Design of Corrosion Protective and Antistatic Hybrid Sol-Gel Coatings on 6XXX AlMgSi Alloys for Aerospace Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Sols
2.3. Substrate Preparation and Coating Deposition
2.4. Characterization
3. Results and Discussion
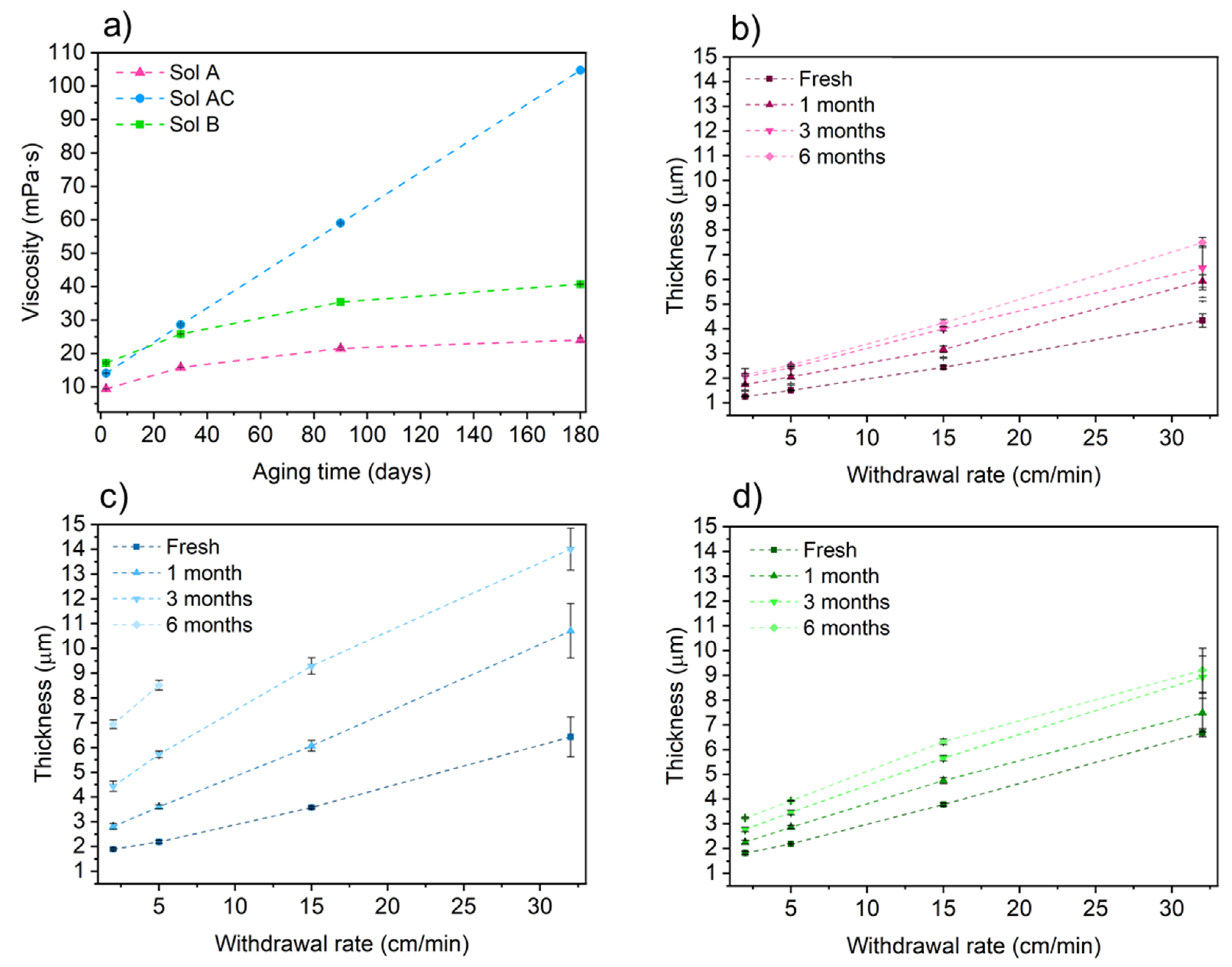
3.1. Effect of Solid Content and Organic/Inorganic Ratio in Viscosity and Thickness
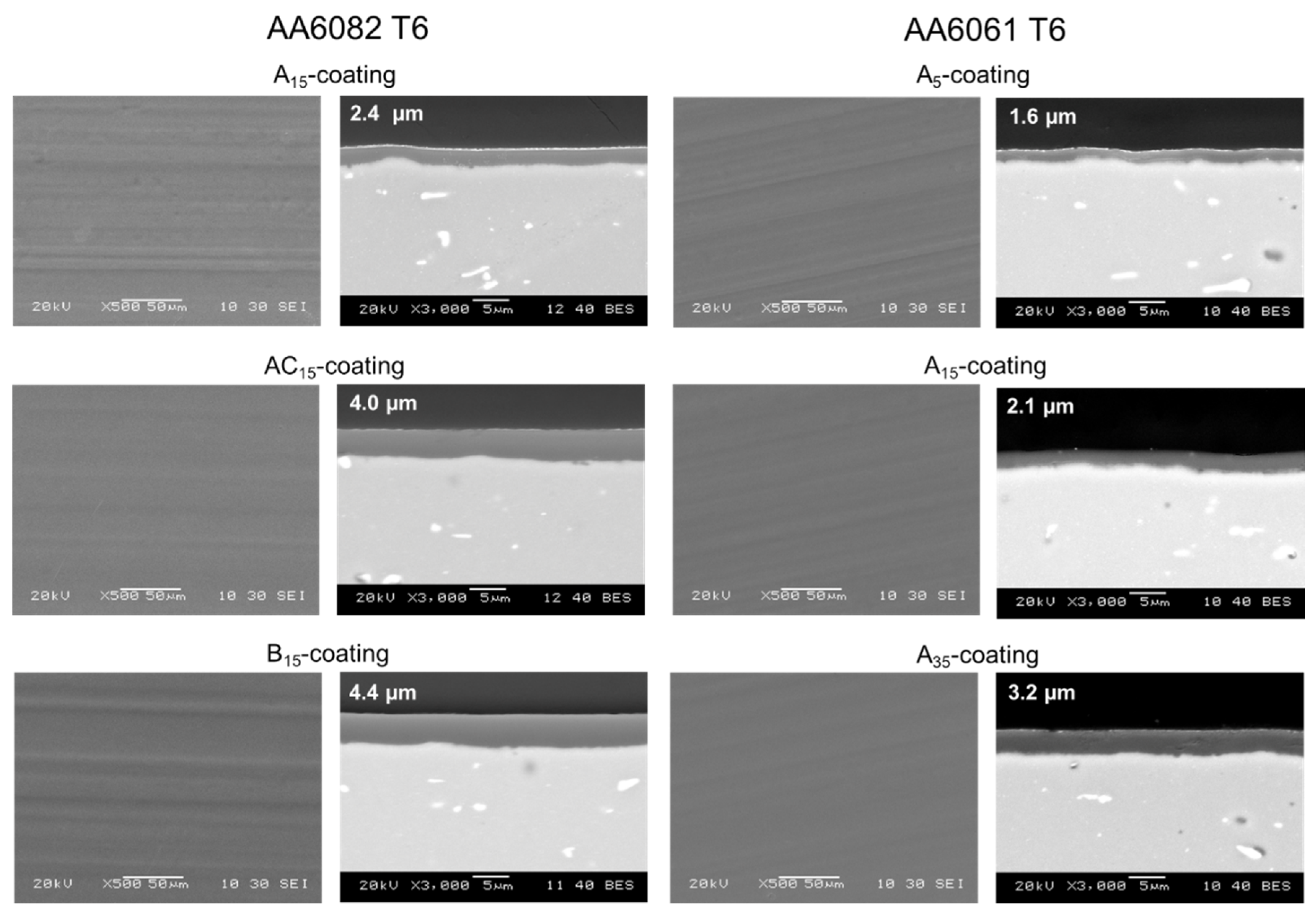
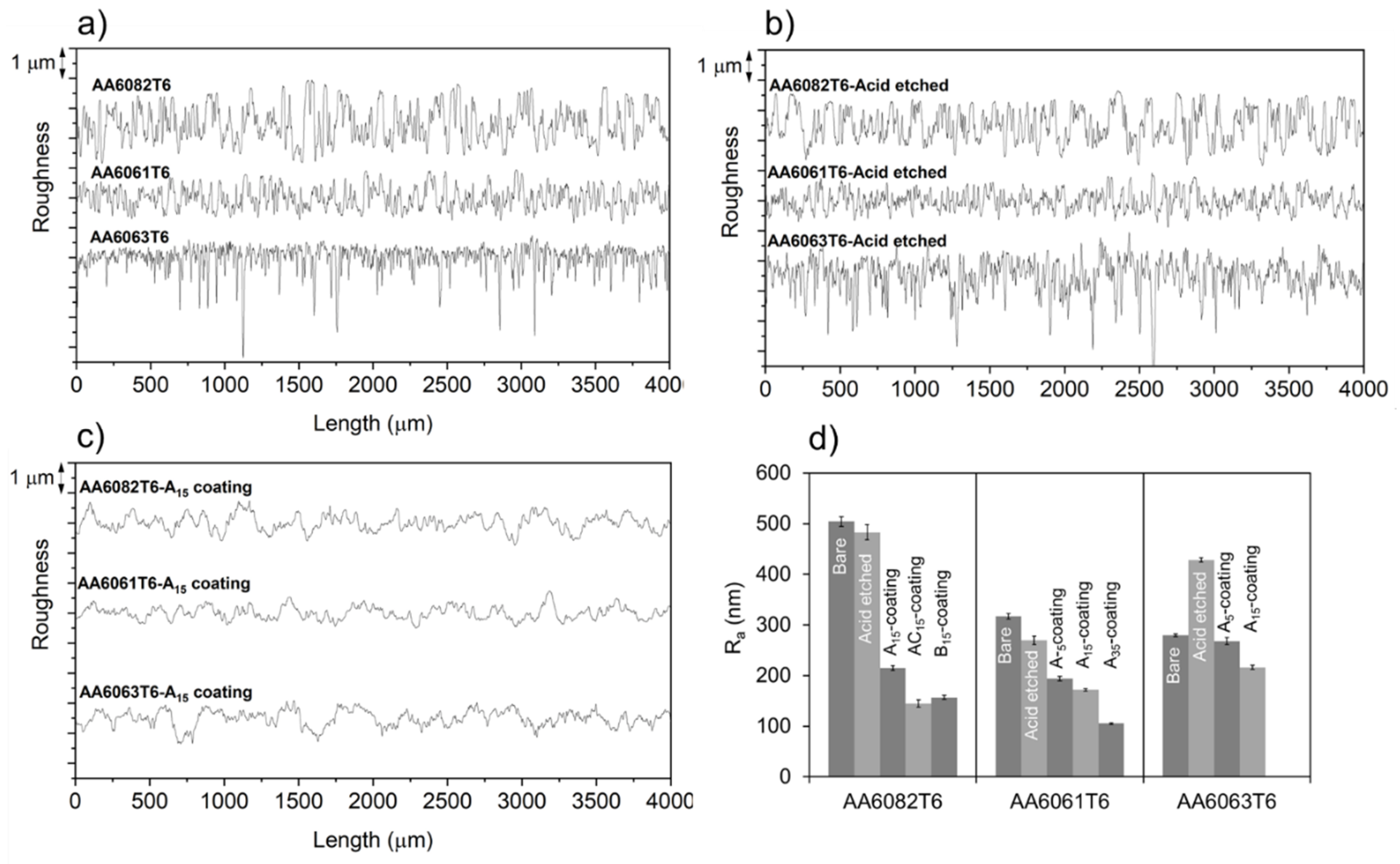
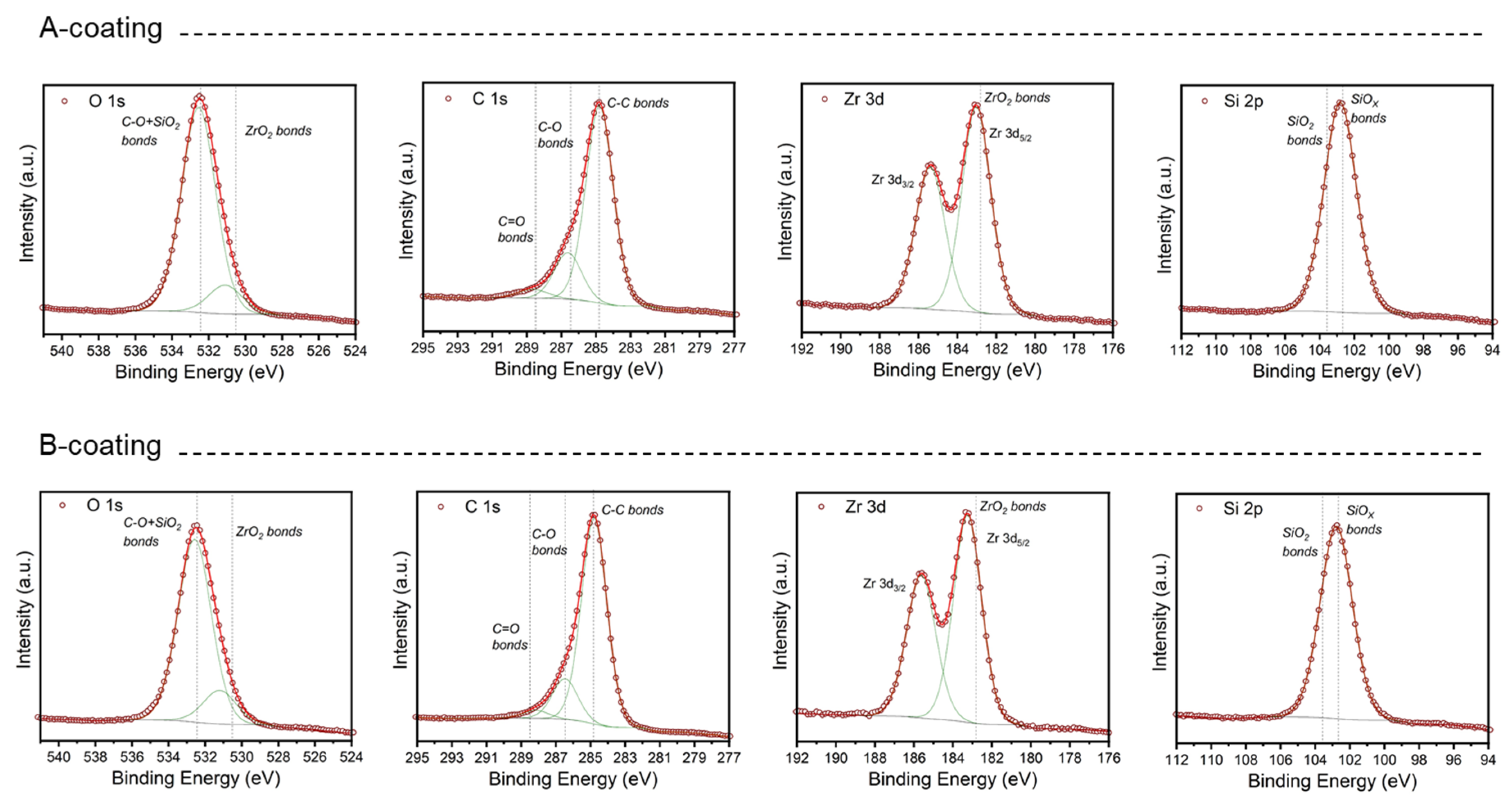
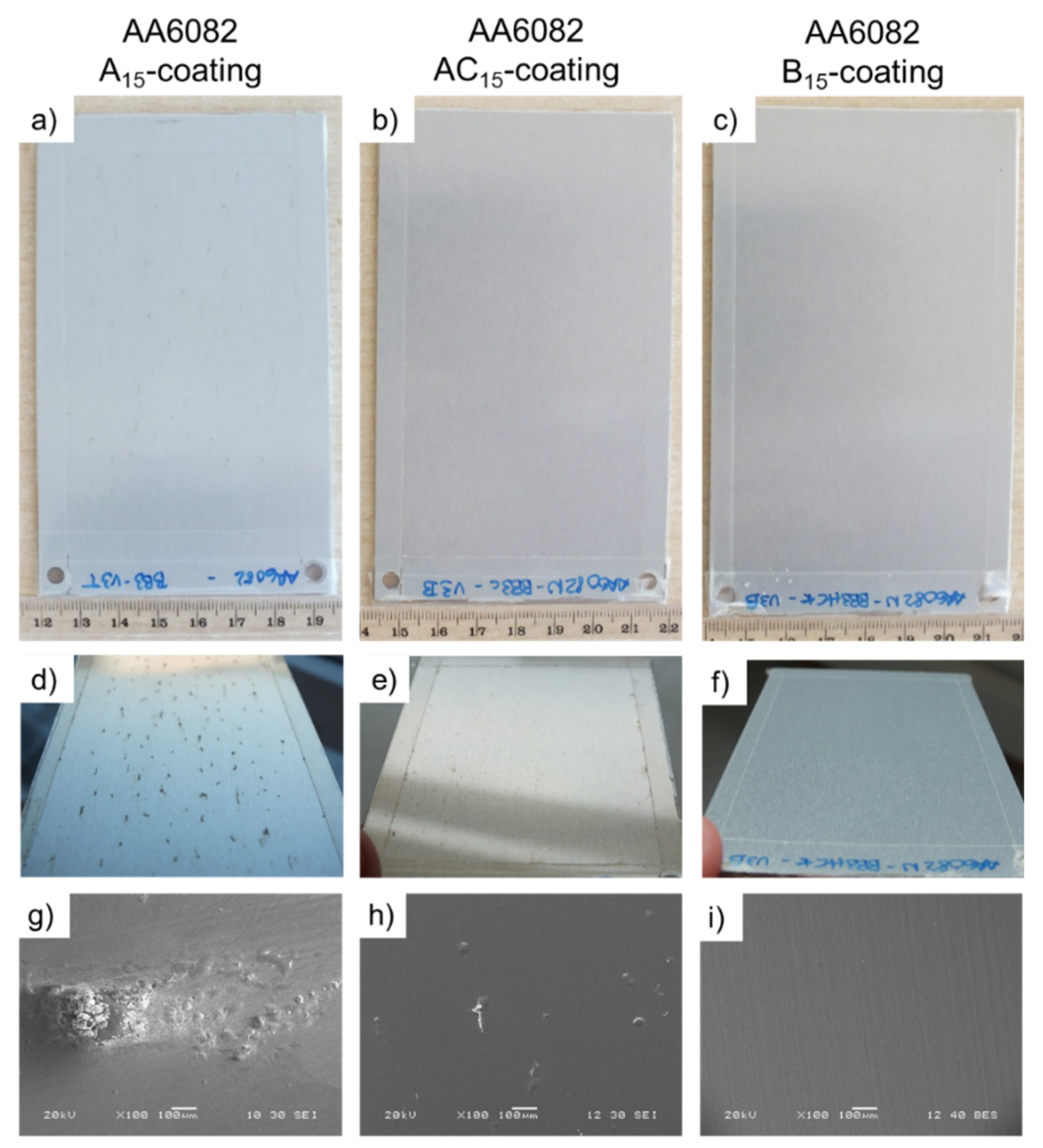
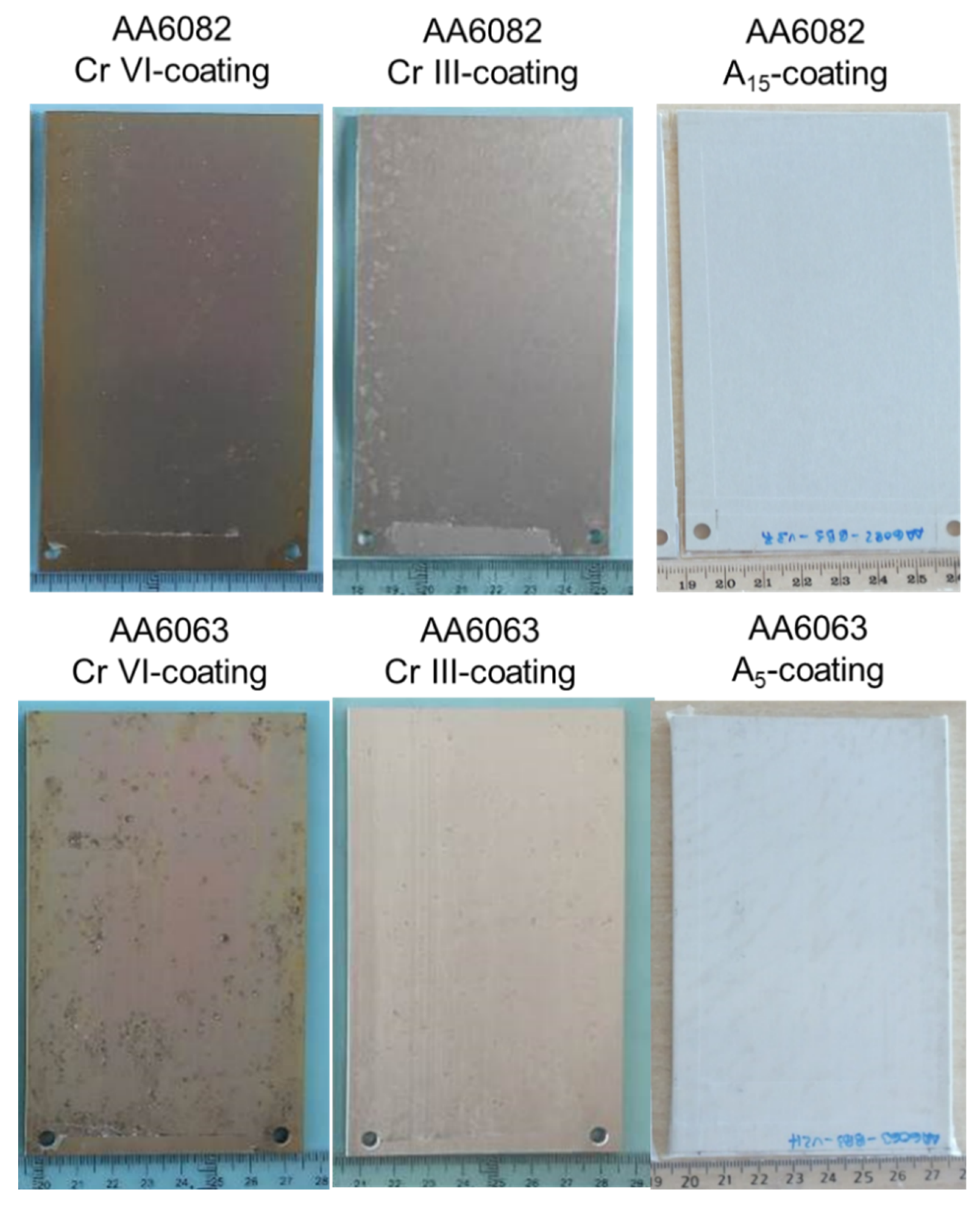
3.2. Coating Composition, Appearance and Roughness
3.3. Contact Electrical Resistance
3.4. Electrochemical Characterization
3.5. Durability Assessment
3.6. Vacuum-Induced Outgassing
4. Conclusions
5. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mondolfo, L.F. Aluminum Alloys: Structure and Properties; Butterworths & Co. Ltd.: London, UK, 1976. [Google Scholar]
- Larsen, M.H.; Walmsley, J.C.; Lunder, O.; Nisancioglu, K. Effect of excess silicon and small copper content on intergranular corrosion of 6000-Series aluminum alloys. J. Electrochem. Soc. 2010, 157, C61. [Google Scholar] [CrossRef]
