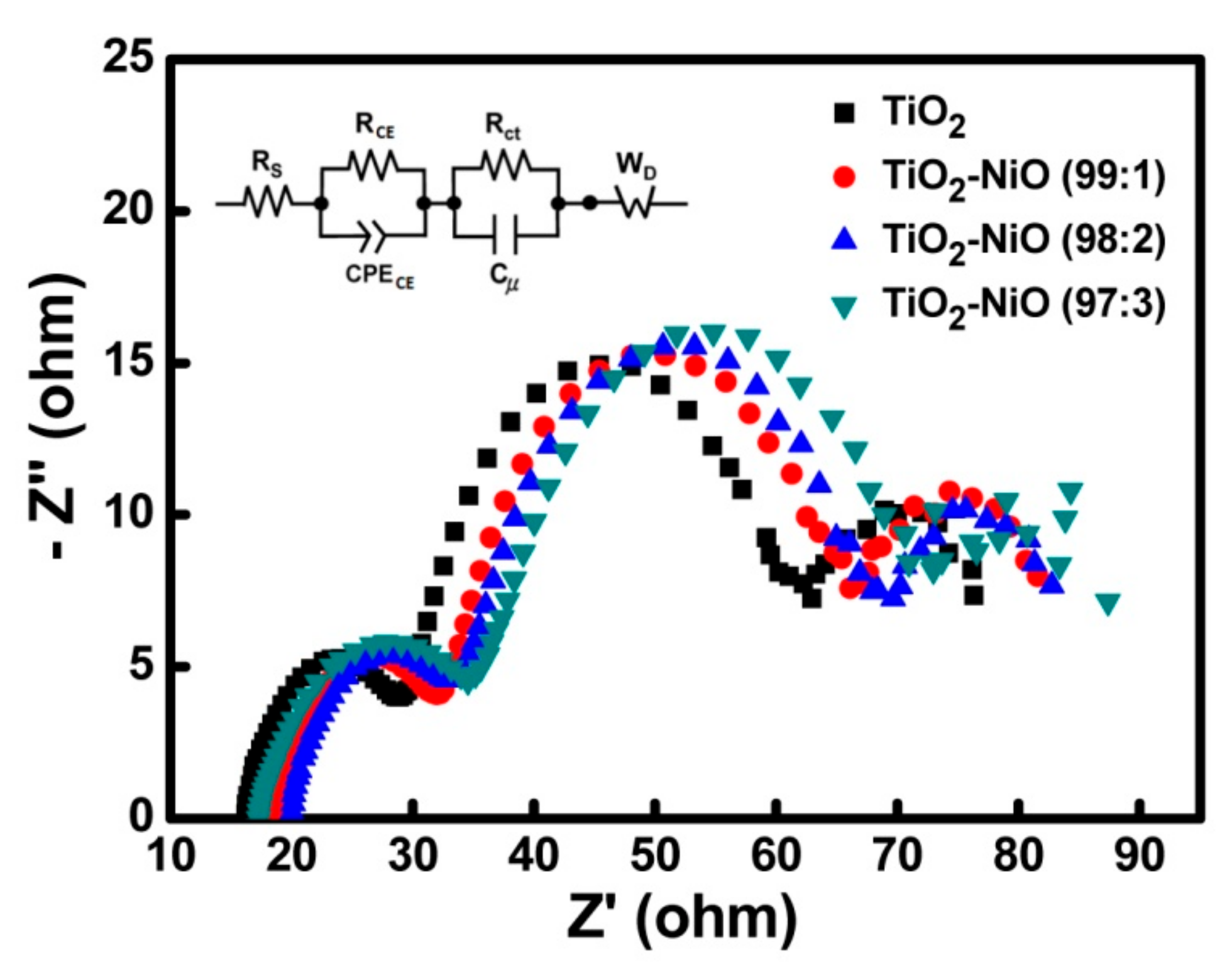
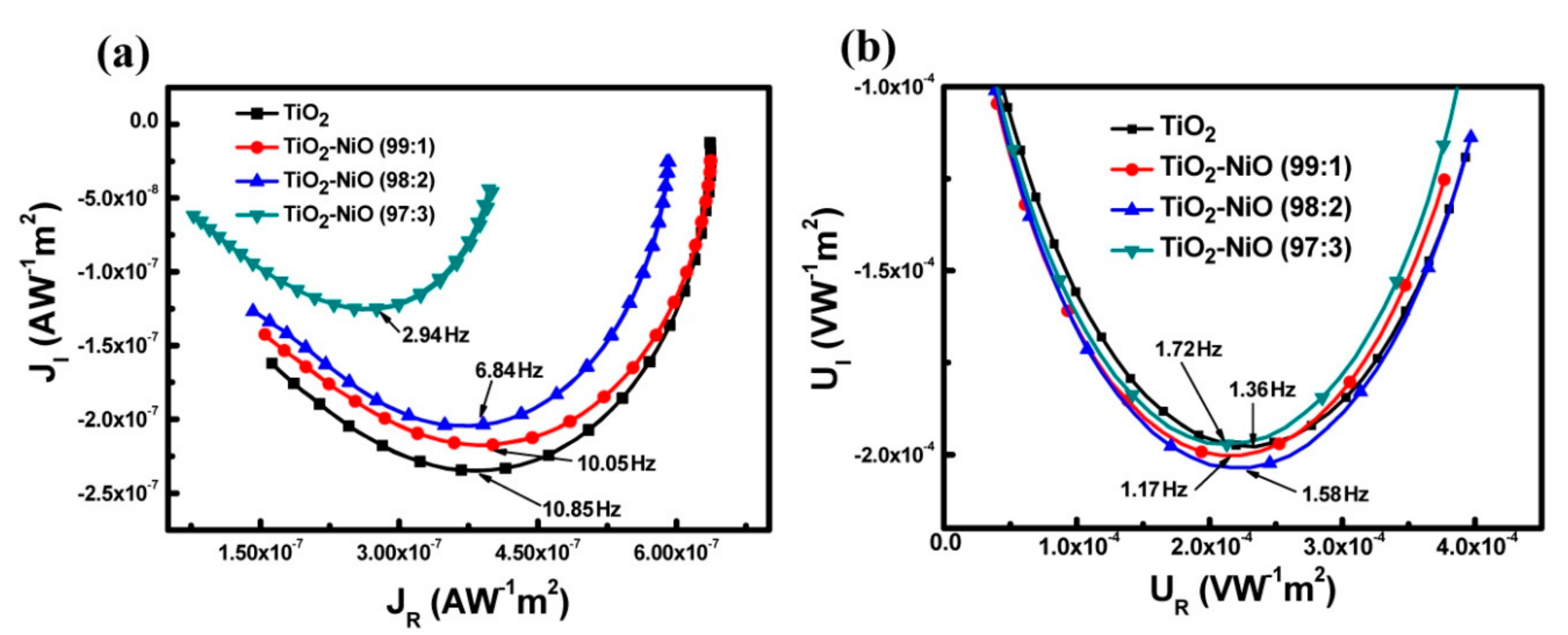
3.3. Characteristics of the Electron Transport
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar]
- Hagfeldt, A.; Grätzel, M. Light-induced redox reactions in nanocrystalline systems. Chem. Rev. 1995, 95, 49–68. [Google Scholar] [CrossRef]
- Jiu, J.; Isoda, S.; Wang, F.; Adachi, M. Dye-sensitized solar cells based on a single-crystalline TiO2 nanorod film. J. Phys. Chem. B 2006, 110, 2087–2092. [Google Scholar] [CrossRef] [PubMed]
- Nazeeruddin, M.K.; Humphry-Baker, R.; Liska, P.; Grätzel, M. Investigation of sensitizer adsorption and the influence of protons on current and voltage of a dye-sensitized nanocrystalline TiO2 solar cell. J. Phys. Chem. B 2003, 107, 8981–8987. [Google Scholar] [CrossRef]
- Grätzel, M. Photoelectrochemical cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Hagfeldt, A.; Grätzel, M. Molecular photovoltaics. Acc. Chem. Res. 2000, 33, 269–277. [Google Scholar] [CrossRef] [Green Version]
- Ting, H.C.; Tsai, C.H.; Chen, C.H.; Lin, L.Y.; Chou, S.H.; Wong, K.T.; Huang, T.W.; Wu, C.C. A novel amine-free dianchoring organic dye for efficient dye-sensitized solar cells. Org. Lett. 2012, 14, 6338–6341. [Google Scholar] [CrossRef]
- Bessho, T.; Zakeeruddin, S.M.; Yeh, C.Y.; Diau, E.W.G.; Grätzel, M. Highly efficient mesoscopic dye-sensitized solar cells based on donor–acceptor-substituted porphyrins. Angew. Chem. Int. Ed. 2010, 49, 6646–6649. [Google Scholar] [CrossRef]
- Chiba, Y.; Islam, A.; Watanabe, Y.; Komiya, R.; Koide, N.; Han, L. Dye-sensitized solar cells with conversion efficiency of 11.1%. Jpn. J. Appl. Phys. 2006, 45, L638. [Google Scholar] [CrossRef]
- Nazeeruddin, M.K.; Zakeeruddin, S.M.; Humphry-Baker, R.; Jirousek, M.; Liska, P.; Vlachopoulos, N.; Shklover, V.; Fischer, C.-H.; Grätzel, M. Acid−base equilibria of (2,2‘-Bipyridyl-4,4‘-dicarboxylic acid)ruthenium(II) complexes and the effect of protonation on charge-transfer sensitization of nanocrystalline titania. Inorg. Chem. 1999, 38, 6298–6305. [Google Scholar] [CrossRef]
- Chen, C.Y.; Wang, M.; Li, J.Y.; Pootrakulchote, N.; Alibabaei, L.; Ngoc-le, C.; Decoppet, J.; Tsai, J.; Grätzel, C.; Wu, C.G.; et al. Highly efficient light-harvesting ruthenium sensitizer for thin-film dye-sensitized solar cells. ACS Nano 2009, 3, 3103–3109. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.L.; Teng, H.; Lee, Y.L. In situ gelation of electrolytes for highly efficient gel-state dye-sensitized solar cells. Adv. Mater. 2011, 23, 4199–4204. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.H.; Hsu, S.Y.; Lu, C.Y.; Tsai, Y.T.; Huang, T.W.; Chen, Y.F.; Jhang, Y.H.; Wu, C.C. Influences of textures in Pt counter electrode on characteristics of dye-sensitized solar cells. Org. Electron. 2012, 13, 199–205. [Google Scholar] [CrossRef]
- Zhang, Q.; Dandeneau, C.S.; Zhou, X.; Cao, G. ZnO nanostructures for dye-sensitized solar cells. Adv. Mater. 2009, 21, 4087–4108. [Google Scholar] [CrossRef]
- Duong, T.T.; Choi, H.J.; He, Q.J.; Le, A.T.; Yoon, S.G. Enhancing the efficiency of dye sensitized solar cells with an SnO2 blocking layer grown by nanocluster deposition. J. Alloys. Compd. 2013, 561, 206–210. [Google Scholar] [CrossRef]
- Congiu, M.; Marco, M.L.D.; Bonomo, M.; Nunes-Neto, O.; Dini, D.; Graeff, C.F.O. Pristine and Al-doped hematite printed films as photoanodes of p-type dye-sensitized solar cells. J. Nanopart. Res. 2017, 19, 7. [Google Scholar] [CrossRef]