- Totten, G.E.; MacKenzie, D.S. Handbook of Aluminum; Marcel Dekker Inc.: New York, NY, USA, 2003; ISBN 9780203912591. [Google Scholar]
- Kairy, S.K.; Alam, T.; Rometsch, P.A.; Davies, C.H.J.; Banerjee, R.; Birbilis, N. Understanding the origins of intergranular corrosion in copper-containing Al-Mg-Si alloys. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2016, 47, 985–989. [Google Scholar] [CrossRef]
- Svenningsen, G.; Lein, J.E.; Bjørgum, A.; Nordlien, J.H.; Yu, Y.; Nisancioglu, K. Effect of low copper content and heat treatment on intergranular corrosion of model AlMgSi alloys. Corros. Sci. 2006, 48, 226–242. [Google Scholar] [CrossRef]
- He, C.; Li, R.; Meng, X.; Lü, S.; Xie, L.; Ma, G.; Wang, J. Effect of Quenching Condition on Corrosion Behavior of 6063 Al Alloy. Int. Conf. Manuf. Sci. Eng. 2015, 1873–1876. [Google Scholar]
- Nazeer, A.A.; Madkour, M. Potential use of smart coatings for corrosion protection of metals and alloys: A review. J. Mol. Liq. 2018, 253, 11–22. [Google Scholar] [CrossRef]