- Barea, E.; Xu, X.; González-Pedro, V.; Ripollés-Sanchis, T.; Fabregat-Santiago, F.; Bisquert, J. Origin of efficiency enhancement in Nb2O5 coated titanium dioxide nanorod based dye sensitized solar cells. Energy Environ. Sci. 2011, 4, 3414–3419. [Google Scholar] [CrossRef] [Green Version]
- Memming, R.; Tributsch, H. Electrochemical investigations on the spectral sensitization of gallium phosphide electrodes. J. Phys. Chem. 1971, 75, 562–570. [Google Scholar] [CrossRef]
- Wang, Z.S.; Kawauchi, H.; Kashima, T.; Arakawa, H. Significant influence of TiO2 photoelectrode morphology on the energy conversion efficiency of N719 dye-sensitized solar cell. Coord. Chem. Rev. 2004, 248, 1381–1389. [Google Scholar] [CrossRef]
- Yoon, J.H.; Jang, S.R.; Vittal, R.; Lee, J.; Kim, K.J. TiO2 nanorods as additive to TiO2 film for improvement in the performance of dye-sensitized solar cells. J. Photochem. Photobiol. A. 2006, 180, 184–188. [Google Scholar] [CrossRef]
- Liu, B.; Aydil, E.S. Growth of oriented single-crystalline rutile TiO2 nanorods on transparent conducting substrates for dye-sensitized solar cells. J. Am. Chem. Soc. 2009, 131, 3985–3990. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Shankar, K.; Varghese, O.K.; Paulose, M.; Latempa, T.J.; Grimes, C.A. Vertically aligned single crystal TiO2 nanowire arrays grown directly on transparent conducting oxide coated glass: Synthesis details and applications. Nano Lett. 2008, 8, 37813786. [Google Scholar]
- Mor, G.K.; Shankar, K.; Paulose, M.; Varghese, O.K.; Grimes, C.A. Use of highly-ordered TiO2 nanotube arrays in dye-sensitized solar cells. Nano Lett. 2006, 6, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Yao, Y.; Huang, T.; Yu, A. Solvothermal growth of well-aligned TiO2 nanowire arrays for dye-sensitized solar cell: Dependence of morphology and vertical orientation upon substrate pretreatment. Int. J. Electrochem. Sci. 2011, 6, 1871–1879. [Google Scholar]