- Santa Coloma, P.; Izagirre, U.; Belaustegi, Y.; Jorcin, J.B.; Cano, F.J.; Lapeña, N. Chromium-free conversion coatings based on inorganic salts (Zr/Ti/Mn/Mo) for aluminum alloys used in aircraft applications. Appl. Surf. Sci. 2015, 345, 24–35. [Google Scholar] [CrossRef] [Green Version]
- Hoebbel, D.; Nacken, M.; Schmidt, H. A NMR study on the hydrolysis, condensation and epoxide ring-opening reaction in sols and gels of the system glycidoxypropyltrimethoxysilane-water-titaniumtetraethoxide. J. Sol-Gel Sci. Technol. 1998, 12, 169–179. [Google Scholar] [CrossRef]
- Yadav, R.; Tirumali, M.; Wang, X.; Naebe, M.; Kandasubramanian, B. Polymer composite for antistatic application in aerospace. Def. Technol. 2020, 16, 107–118. [Google Scholar] [CrossRef]
- Larsson, A. The interaction between a lightning flash and an aircraft in flight. C. R. Phys. 2002, 3, 1423–1444. [Google Scholar] [CrossRef]
- Gagné, M.; Therriault, D. Lightning strike protection of composites. Prog. Aerosp. Sci. 2014, 64, 1–16. [Google Scholar] [CrossRef]
- Agustín-Sáenz, C.; Martín-Ugarte, E.; Jorcin, J.B.; Imbuluzqueta, G.; Santa Coloma, P.; Izagirre-Etxeberria, U. Effect of organic precursor in hybrid sol-gel coatings for corrosion protection and the application on hot dip galvanised steel. J. Sol-Gel Sci. Technol. 2019, 89, 264–283. [Google Scholar] [CrossRef]