- Li, Z.; Yu, L. The Size Effect of TiO2 hollow microspheres on photovoltaic performance of ZnS/CdS quantum dots sensitized solar cell. Materials 2019, 12, 1583. [Google Scholar] [CrossRef] [Green Version]
- Mahmoudabadi, Z.D.; Eslami, E. One-step synthesis of CuO/TiO2 nanocomposite by atmospheric microplasma electrochemistry – Its application as photoanode in dye-sensitized solar cell. J. Alloys Compd. 2019, 793, 336–342. [Google Scholar] [CrossRef]
- Chava, R.K.; Lee, W.M.; Oh, S.Y.; Jeong, K.U.; Yu, Y.T. Improvement in light harvesting and device performance of dye sensitized solar cells using electrophoretic deposited hollow TiO2 NPs scattering layer. Sol. Energ. Mat. Sol. C. 2017, 161, 255–262. [Google Scholar] [CrossRef]
- Haque, S.A.; Palomares, E.; Cho, B.M.; Green, A.N.M.; Hirata, N.; Klug, D.R.; Durrant, J.R. Charge separation versus recombination in dye-sensitized nanocrystalline solar cells: The minimization of kinetic redundancy. J. Am. Chem. Soc. 2004, 127, 3456–3462. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, S.; Zhao, H.; Will, G.; Liu, P. An efficient and low-cost TiO2 compact layer for performance improvement of dye-sensitized solar cells. Electrochim. Acta 2009, 54, 1319–1324. [Google Scholar] [CrossRef] [Green Version]
- Chou, C.S.; Yang, R.Y.; Yeh, C.K.; Lin, Y.J. Preparation of TiO2/Nano-metal composite particles and their applications in dye-sensitized solar cells. Powder Technol. 2009, 194, 95–105. [Google Scholar] [CrossRef]
- Habibi, M.H.; Karimi, B.; Zendehdel, M.; Habibi, M. Fabrication, characterization of two nano-composite CuO–ZnO working electrodes for dye-sensitized solar cell. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2013, 116, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Din, M.I.; Rani, A. Recent advances in the synthesis and stabilization of nickel and nickel oxide nanoparticles: A green adeptness. Int. J. Anal. Chem. 2016, 2016, 3512145. [Google Scholar]
- Danial, A.S.; Saleh, M.M.; Salih, S.A.; Awad, M.I. On the synthesis of nickel oxide nanoparticles by sol–gel technique and its electrocatalytic oxidation of glucose. J. Power Sources 2015, 293, 101–108. [Google Scholar] [CrossRef]
- El-Kemary, M.; Nagy, N.; El-Mehasseb, I. Nickel oxide nanoparticles: Synthesis and spectral studies of interactions with glucose. Mater. Sci. Semicond. Process. 2013, 16, 1747–1752. [Google Scholar] [CrossRef]
- Bonomo, M. Synthesis and characterization of NiO nanostructures: A review. J. Nanopart. Res. 2018, 20, 222. [Google Scholar] [CrossRef]
- Bonomo, M.; Mariani, P.; Mura, F.; Carlo, A.D.; Dini, D. Nanocomposites of nickel oxide and zirconia for the preparation of photocathodes with improved performance in p-type dye-sensitized solar cells. J. Electrochem. Soc. 2019, 166, D290–D300. [Google Scholar] [CrossRef]
- Bandara, J.; Pradeep, U.W.; Bandara, R.G.S.J. The role of n–p junction electrodes in minimizing the charge recombination and enhancement of photocurrent and photovoltage in dye sensitized solar cells. J. Photochem. Photobiol. A 2005, 170, 273–278. [Google Scholar] [CrossRef]
- Liu, B.; Sun, Y.; Wang, X.; Zhang, L.; Wang, D.; Fu, Z.; Lin, Y.; Xie, T. Branched hierarchical photoanode of anatase TiO2 nanotubes on rutile TiO2 nanorod arrays for efficient quantum dot-sensitized solar cells. J. Mater. Chem. A 2015, 3, 4445–4452. [Google Scholar] [CrossRef]
- Chae, S.Y.; Hwang, Y.J.; Joo, O.S. Role of HA additive in quantum dot solar cell with Co[(bpy)3]2+/3+-based electrolyte. RSC Adv. 2014, 4, 26907–26911. [Google Scholar] [CrossRef]