- Verma, S.; Gupta, M.; Misra, J.P. Friction stir welding of aerospace materials: A state of art review. In Chapter 13 in DAAAM International Scientific Book 2016; Katalinic, B., Ed.; DAAAM International: Vienna, Austria, 2016; pp. 135–150. ISBN 978-3-902734-09-9. [Google Scholar]
- MIL-DTL-81706B. Chemical Conversion Materials for Coating Aluminium and Aluminium Alloys; Naval Air Warfare Center Aircraft Division: Lakehurst, NJ, USA, 2006. [Google Scholar]
- ECSS-Q-ST-70-02C. Space Product Assurance, Thermal Vacuum Outgassing Test for the Screening of Space Materials; ECSS Secretariat ESA-ESTEC, Requirements & Standards Division: Noordwijk, The Netherlands, 2008. [Google Scholar]
- MIL-DTL-5541F. Chemical Conversion Coatings on Aluminum and Aluminum Alloys; Naval Air Warfare Center Aircraft Division: Lakehurst, NJ, USA, 2006. [Google Scholar]
- ISO 4288. Geometrical Product Specifications (GPS)—Surface Texture: Profile Method—Rules and Procedures for the Assessment of Surface Texture; International Organization for Standardization: Geneva, Switzerland, 1996. [Google Scholar]
- ECSS-Q-ST-70-04C. Thermal Testing for the Evaluation of Space Materials, Processes, Mechanical Parts and Assemblies; ECSS Secretariat ESA-ESTEC, Requirements & Standards Division: Noordwijk, The Netherlands, 2008. [Google Scholar]
- Scriven, L. Physics and applications of dip coating and spin coating. Mater. Res. Soc. Symp. Proc. 1988, 121, 717–729. [Google Scholar] [CrossRef]
- Faustini, M.; Louis, B.; Albouy, P.A.; Kuemmel, M.; Grosso, D. Preparation of sol-gel films by dip-coating in extreme conditions. J. Phys. Chem. C 2010, 114, 7637–7645. [Google Scholar] [CrossRef]
- Feng, Z.; Frankel, G.S. Evaluation of coated Al alloy using the breakpoint frequency method. Electrochim. Acta 2016, 187, 605–615. [Google Scholar] [CrossRef] [Green Version]
- Martins, N.C.T.; Moura e Silva, T.; Montemor, M.F.; Fernandes, J.C.S.; Ferreira, M.G.S. Electrodeposition and characterization of polypyrrole films on aluminium alloy 6061-T6. Electrochim. Acta 2008, 53, 4754–4763. [Google Scholar] [CrossRef]