- Hsu, S.H.; Li, C.T.; Chien, H.T.; Salunkhe, R.R.; Suzuki, N.; Yamauchi, Y.; Ho, K.C.; Wu, K.C.W. Platinum-free counter electrode comprised of metal-organic-framework (MOF)-derived cobalt sulfide nanoparticles for efficient dye-sensitized solar cells (DSSCs). Sci. Rep. 2014, 4, 6983. [Google Scholar] [CrossRef]
- Dharmaraj, N.; Prabu, P.; Nagarajan, S.; Kim, C.H.; Park, J.H.; Kim, H.Y. Synthesis of nickel oxide nanoparticles using nickel acetate and poly(vinyl acetate) precursor. Mater. Sci. Eng. B 2006, 128, 111–114. [Google Scholar] [CrossRef]
- Mansour, A.N. Characterization of NiO by XPS. Surf. Sci. Spectra 1994, 3, 231. [Google Scholar] [CrossRef]
- Diebold, U.; Madey, T.E. TiO2 by XPS. Surf. Sci. Spectra 1996, 4, 227. [Google Scholar] [CrossRef]
- Chou, C.S.; Lin, Y.J.; Yang, R.Y.; Liu, K.H. Preparation of TiO2/NiO composite particles and their applications in dye-sensitized solar cells. Adv. Powder Technol. 2011, 22, 31–42. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef]
- Liao, J.Y.; Lei, B.X.; Kuang, D.B.; Su, C.Y. Tri-functional hierarchical TiO2 spheres consisting of anatase nanorods and nanoparticles for high efficiency dye-sensitized solar cells. Energy Environ. Sci. 2011, 4, 4079–4085. [Google Scholar] [CrossRef]
- Fakharuddin, A.; Ahmed, I.; Khalidin, Z.; Yusoff, M.M.; Jose, R. Channeling of electron transport to improve collection efficiency in mesoporous titanium dioxide dye sensitized solar cell stacks. Appl. Phys. Lett. 2014, 104, 053905. [Google Scholar] [CrossRef]
- Liu, T.; Yu, K.; Gao, L.; Chen, H.; Wang, N.; Hao, L.; Li, T.; He, H.; Guo, Z. A graphene quantum dot decorated SrRuO3 mesoporous film as an efficient counter electrode for high-performance dye-sensitized solar cells. J. Mater. Chem. A 2017, 5, 17848–17855. [Google Scholar] [CrossRef]