- Martins, N.C.T.; Moura e Silva, T.; Montemor, M.F.; Fernandes, J.C.S.; Ferreira, M.G.S. Polyaniline coatings on aluminium alloy 6061-T6: Electrosynthesis and characterization. Electrochim. Acta 2010, 55, 3580–3588. [Google Scholar] [CrossRef]
- Trueba, M.; Trasatti, S.P. Pyrrole-based silane primer for corrosion protection of commercial Al alloys. Part I: Synthesis and spectroscopic characterization. Prog. Org. Coat. 2009, 66, 254–264. [Google Scholar] [CrossRef]
- Trueba, M.; Trasatti, S.P. Pyrrole-based silane primer for corrosion protection of commercial Al alloys. Part II. Corrosion performance in neutral NaCl solution. Prog. Org. Coat. 2009, 66, 265–275. [Google Scholar] [CrossRef]





| AlMgSi Alloy | Si | Fe | Cu | Mn | Mg | Cr | Zn | Ti | Al |
|---|---|---|---|---|---|---|---|---|---|
| 6082 T6 | 0.90 | 0.43 | 0.09 | 0.45 | 0.90 | 0.03 | 0.09 | 0.03 | Balance |
| 6061 T6 | 0.68 | 0.36 | 0.18 | 0.08 | 1.1 | 0.17 | <0.02 | <0.03 | Balance |
| 6063 T6 | 0.46 | 0.23 | <0.05 | 0.05 | 0.52 | <0.05 | 0.03 | <0.03 | Balance |
| Si Part | Zr Part | |
|---|---|---|
| Sol A | TEOS/GPTMS/BPA/n-propanol/H2SO4 0.1M Molar ratio 1:1:0.5:2:4 | TPOZ/AcAc/n-propanol/H2SO4 0.1M Molar ratio 1:1.4:16:3 |