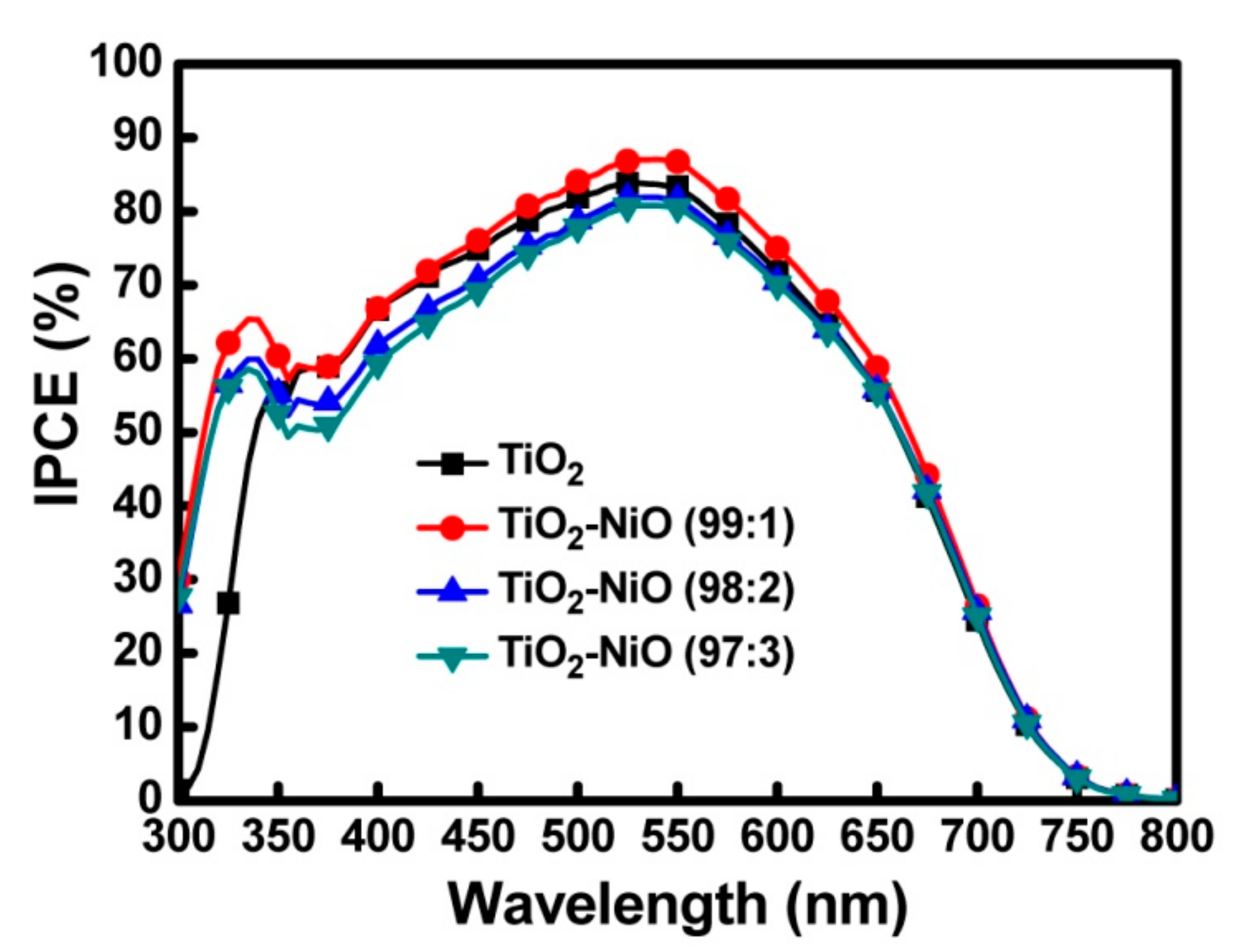

| Working Electrode | JSC (mA/cm2) | VOC (V) | Fill Factor | PCE (%) |
|---|---|---|---|---|
| TiO2 | 16.49 ± 0.05 | 0.73 ± 0.01 | 0.64 | 7.70 ± 0.13 |
| TiO2–NiO (99:1) | 17.15 ± 0.05 | 0.73 ± 0.01 | 0.67 | 8.39 ± 0.14 |
| TiO2–NiO (98:2) | 15.69 ± 0.04 | 0.73 ± 0.01 | 0.67 | 7.67 ± 0.13 |
| TiO2–NiO (97:3) | 15.34 ± 0.05 | 0.73 ± 0.01 | 0.66 | 7.39 ± 0.12 |
| Working Electrode | RS (ohm) | RCE (ohm) | Rct (ohm) | WD (ohm) |
|---|---|---|---|---|
| TiO2 | 16.12 | 10.74 | 29.94 | 13.28 |
| TiO2–NiO (99:1) | 18.32 | 11.04 | 30.44 | 13.73 |
| TiO2–NiO (98:2) | 19.61 | 11.26 | 31.48 | 14.14 |
| TiO2–NiO (97:3) | 17.15 | 11.78 | 32.50 | 14.39 |
| Working Electrode | τd (ms) | τn (ms) | D (m2/s) | ηcc |
|---|---|---|---|---|
| TiO2 | 14.67 | 116.53 | 3.19 × 10−9 | 0.87 |
| TiO2–NiO (99:1) | 15.84 | 135.86 | 2.96 × 10−9 | 0.88 |
| TiO2–NiO (98:2) | 23.25 | 100.42 | 2.01 × 10−9 | 0.77 |
| TiO2–NiO (97:3) | 54.09 | 92.56 | 8.66 × 10−10 | 0.42 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, C.-H.; Lin, C.-M.; Liu, Y.-C. Increasing the Efficiency of Dye-Sensitized Solar Cells by Adding Nickel Oxide Nanoparticles to Titanium Dioxide Working Electrodes. Coatings 2020, 10, 195. https://doi.org/10.3390/coatings10020195
Tsai C-H, Lin C-M, Liu Y-C. Increasing the Efficiency of Dye-Sensitized Solar Cells by Adding Nickel Oxide Nanoparticles to Titanium Dioxide Working Electrodes. Coatings. 2020; 10(2):195. https://doi.org/10.3390/coatings10020195
Chicago/Turabian StyleTsai, Chih-Hung, Chia-Ming Lin, and Yen-Cheng Liu. 2020. "Increasing the Efficiency of Dye-Sensitized Solar Cells by Adding Nickel Oxide Nanoparticles to Titanium Dioxide Working Electrodes" Coatings 10, no. 2: 195. https://doi.org/10.3390/coatings10020195