| Sol AC | TEOS/GPTMS/BPA/n-propanol/H2SO4 0.1M Molar ratio 1:1:0.5:2:4 | TPOZ/AcAc/n-propanol/H2SO4 0.1M Molar ratio 1:1.4:5.4:3 |
| Sol B | TEOS/GPTMS/BPA/n-propanol/H2SO4 0.1M Molar ratio 1:2:1:2:6 | TPOZ/AcAc/n-propanol/H2SO4 0.1M Molar ratio 1:1.4:16:3 |
| AlMgSi Alloy | Sol Formulation | Withdrawal Rate (cm/min) | Coating |
|---|---|---|---|
| AA6082 T6 | Sol A | 5 | A5-coating |
| 15 | A15-coating | ||
| Sol AC | 5 | AC5-coating | |
| 15 | AC15-coating | ||
| Sol B | 5 | B5-coating | |
| 15 | B15-coating | ||
| AA6061 T6 | Sol A | 5 | A5-coating |
| 15 | A15-coating | ||
| 35 | A35-coating | ||
| AA6063 T6 | Sol A | 5 | A5-coating |
| 15 | A15-coating | ||
| Sol A-AgNW | 5 | AgNW-coating |
| Zr (at. %) | Si (at. %) | C (at. %) | O (at. %) | Stoichiometry | ||
|---|---|---|---|---|---|---|
| A-coating | 3.6 1 | 18.1 1 | 45.4 1 | 32.9 1 | ZrO1.5 | SiO1.4 |
| B-coating | 2.7 | 13.9 | 53.5 | 30.0 | ZrO1.8 | SiO1.2 |
| mg/cm2 | g/cm3 | TML (%) | RML (%) | WVR (%) | CVCM (%) | |
|---|---|---|---|---|---|---|
| A15-coating | 0.54 | 1.13 | 5.157 ± 0.392 | 1.404 ± 0.033 | 3.753 ± 0.455 | 0.009 ± 0.002 |
| AC15-coating | 0.80 | 1.03 | 5.483 ± 0.400 | 1.318 ± 0.153 | 4.165 ± 0.495 | 0.006 ± 0.003 |
| B15-coating | 1.35 | 1.53 | 3.551 ± 0.035 | 1.791 ± 0.037 | 1.760 ± 0.059 | 0.303 ± 0.132 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agustín-Sáenz, C.; Santa Coloma, P.; Fernández-Carretero, F.J.; Brusciotti, F.; Brizuela, M. Design of Corrosion Protective and Antistatic Hybrid Sol-Gel Coatings on 6XXX AlMgSi Alloys for Aerospace Application. Coatings 2020, 10, 441. https://doi.org/10.3390/coatings10050441
Agustín-Sáenz C, Santa Coloma P, Fernández-Carretero FJ, Brusciotti F, Brizuela M. Design of Corrosion Protective and Antistatic Hybrid Sol-Gel Coatings on 6XXX AlMgSi Alloys for Aerospace Application. Coatings. 2020; 10(5):441. https://doi.org/10.3390/coatings10050441
Chicago/Turabian StyleAgustín-Sáenz, Cecilia, Patricia Santa Coloma, Francisco J. Fernández-Carretero, Fabiola Brusciotti, and Marta Brizuela. 2020. "Design of Corrosion Protective and Antistatic Hybrid Sol-Gel Coatings on 6XXX AlMgSi Alloys for Aerospace Application" Coatings 10, no. 5: 441. https://doi.org/10.3390/coatings10050441





